Abstract
Maternal behavior in rats undergoes considerable plasticity in parallel to the developmental stage of the pups, resulting in distinct patterns of maternal behavior and care at different postpartum time points. The medial preoptic area (mPOA) of the hypothalamus is one critical neural substrate underlying the onset and early expression of maternal behavior in rats but little is known about its specific functional role in the evolving expression of maternal behavior across the postpartum period. The present study uses a reversible local neural inactivation method to examine the role of the mPOA in the regulation of maternal behavior throughout the postpartum period, particularly extending into the late postpartum, a little examined period. This approach avoids the compensatory plasticity in CNS that occurs after permanent lesions, and allows the repeated testing of same individuals. Early (PPD7-8) and late (PPD 13-14) postpartum maternal behavior was evaluated in female rats following infusions of bupivacaine or vehicle into the mPOA or into control areas. As expected, mPOA inactivation severely but transiently disrupted early postpartum maternal behavior whereas infusion of vehicle or inactivation of adjacent control sites did not. Later in the postpartum period, however, transient mPOA inactivation facilitates the expression of maternal behaviors, highly contrasting the behavioral expression levels characteristic of late postpartum. Results strongly demonstrate that the mPOA is differentially engaged throughout postpartum in orchestrating appropriate maternal responses with the developmental stage of the pups.
Keywords: bupivacaine, maternal behavior, medial preoptic area, neural inactivation, postpartum period, pup-related stimuli
Introduction
The maternal behavior of female rats during the progression of the postpartum period is not static and unchanging, but rather is dynamic and plastic, changing in response to the developing behavioral capacities and physiological needs of the pups. These changes result in distinct patterns of maternal behavior and care at different postpartum time points [25, 28, 29, 57, 79, 81, 86, 87, 104]. This exquisite capacity for plasticity in the behavioral repertoire of postpartum females must necessarily involve structures in the neural network that mediate maternal responsiveness, which are sensitive to the incoming changing information from the developing pups. To date, however, no study has considered the behavioral flexibility characteristic of mother rats throughout the postpartum period when examining the neural basis of postpartum maternal behavior. The majority of research in this area has focused on the neural basis underlying either the onset of maternal behavior at parturition or its isolated expression at a unique time point in the immediate early postpartum period. Consequently, virtually nothing is known about the functional reorganization the maternal circuitry undergoes with the progression of the postpartum period to allow for the behavioral evolution of maternal care-giving responses across postpartum.
Among the brain structures critically involved in postpartum maternal responsiveness, it is widely believed that the medial preoptic area (mPOA) acts as a primary locus of integration, orchestrating the effective expression of maternal behavior to the developmental stage of the pups across postpartum. In support of this, the mPOA has been demonstrated to be necessary for both the onset [12, 26, 39, 40, 60, 73, 88], and early expression [12, 26, 39, 43, 59, 60, 65, 73] of maternal behavior in rats. The mPOA receives converging pup-related information from multiple sensory modalities [62] and is a primary neural site where the hormones of pregnancy act to promote maternal responsiveness to pup-related stimuli at parturition [6, 7, 73, 78]. Further, important connections with the mesolimbic dopamine system, known to mediate the activational aspects of maternal behavior [9, 11, 17, 23, 24, 31, 32, 41, 45, 74, 79, 81, 93, 94, 100, 101], potentially allow pup-responsive mPOA neurons to influence (the motor processes of the limbic motor system underlying maternal behavior, promoting) the female’s responsivity to pups [61] with expression of appropriate maternal behaviors across postpartum.
While it is generally postulated that the mPOA is a crucial area responsible for integrating the sensory feedback from the pups to tailor appropriate maternal responsiveness through mPOA output circuits, including the mesolimbic dopamine system, this postulate rests on data collected from initial phases of the postpartum period. The aim of the present study was to investigate the functional role of the mPOA in the expression of maternal behavior at two distinct behavioral stages characterizing early and late postpartum period.
A methodological approach using transient site specific neuronal inactivation by infusion of bupivacaine hydrochloride (an amide local anesthetic which blocks voltage-dependent sodium channels) was chosen because it allows the analysis of the function of mPOA in a temporally specific manner. This approach avoids the complication of the compensatory plasticity that likely results from permanent lesions, and thus avoids the impact of time-dependent neural reorganization of the remainder of the neural circuit in question and the potential complications in the interpretation of the outcome [1, 38, 46]. Furthermore, reversible inactivation can be repeated during multiple experimental sessions in the same animal, allowing the longitudinal study of maternal behavior of the same individual females across postpartum (i.e. to serve as their own controls), which increases data reliability [46, 52]. Bupivacaine was selected over other pharmacological agents (i.e. other NA+ channel blockers like TTX or specific to a particular receptor, such as the GABAA receptor agonist muscimol), to effectively suppress neural activity of the entire mPOA, based on its (1) fast induction time/onset of action (within minutes), (2) a duration of effect up to 1 h versus several hours or days [33, 52, 111], and (3) considerably smaller functional spread relative to muscimol or TTX, since the same infusion volumes of commonly-used doses of muscimol or TTX are established to functionally inactivate at least twice as much brain tissue [2, 5, 13, 15, 36, 51, 52, 75, 76, 82, 102]. Further, given the neurochemical heterogeneity within the mPOA we were interested in a total inactivation rather than partial interference related with a specific neurotransmitter action or to specific subcellular mechanisms.
Materials and Methods
Animals
Primiparous postpartum Sprague-Dawley female rats (original stock from Charles River Laboratories, Kingston, NY) bred in our colony at the Rutgers University Laboratory Animal Facility (accredited by the American Association for Accreditation of Laboratory Animal Care) were used in this study. Before giving birth, pregnant females were housed in individual transparent maternity cages (48.5 cm long×38.5 cm wide×20.5 cm high) lined with fresh woodchip bedding (Beta chip, Northeastern Products Corp., Warrensburg, NY) and containing shredded paper towels as nest-building material. The floors of these cages were divided into four equal compartments by 5-cm-high Plexiglas dividers. All females were kept on a 12-hr light/dark cycle (light on at 0700AM) at 22 ± 1 °C, with ad libitum access to water, rat chow (PMI Lab Diet 5008, Nutrition International, LLC, Brentwood, MO) and sunflowers seeds. On postpartum day 1 (birth=day 0), litters were culled to four male and four female pups per dam, and all postpartum females were maintained and tested with their own pups.
All animal care and experimental procedures performed in this study were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), and were reviewed and approved by the Rutgers University Animal Care and Facilities Committee. All efforts were made to minimize the number of animals used and their suffering.
Experimental Groups and General procedures
Females were randomly assigned to one of the following groups: (1) females stereotaxically implanted with bilateral guide cannulae aimed at the mPOA, or (2) at locations aimed immediately outside the mPOA: (a) dorsal control and (b) lateral, rostral, or caudal control groups, and (3) females not subjected to stereotaxic surgery (behavioral control group).
A within-subject design was used to examine the functional role of the mPOA in maternal behavior over two distinct stages of the postpartum period. Each postpartum female was tested with her own pups for maternal behavior at both early (PPD7 and 8) and late (PPD13 and 14) postpartum days, following intracranial infusion of either 2% bupivacaine HCl or saline vehicle. Within each independent group, half of the females were first injected with bupivacaine and the other half with saline. The treatment conditions were reversed the next day in a counterbalanced design. Thus, postpartum females received two bupivacaine infusions, one on PPD7 or 8, and the second one on either PPD13 or 14; and two saline infusions, one during early postpartum and the second one during late postpartum.
To investigate the possibility that repeated bupivacaine infusion could affect the subsequent expression of late postpartum maternal behavior, an additional group of mPOA-cannulated females was prepared. These females were treated in the exact same way as the other groups, except that they only received mPOA infusions in late postpartum. Their maternal behavior toward their own pups was only tested at PPD13 and 14, following intracranial infusion of either bupivacaine or saline vehicle.
Stereotaxic Surgery
On day 1 postpartum, females were anesthetized with 1.0mL/kg of a solution that contained ketamine HCl (75.0 mg/mL), xylazine (7.5 mg/mL) and acepromazine maleate (1.5mg/mL). All coordinates are for animals first set with flat skull readings for bregma and lambda. All females received bilateral implantations of 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA). For the mPOA site, guide cannulae were implanted 2.0 mm dorsal to target at the following coordinates: AP-0.5 (from bregma), ML±0.5 (from midline), and DV-6.5 (from the skull surface). For the dorsal control site, guide cannulae were implanted at the following coordinates: AP-0.5 (from bregma), ML±0.5 (from midline), and DV-5.0 (from the skull surface); lateral POA controls were carried out with AP-0.5 (from bregma), ML±1.5 (from midline), and DV-6.5 (from the skull surface). The guide cannulae were secured to the skull with stainless steel screws and cranioplastic cement. To maintain patency of the cannulae prior to injection, stylets were inserted. Immediately after surgery, females were reunited with their pups in their home cages, and allowed to recover for one week. Overall condition and health were evaluated daily, particularly after surgery and up to the end of the experiment. All females remained healthy across the experiment, and their pups gained weight and developed normally.
Intracranial Injection Procedures
Habituation
One day before behavioral testing, females were habituated to the intracranial infusion and testing procedure. After a 10-min separation from their litters, females were removed from their home cage, transported to an adjacent room where they were gently handled for approximately 5 min, during which their dummy stylets were removed and reinserted. Immediately after, they were brought back to their home cage. Five min later, their eight pups were scattered in the home cage across the nest site and a maternal behavior test was performed. Behavioral control females did not undergo surgery but were similarly handled and tested. All cannulated females exhibited typical maternal behaviors that were identical to those displayed by behavioral control females.
Intracranial Infusions
On behavioral test days, after a 10-min separation from their litters, females were removed from their home cage and transported to the adjacent room. The stylets were removed and 28-gauge stainless steel injectors, extending 2.0 mm beyond the tip of the guide cannulae were inserted. Injectors were connected by PE-10 tubing to 10μL Hamilton syringes, and bilateral infusions were driven simultaneously by a two-syringe infusion pump (Harvard 22 syringe pump; Harvard Apparatus, Holliston, MA). Infusions of bupivacaine (2% solution; Sigma, St. Louis, MO) or saline vehicle were delivered at a rate of 0.5μL/min. Each side received 1.0 μL total volume, and the injectors were left in place for an additional 1 min to allow for diffusion of the drug. Immediately after, stylets were replaced, the females were brought back into their home cage and a 30-min maternal behavior test was performed.
Bupivacaine hydrochloride in a dose of 20mg/mL dissolved in 0.9% saline solution was used to reversibly produce site specific neural inactivation. Bupivacaine, like the structurally similar amide-linked anesthetic lidocaine, blocks voltage-dependent sodium channels, and hence action potential initiation and conduction, both in neuronal cell bodies and axons [34, 35]. We chose bupivacaine due to its longer duration of action over lidocaine (lidocaine approximately 10–30 min range, bupivacaine approximately 30–50 min range; [10]). The bupivacaine dose and volume used in this study were selected on the basis of previous studies of estimates of the effective spread (i.e. the distance over which there is a physiological effect) and time course of neural inactivation caused by intracranial lidocaine infusion [5, 50, 82, 90, 91, 102]. Furthermore, relative to a smaller, 0.5 uL infusion volume, an infusion volume of 1.0uL has been demonstrated to have a similar spread of functional inactivation in terms of effectiveness, but produced a more stable inactivation over time [102, 108]. Accordingly, in a preliminary experiment, a more robust and consistent effect over time in the maternal behavior of postpartum females was observed following 1.0uL versus 0.5 uL infusion of 2% bupivacaine into the mPOA. For these reasons, an infusion of 1.0uL of 2% bupivacaine was selected for the present studies to effectively suppress neural activity of the entire mPOA and during the entire 30-min behavioral test.
Maternal Behavior Testing
All testing was conducted during the light phase of the light/dark cycle. Following infusions of either bupivacaine or saline, postpartum females were returned to their home cage. Five minutes after, their eight pups were scattered in the home cage opposite to the female’s nest. The number of the following maternal behavioral components was continuously recorded for 30 min: retrievals of the pups into the nest, mouthings (oral repositioning of the pups into the nest), sniffings, full body and anogenital lickings, and nest building. In addition, the total duration of huddling behaviors, including lying in contact with pups and hovering over the pups in the nest while actively performing other behaviors (i.e. licking of pups or self grooming), and the nursing posture kyphosis, a quiescent upright crouching over pups [99] were recorded. Total time in contact with pups was the summed durations of huddling plus nursing behaviors. Also, the latency (i.e. time from the introduction of the pups to the first behavioral response by the female) to begin retrieving pups, to reunion of the entire litter into the nest as well as to begin hovering over and nursing was registered. Only those postpartum females that retrieved each of their eight pups into the nest were considered to have grouped the litter. The latency to begin hovering over or nursing the pups was the first occurrence of a bout of each behavior ≥2 min in duration. A latency of 1800 s was given for any category of behavior that was not initiated (or completed, i.e. reunion of the litter) within the 30-min observation period. Other behaviors recorded included general exploration (line crosses and rearings), self-grooming and eating/drinking.
On the morning following each intracranial injection, the maternal behavior of postpartum females was observed to verify that there were no remaining behavioral effects from the previous day’s treatment. No behavioral impairments were found, i.e. the maternal behavior of all subjects was completely normal by each post-injection morning.
Locomotor activity
To identify any nonspecific locomotor effect induced by intracranial bupivacaine infusions, the locomotor activity of additional mPOA cannulated postpartum females was evaluated in computerized photocell activity boxes (VersaMax Animal Activity Monitor system, AccuScan Instruments Inc., Columbus, OH) over a 60-min test following infusion of either bupivacaine (n=5) or vehicle (n=4).
Histology and Analysis of infusion Sites
Upon completion of the study, postpartum females were deeply anesthetized with Nembutal and perfused intracardially with physiological saline followed by a 4.0% formaldehyde solution. Brains were removed and stored in 4.0% formaldehyde for 48h. The brains were frozen using dry ice and serial cross sections of 30μm-thick were cut around the implantation site on a cryostat. The sections were mounted on slides, stained with Cresyl violet, and cover slipped. Cannulae placements were verified with the aide of a Zeiss Axioplan microscope equipped with a Neurolucida imaging system, according to the neuroanatomical atlas of Paxinos and Watson [77].
Statistics
Maternal behavior data are expressed as median (semi-interquartile ranges [SIQR]). As variances were not homogeneous, behavioral data were analyzed by means of non-parametric tests [92]. Kruskal-Wallis one-way analysis of variance followed by Mann—Whitney U tests were used for comparisons of independent groups. Wilcoxon matched-pairs signed-ranks test were used for intragroup comparisons. Locomotor activity data are expressed as mean ± SEM, and were analyzed using one-way repeated measures ANOVA. Statistical significance in all cases was P<0.05, two-tailed probabilities.
Results
Location of the Cannulae Implantation Site
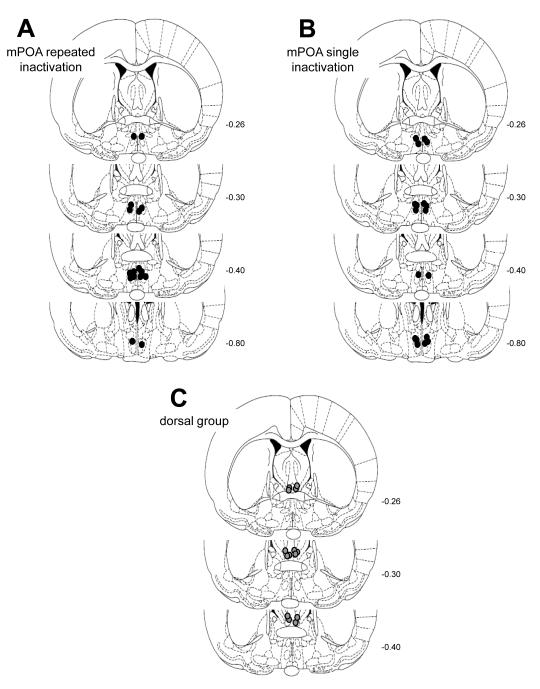
Figs. 1A-C represents cross sections of the rat brain showing the placement of the injection cannulae tips based on the microscopic analysis of cresyl violet-stained sections. Figure 1A show the placement of the injection cannula tips for the mPOA group providing repeated measure testing at both early (PPD7-8) and late postpartum (PPD13-14) stages (n=8). Figure 1B shows the location of the mPOA infusion sites for the group of postpartum females tested only during PPD13-14 (n=9). Histological analysis indicated that both mPOA groups had infusion sites distributed in a similar pattern, with most of the bilateral infusion sites located between -0.26 and -0.88 mm from bregma.
Figure 1.
Schematic representation, based on the microscopic analysis of cresyl violet-stained sections, of infusion sites for female rats tested for maternal behavior following infusion treatments. (A) mPOA cannulated postpartum females tested at both early and late postpartum stages; (B) mPOA cannulated postpartum females tested only in late postpartum, and (C) postpartum females with cannulae located immediately dorsal to the mPOA (dorsal control). Circles represent the most ventral extent of the injector tracks in the brain. Plates were taken from the atlas of Paxinos and Watson (1997). Numbers beside each plate indicate the distance from bregma in millimeters.
Postpartum females included in the dorsal control group (n=7) had cannulae bilaterally placed dorsal to the anterior commissure, ranging between -0.26 and -0.40mm from bregma (Figure 1C). Nine additional postpartum females had infusion sites that bilaterally targeted control structures outside of the mPOA. One female had an asymmetrical placement that resulted in a unilateral infusion into the lateral mPOA and a unilateral infusion into the third ventricle, 3 females had their cannulae lateral to the mPOA into the LPOA, 2 females had their cannulae rostral to the mPOA into the diagonal band of Broca; and 3 females had their cannulae caudal to the mPOA into the anterior hypothalamus.
Transient mPOA inactivation reversibly inhibited early postpartum maternal behavior
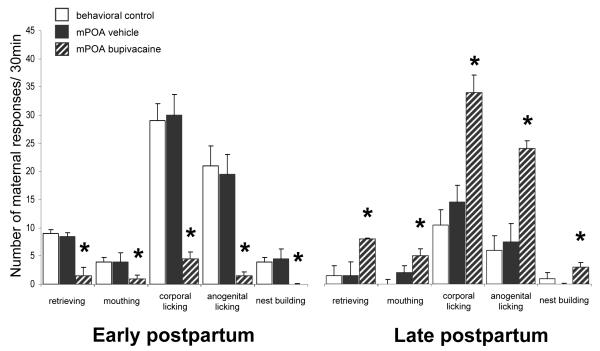
Early postpartum females receiving bupivacaine infusion into the mPOA exhibited severe deficits in their maternal behavior. As shown in Figure 2, bupivacaine-treated postpartum females exhibited significantly fewer retrievals (T=0.0, N=8, P=0.012, Wilcoxon’s matched pairs signed-ranks test) and mouthings (T=0.0, P=0.012) than after receiving saline infusions into mPOA (Figure 2). In fact, during mPOA inactivation, none of the 8 postpartum females completed retrieving and grouping the pups into the nest; 4 of 8 did not retrieve any pups, and the remaining 4 retrieved ≤3 pups of their 8-pup litter. Moreover, the number of corporal (T=0.0, P=0.012) and anogenital lickings (T=0.0, P=0.012) was also dramatically reduced, and nest building was virtually absent (T=0.0, P=0.02). It is important to mention that the inserted cage barriers delayed, but did not prevent the pups from crawling into the nest area and grouping together in a huddle. Thus, even though none of the bupivacaine-treated postpartum females completed retrieving and grouping of the pups, their litters did group themselves into the nest within 594.5 ± 118.38 s, where they generally stayed together until the end of the behavioral test. Although most mPOA-inactivated females did not group their pups into the nest, they did approach and investigate them, and eventually hovered over the pups and nursed them (Table). In fact, the percentage of females that did hover over and nurse their litters was not significantly different after bupivacaine and vehicle infusions (6/8 and 8/8, respectively; Fisher exact probability test P=ns). However, mPOA-inactivated females spent significantly less time in contact with pups (T=0.0, P=0.012), due to an increased latency for dams to begin hovering over the pups (T=0.0, P=0.012; see Table).
Figure 2.
Effect of transient inactivation of the mPOA on median ± SIQR number of active maternal responses over a 30 min-behavioral test. Female rats were tested on early PPD 7 and 8 and then again on late PPD13 and 14. Data shown is for the behavioral control females and for mPOA cannulated females following either vehicle or inactivation treatment with 2% bupivacaine. *Significant difference at P<0.05 on bupivacaine versus saline test days (within-group comparison, Wilcoxon matched-pairs signed-ranks test) and versus the behavioral control females (between-group comparison, Mann-Whitney U test).
Latencies and durations of maternal behaviors
| EARLY POSTPARTUM | LATE POSPARTUM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects with infusions | Subjects with infusions | ||||||||
| Non-surgical control |
Infusion | mPOA repeated inactivation |
dorsal control |
Non-surgical control |
Infusion | mPOA repeated inactivation |
mPOA single inactivation |
dorsal control |
|
| Latency (seconds) | |||||||||
| First retrieval | 5.0±3.0 | vehicle | 2.0±0.7 | 3.0±1.0 | 88.5±887.5 | vehicle | 93.5±877.4 | 87.0±893.0 | 113.0±876.0 |
| bupivacaine | 648.5±556.5*# | 4.0±2.5 | bupivacaine | 14.0±5.1*# | 2.0±19.0*# | 90.0±915.0 | |||
| Reunion litter | 65.0±11.7 | vehicle | 75.0±14.8 | 63.0±3.5 | 1800.0±825.9 | vehicle | 1800.0±217.7 | 1800.0±0.0 | 1800.0±436.7 |
| bupivacaine | 1800±0*# | 68.0±6.0 | bupivacaine | 103.0±35.0*# | 105.0±77.0*# | 1800.0±443.0 | |||
| Hover over | 273.0±98.7 | vehicle | 174.0±37.0 | 188.0±74.0 | 270.5±121.1 | vehicle | 251.0±107.5 | 242.0±50.0 | 342.0±146.7 |
| bupivacaine | 959.5±348.7*# | 238.0±82.5 | bupivacaine | 198.0±33.5 | 201.0±56.5 | 285.0±105.0 | |||
| Nursing posture | 910.0±155.2 | vehicle | 830.0±72.0 | 900.0±129.0 | 874.0±294.0 | vehicle | 798.0±215.7 | 849.0±202.5 | 801.0±82.0 |
| bupivacaine | 1105.0±455.6 | 870.0±35.0 | bupivacaine | 732.0±142.5 | 827.0±58.0 | 798.0±252.5 | |||
| Duration (seconds) | |||||||||
| Hover over | 788.0±170.0 | vehicle | 808.5±109.2 | 765.0±115.5 | 495.5±103.1 | vehicle | 518.7±65.4 | 580.0±155.5 | 559.0±117.0 |
| bupivacaine | 179.0±138.4*# | 888±119.5 | bupivacaine | 617.0±119.5 | 647.0±127.5 | 582.0±136.2 | |||
| Nursing posture | 590.0±102.0 | vehicle | 630.0±147.7 | 612.0±40 | 623.0±168.4 | vehicle | 716.5±85.125 | 599.0±144.0 | 676.0±166.2 |
| bupivacaine | 404.5±359.6 | 444±143.5 | bupivacaine | 958.0±192.25 | 752.0±138.5 | 652.0±86.0 | |||
| Total time with pups | 1358.0±217.5 | vehicle | 1563.5±87.5 | 1581.0±94.5 | 1180.5±208.1 | vehicle | 1265.0±145.25 | 1421.0±315.0 | 1458.0±96.2 |
| bupivacaine | 784.0±348.7*# | 1427.0±189.5 | bupivacaine | 1308.0±209.2*# | 1453.0±1115.0*# | 1375.0±82.5 | |||
Data are expressed as median ± SIQR.
Significant (P<0.05) difference in responding relative to the vehicle infusion (within group comparison, Wilcoxon matched-pairs signed-ranks test )
significant difference relative to non-surgical control females (between group comparison, Mann—Whitney U test ).
Importantly, infusion of saline vehicle into mPOA had no significant effect on any maternal component measured (Figure 2 and Table). Thus, early postpartum females receiving vehicle infusion into the mPOA exhibited full maternal behavior that was not different from the unoperated control group (Mann-Whitney U test, all Ps=ns).
Transient mPOA inactivation reversibly increased late postpartum maternal behavior
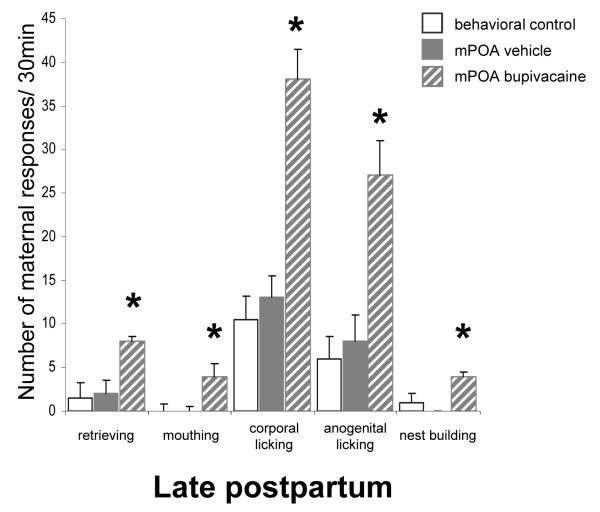
Inactivation at the very same mPOA location had a completely different effect during late postpartum, causing a significant increase in maternal behavior compared to that seen after vehicle infusion (Figure 2). Thus, bupivacaine-treated postpartum females that exhibited impaired maternal behavior during early postpartum showed notably increased maternal behavior when infused with bupivacaine in late postpartum (Figure 2 and Table). Importantly, the additional mPOA group tested only in late postpartum similarly showed increased maternal behavior toward pups following bupivacaine compared to saline infusions (Figure 3 and Table), in a manner that did not differ from the group that received repeated bupivacaine infusions. (Repeated mPOA inactivation vs. Single mPOA inactivation, all Ps=ns, Mann-Whitney U test). The great majority of bupivacaine-treated late postpartum females completed retrievals and grouped all pups into the nest (Table). Of the 17 late postpartum females (both mPOA groups), 16 retrieved all pups and the remaining subject retrieved 3 of the 8-pup litter after infusion of bupivacaine, while after saline infusion only 3 of these same 17 females grouped all pups, 9 retrieved ≤4 pups and the remaining 5 did not retrieve any pups at all. Concerning those 14 vehicle-treated females that did not complete retrieving, their pups grouped together into the nest by themselves with a median latency of 283.0 ± 81.0 s. In marked contrast, following bupivacaine infusion into the mPOA these same late postpartum females retrieved and grouped their litter into the nest, and did so significantly faster. Once the pups were grouped into the nest, they usually stayed together in a close group. Furthermore, it was observed that 13-14 day-old pups sometimes struggled when picked up by their mother, but this behavior from the pups neither prevented bupivacaine-treated late postpartum females to complete retrievals, nor increased the number of retrievals per pup needed by the mothers to group the entire litter into the nest (Figures 2 and 3, Table). This highlights the robust activational effect on the maternal behavior of late postpartum females following bupivacaine infusion into the mPOA. The numbers of mouthings (T=0.0, N=17, P=0.001, Wilcoxon’s matched pairs signed-ranks test), corporal (T=0.0, P=0.0003) and anogenital licking (T=0.0, P=0.0003), and nest building (T=6.0, P=0.001) behaviors also increased significantly when females received bupivacaine versus when these same females received a saline infusion into the mPOA (Figures 2 and 3). Further, late postpartum females spent more time in contact with pups after mPOA infusion of bupivacaine than saline vehicle (T=15.0, P=0.004).
Figure 3.
Replication of late postpartum group: Effect of transient inactivation of the mPOA on median ± SIQR number of active maternal responses over a 30 min-behavioral test. An additional independent group of mPOA cannulated female rats was tested only on late PPD13 and 14, following either vehicle or bupivacaine treatment. *Significant difference at P<0.05 on bupivacaine versus saline test days (within-group comparison, Wilcoxon matched-pairs signed-ranks test) and versus the behavioral control females (between-group comparison, Mann-Whitney U test).
Importantly, no significant differences were found between behavioral control and either vehicle-treated late postpartum female group, in any maternal behavior measured (Mann-Whitney U tests: all Ps=ns; Figures 2 and 3, Table).
Demonstration of regional specificity
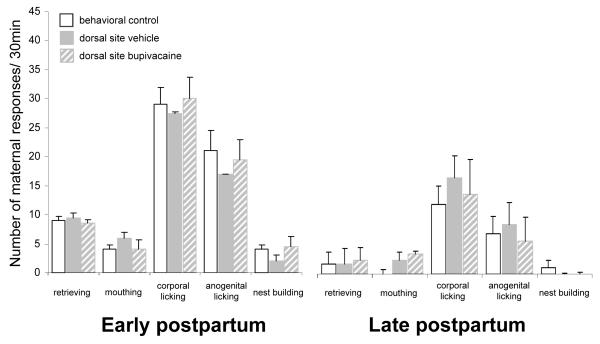
Females with infusion sites that bilaterally targeted control structures outside of the mPOA did not show any change in maternal behavior after bupivacaine or saline infusions (dorsal, n=7; rostral, n=2; lateral n=3; and caudal to the mPOA, n=3). In particular, neither bupivacaine nor saline infusion into adjacent dorsal sites to the mPOA had any effect on the expression of early and late maternal behavior (Wilcoxon’s matched pairs signed-ranks tests: N=7, all Ps=ns; Figure 4 and Table). Moreover, the maternal behavior of these anatomical control females was not different from that of behavioral control females (Mann-Whitney U tests: all Ps=ns; Figure 4 and Table).
Figure 4.
Effect of transient inactivation of dorsal sites to the mPOA on median ± SIQR number of active maternal responses over a 30 min-behavioral test. No significant differences in early and/or late postpartum maternal behavior were found in females following vehicle and bupivacaine treatment.
Locomotor activity
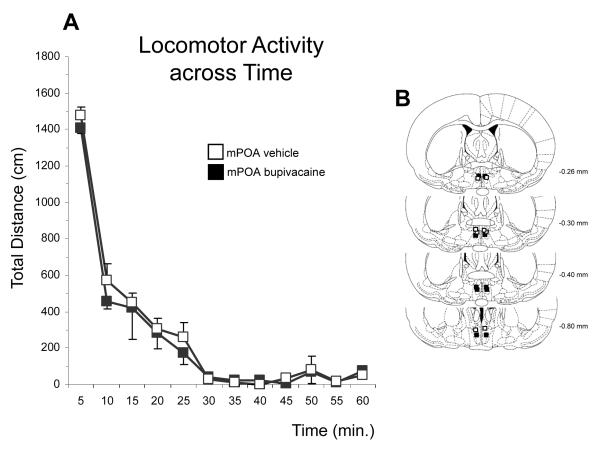
Home cage activity, including general exploration and self grooming did not differ both within and between groups (all Ps=ns, data not shown). Moreover, mPOA inactivation did not alter the locomotor activity of postpartum females relative to vehicle treatment, measured in the automated apparatus (time by treatment interaction: F (11, 77) = 0.2684, P=ns; Figure 5).
Figure 5.
A. Locomotor activity (mean ± SEM) graphed in 5-min intervals over a 60min-test session for postpartum females with bilateral infusions of either 2% bupivacaine or vehicle into the mPOA. B. Schematic representation, based on the microscopic analysis of cresyl violet-stained sections, of infusion sites for female rats tested for locomotor activity. Plates were taken from the atlas of Paxinos and Watson (1997). Numbers beside each plate indicate the distance from bregma in millimeters.
In summary, inactivation of dorsal, lateral, anterior or posterior structures to the mPOA was ineffective in affecting maternal behavior, thereby confirming spatial specificity. Saline injections within mPOA had no effect indicating that neuronal inactivation, not mechanical or osmotic effects of drug injection, affected expression of maternal behavior in postpartum females. Moreover, that fact that saline infusions did not produce any behavioral effects even when preceded by one or two bupivacaine injections - while bupivacaine injections continued to have behavioral effects — shows that the target tissue remains stable over multiple injections. Unilateral mPOA inactivation was also without behavioral effect. Thus, the above described changes in maternal behavior produced by bupivacaine infusion into the mPOA are mainly due to effects within and not beyond mPOA.
Discussion
The present study demonstrates that the mPOA is differentially engaged throughout postpartum in fine-tuning/orchestrating appropriate maternal responses with the developmental stage of the pups. During early postpartum, the mPOA plays a necessary positive role in the regulation of maternal responsiveness (i.e. functional integrity of the mPOA is necessary for the expression of maternal behavior), since its neural inactivation, produced by infusion of bupivacaine, transiently and severely impaired the maternal behavior of early postpartum female rats. In contrast, temporary inactivation of the mPOA in late postpartum reversibly facilitated the expression of maternal behaviors, suggesting that with the progression of the postpartum period, the necessary excitatory role/influence of mPOA outputs wanes in its importance. Further, results suggest that later in the postpartum period, the mPOA might act to suppress other brain structures that may interfere with appropriate expression of maternal behavior. Importantly, the behavioral effects of bupivacaine were specific for maternal behavior, as other behaviors, including locomotor activity in both familiar and unfamiliar environments were not affected by the treatment. Collectively, these data strongly suggest that substantial functional reorganization of the mPOA and connections (i.e. region-specific and pathway-specific changes in synaptic strength), likely mediated by the continuous experience of interaction with developing pups, occur with the progression of the postpartum period to allow for the behavioral evolution of maternal care-giving responses across postpartum.
Transient inactivation of the mPOA severely disrupts early postpartum maternal behavior
As expected, mPOA inactivation severely disrupted the display of early postpartum maternal behaviors whereas infusion of vehicle into the mPOA or inactivation of adjacent dorsal, lateral, rostral or caudal sites to the mPOA did not. It is worth noting, however, that the active components of maternal behavior, including retrieving, licking and nest building were more severely disrupted by bupivacaine inactivation of the mPOA than was nursing behavior. Consistent with the present results, similar findings have been reported following permanent mPOA lesions [39, 40, 60, 63, 103] and pharmacological manipulations [3, 53, 84, 105]. This selective involvement of the mPOA in the expression of active maternal components is further supported by findings showing increased induction of the Fos protein product of the immediate early gene c-fos in the mPOA of early postpartum females following active maternal interaction with pups [18, 20, 47, 48, 67, 68, 70, 71, 106].
Transient inactivation of the mPOA facilitates late postpartum maternal behavior
Remarkably, all bupivacaine-treated late postpartum females retrieved and grouped pups into the nest, and engaged in more licking and nest building relative to controls during the 30-min maternal behavior test. The present results provide strong evidence for the facilitatory effect of mPOA inactivation on late postpartum maternal behavior, as both repeated and single mPOA inactivation groups showed a notable increase in maternal behavior. To our knowledge, this is the first study to demonstrate that the mPOA is differentially engaged during early vs late postpartum in the regulation of maternal responsiveness. For instance, inactivation of the mPOA did not inhibit maternal behavior in late postpartum, suggesting that with the progression of the postpartum period, maternal responsiveness is progressively less dependent on mPOA activity. Not only do the present results demonstrate that mPOA is not necessary for the expression of maternal behavior in late postpartum, the finding that mPOA inactivation facilitated late postpartum maternal behavior suggests that the regulation of maternal responsiveness is more at this point distributed in the maternal circuitry such that additional network components are recruited with maternal experience. An important implication of this is that mPOA may play an active role in suppressing the ability of other brain structures to inappropriately influence maternal behavior in late postpartum.
Importantly, the effects of mPOA inactivation across postpartum were specific to maternal behavior, in that early and late postpartum females did modify the nature of their maternal behavior, but showed similar levels of locomotor activity, exploration, and self-grooming relative to controls. Moreover, no compensatory behaviors, typically displayed by postpartum females to override oromotor impairments [100], were exhibited by any of the bupivacaine-treated females. This is consistent with results from others showing that postpartum females bearing permanent lesions of the mPOA show normal levels of locomotor activity, body weight regulation, and hoarding behavior [26, 64].
Locations and spread of injections/Regional Specificity
Bupivacaine inactivation has been successfully used in behavioral studies to induce effective temporary neural inhibition of discrete brain areas [8, 16, 21, 37, 85, 108]. The effective spread of neural inactivation (i.e. the distance over which there is a physiological effect) of the structurally similar amide-linked anesthetic lidocaine has been well characterized both in cortical and subcortical structures using electrophysiological and other indices of neural activity [5, 50, 82, 90, 91, 102]. Accordingly, the 1.0μL infusion volume used in the present experiments likely produced a spherical area of functional inactivation with an approximate radius of 500-620 μm from the tip of the injector [5, 102], which approximately corresponds to the volume of the mPOA. Given (1) the central location of infusion sites within the mPOA, (2) the limited diffusion of bupivacaine, and (3) the robust effect of bupivacaine on maternal behavior with little intergroup variability, it is highly likely that the vast majority of the physiological effects of bupivacaine injections in the majority of our females remained primarily within, but not beyond the mPOA. Nevertheless, considering the distribution of the mPOA infusion sites in the rostro-caudal axis, in some limited cases, bupivacaine might have diffused rostrally to the caudal aspect of the diagonal band of Broca, and in others, caudally to the most rostral parts of the anterior hypothalamus and anterior paraventricular nucleus of the hypothalamus. However, bupivacaine infusions into the above-mentioned sites, at both early and late postpartum stages, had no effect on the maternal behavior of postpartum females (anatomical controls). In agreement with these findings, previous studies have shown that discrete permanent bilateral lesions of either the anterior hypothalamic area [26], the paraventricular nucleus of the hypothalamus [64], or the ventromedial hypothalamus [30] of early postpartum females did not affect the expression of maternal behavior.
Axon-sparing neurotoxic lesion of either the mPOA [65] or the lateral POA [4, 65], or knife cuts specifically severing the dorsolateral connections between the mPOA and LH/POA [60, 63, 64, 66, 103] have been shown to cause similar disruptions in early postpartum maternal behavior. While it is formally possible that in some cases our bupivacaine infusions could have included the medial regions of the LPOA, these cases are limited in number as most of the mPOA infusion sites were medial enough so that the proposed sphere of spread would be contained within the mPOA. Furthermore, direct bilateral inactivation of the LPOA had no effect on any measure of maternal behavior (present results).
Of greatest concern was spread into regions dorsal to the mPOA, which includes the bed nucleus of the stria terminalis, the anterior commissure and septum, since bupivacaine might have preferentially taken the path of least physical resistance and flow up the injection track dorsally. However, the maternal behavior of postpartum females with injector tips located dorsal to the mPOA, approximately 1.5mm above from those within mPOA, was unaffected by bupivacaine infusions. Nevertheless, it could be argued that mPOA bupivacaine infusions might have spread into the adjacent ventral bed nucleus of the stria terminalis (vBNST), which itself play a positive role in early postpartum maternal behavior [69, 83]. However, as neurochemical lesions that specifically destroy the vBNST without damaging the mPOA are not as critical in disrupting early postpartum maternal behavior as those after mPOA permanent lesions [69, 83], it is reasonable to argue that the robust bupivacaine—induced behavioral effects were primarily due to mPOA inactivation.
As a sodium channel blocker, bupivacaine inactivates cell bodies along with axons terminating in and passing through the inactivated area [34, 35]. However, given that axon-sparing neurotoxic lesions of the mPOA (i.e. permanent lesion targeting only cell bodies; [65] have virtually identical effects on early postpartum maternal behavior as those obtained in the present study with bupivacaine inactivation, we posit that the behavioral effects observed after bupivacaine infusion are attributable to reduced activity of mPOA neurons and their efferent connections.
What is the role of the mPOA in maternal behavior across postpartum?
Collectively, the present data suggest that experience-based reorganization of the maternal circuit occurs to allow for the natural evolution of maternal behavior throughout the postpartum period. In particular, the present results suggest that alterations in mPOA function occur as the postpartum progresses to enable appropriate maternal responses to the different needs of the developing pups. We posit that the rapid and dramatic developmental changes of the pups that the mother rat experiences over the course of the postpartum period impact mPOA processes that consequently result in different patterns of maternal behavior and care at different postpartum time points. Substantial evidence suggests that the mPOA regulates the motivational aspects of early postpartum maternal responsiveness to pups through activation of the mesolimbic dopamine system [61, 62, 72, 74, 95]. In agreement, the impairments in early postpartum maternal behavior seen after transient mPOA inactivation (present results) are remarkably similar to those reported after disruption of mesolimbic dopaminergic system activity [31, 32, 41, 53, 72, 79, 93, 94]. One interpretation of the present results is that mPOA responsiveness to pup-related stimuli progressively declines as the pups develop and consequently a deactivation of the mesolimbic dopamine system occurs as the postpartum period progresses. In other words, mPOA inhibition of the nucleus accumbens progressively declines as pups grow older, consequently reducing the active components of maternal behavior. Consistent with this, we have provided evidence that the characteristic waning of postpartum maternal responsiveness can be considerably postponed if late postpartum females are treated with DA agonists [80, 81] or if they cohabited with young pups [81]. Ongoing work is examining if altered sensitivity of D1 and/or D2 DA receptors within key components of the maternal circuitry is related to the changing expression of maternal responsiveness with the progression of the postpartum period.
In parallel, it appears that with the progression of postpartum, mPOA inhibition of additional network components, presumably involved in stimuli recognition and maternal memory, also importantly contributes to the expression of appropriate maternal behaviors to the particular requirements of the young. Interestingly, the disinhibitory effect of mPOA inactivation on maternal behavior resembled a type of habitual responding, as indicated by a lack of sensitivity of the maternal response to the developmental stage of the pups. Additional circuitry components that may be involved in habitual responding may include medial prefrontal cortex and dorsal striatum, which are interconnected with components of the maternal circuitry, including the mPOA and the NA [27, 110], and have been shown to be respectively involved in stimuli recognition and motor/action learning and habituation [14, 42, 54, 109]. Currently, adequate evidence is not available with respect to this interesting possibility, although previous studies have indicated that maternal behavior relies less on mPOA influence in multiparous female rats [22, 43, 55, 56, 83]. Ongoing work in our laboratory is examining the functional role of these brain areas to further refine the understanding of the neural circuit that supports maternal responsiveness throughout the postpartum period.
Conclusion
Taken together, present results include the novel finding that the mPOA is differentially engaged throughout the postpartum period to orchestrate appropriate maternal responses to the particular needs of the developing pups. During the first week postpartum, the mPOA is a necessary component of the maternal circuit, facilitating the intensive care-giving activity required by the newborn pups. As the postpartum period progresses and the pups grow older, the regulation of maternal responsiveness becomes (with maternal experience) more distributed in the maternal circuit. Still, the mPOA remains sufficiently involved in aspects of maternal responsiveness, allowing the changing expression of maternal behavior that occurs with the progression of the postpartum period.
Acknowledgements
This research was supported by a 2008 NARSAD Young Investigator Award to MP and NIH DA014025 awarded to J.I.M. The authors thank Dr. Andrew M. Farrar for critical comments on the manuscript, and the Laboratory Animal Facility staff at Rutgers University, Newark Campus for animal breeding and care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lorenzini CG Ambrogi, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Neural topography and chronology of memory consolidation: a review of functional inactivation findings. Neurobiol Learn Mem. 1999;71(1):1–18. doi: 10.1006/nlme.1998.3865. [DOI] [PubMed] [Google Scholar]
- [2].Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods. 2002;118(1):51–7. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- [3].Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiol Behav. 2006;87(1):51–65. doi: 10.1016/j.physbeh.2005.08.048. [DOI] [PubMed] [Google Scholar]
- [4].Avar Z, Monos E. Biological role of lateral hypothalamic structures participating in the control of maternal behaviour in the rat. Motility, explorative behaviour, lactation, and the effect of reduced food intake. Acta Physiol Acad Sci Hung. 1969;35(3):285–94. [PubMed] [Google Scholar]
- [5].Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Methods. 2001;105(2):133–41. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- [6].Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87(20):8003–7. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bridges RS, Robertson MC, Shiu RP, Friesen HG, Stuer AM, Mann PE. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology. 1996;64(1):57–64. doi: 10.1159/000127098. [DOI] [PubMed] [Google Scholar]
- [8].Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn Mem. 2006;13(2):187–91. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav. 2002;73:869–75. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- [10].Catterall WA, Mackie K. Local Anesthetics. In: Hardman JG, Limbard LE, Molinoff PB, Ruddon RW, Gilman A, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th Edition McGraw-Hill; New York: 1996. pp. 321–347. [Google Scholar]
- [11].Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cohn J, Gerall AA. Pre- and postpuberal medial preoptic area lesions and maternal behavior in the rat. Physiol Behav. 1989;46(2):333–6. doi: 10.1016/0031-9384(89)90276-x. [DOI] [PubMed] [Google Scholar]
- [13].Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Neurosci. 2005;25(42):9821. Erratum in: [Google Scholar]
- [14].Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [15].Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem. 2002;78(1):100–24. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- [16].Evans SB, Wilkinson CW, Gronbeck P, Bennett JL, Taborsky GJ, Jr, Figlewicz DP. Inactivation of the PVN during hypoglycemia partially simulates hypoglycemia-associated autonomic failure. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R57–65. doi: 10.1152/ajpregu.00439.2002. [DOI] [PubMed] [Google Scholar]
- [17].Ferreira A, Picazo O, Uriarte N, Pereira M, Fernández-Guasti A. Inhibitory effect of buspirone and diazepam, but not 8-OH-DPAT, on maternal behavior and aggression. Pharmacol Biochem Behav. 2000;66:389–96. doi: 10.1016/s0091-3057(00)00211-2. [DOI] [PubMed] [Google Scholar]
- [18].Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110(3):567–82. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- [19].Fleming AS, Sarker J. Experience-hormone interactions and maternal behavior in rats. Physiol Behav. 1990;47(6):1165–73. doi: 10.1016/0031-9384(90)90368-e. [DOI] [PubMed] [Google Scholar]
- [20].Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci. 1994;108(4):724–34. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- [21].Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- [22].Franz JR, Leo RJ, Steuer MA, Kristal MB. Effects of hypothalamic knife cuts and experience on maternal behavior in the rat. Physiol Behav. 1986;38(5):629–40. doi: 10.1016/0031-9384(86)90256-8. [DOI] [PubMed] [Google Scholar]
- [23].Gaffori O, Le Moal LE. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol Behav. 1979;23:317–23. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- [24].Giordano AL, Johnson AE, Rosenblatt JS. Haloperidol-induced disruption of retrieval behavior and reversal with apomorphine in lactating rats. Physiol Behav. 1990;48:211–4. doi: 10.1016/0031-9384(90)90288-f. [DOI] [PubMed] [Google Scholar]
- [25].Giovenardi M, Consiglio AR, Barros HM, Lucion AB. Pup age and aggressive behavior in lactating rats. Braz J Med Biol Res. 2000;33(9):1083–1088. doi: 10.1590/s0100-879x2000000900015. [DOI] [PubMed] [Google Scholar]
- [26].Gray P, Brooks PJ. Effect of lesion location within the medial preoptic-anterior hypothalamic continuum on maternal and male sexual behaviors in female rats. Behav Neurosci. 1984;98(4):703–11. doi: 10.1037//0735-7044.98.4.703. [DOI] [PubMed] [Google Scholar]
- [27].Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- [28].Grota LJ, Ader R. Continuous recording of maternal behavior of Rattus norvegicus. Animal Behavior. 1969;17:722–729. doi: 10.1016/0003-3472(70)90083-7. [DOI] [PubMed] [Google Scholar]
- [29].Grota LJ, Ader R. Behavior of lactating rats in a dual-chambered maternity cage. Horm Behav. 1974;5:275–282. doi: 10.1016/0018-506x(74)90014-2. [DOI] [PubMed] [Google Scholar]
- [30].Hansen S. Medial hypothalamic involvement in maternal aggression of rats. Behav Neurosci. 1989;103(5):1035–46. doi: 10.1037//0735-7044.103.5.1035. [DOI] [PubMed] [Google Scholar]
- [31].Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- [32].Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991;39:71–7. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- [33].Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–91. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- [34].Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966;210(5042):1220–2. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- [35].Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977;69(4):475–96. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Holahan MR, White NM. Intra-amygdala muscimol injections impair freezing and place avoidance in aversive contextual conditioning. Learn Mem. 2004;11(4):436–46. doi: 10.1101/lm.64704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hurtazo HA, Paredes RG, Agmo A. Inactivation of the medial preoptic area/anterior hypothalamus by lidocaine reduces male sexual behavior and sexual incentive motivation in male rats. Neuroscience. 2008;152(2):331–7. doi: 10.1016/j.neuroscience.2007.10.063. [DOI] [PubMed] [Google Scholar]
- [38].Izquierdo I, Medina JH. On brain lesions, the milkman and Sigmunda. Trends Neurosci. 1988;21(10):423–6. doi: 10.1016/s0166-2236(98)01279-x. [DOI] [PubMed] [Google Scholar]
- [39].Jacobson CD, Terkel J, Gorski RA, Sawyer CH. Effects of small medial preoptic area lesions on maternal behavior: retrieving and nest building in the rat. Brain Res. 1980;194(2):471–8. doi: 10.1016/0006-8993(80)91226-3. [DOI] [PubMed] [Google Scholar]
- [40].Kalinichev M, Rosenblatt JS, Morrell JI. The medial preoptic area, necessary for adult maternal behavior in rats, is only partially established as a component of the neural circuit that supports maternal behavior in juvenile rats. Behav Neurosci. 2000;114(1):196–210. doi: 10.1037//0735-7044.114.1.196. [DOI] [PubMed] [Google Scholar]
- [41].Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- [42].Kolb B, Cioe J. Recovery from early cortical damage in rats. IX. Differential behavioral and anatomical effects of temporal cortex lesions at different ages of neural maturation. Behav Brain Res. 2003;144(12):67–76. doi: 10.1016/s0166-4328(03)00068-8. [DOI] [PubMed] [Google Scholar]
- [43].Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: Effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100(1–2):15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]; Behav Brain Res. 2000;108(2):215–231. doi: 10.1016/s0166-4328(99)00170-9. Corrected and republished in: [DOI] [PubMed] [Google Scholar]
- [44].Leon M, Croskerry PG, Smith G. Thermal control of mother-young contact in rats. Physiol Behav. 1978;21:793–811. doi: 10.1016/0031-9384(78)90021-5. [DOI] [PubMed] [Google Scholar]
- [45].Li M, Davidson P, Budin R, Kapur S, Fleming AS. Effects of typical and atypical antipsychotic drugs on maternal behavior in postpartum female rats. Schizophr Res. 2004;70:69–80. doi: 10.1016/j.schres.2003.09.013. [DOI] [PubMed] [Google Scholar]
- [46].Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods. 1999;86(2):109–17. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- [47].Lonstein JS, De Vries GJ. Maternal behaviour in lactating rats stimulates c-fos in glutamate decarboxylase-synthesizing neurons of the medial preoptic area, ventral bed nucleus of the stria terminalis, and ventrocaudal periaqueductal gray. Neuroscience. 2000;100(3):557–68. doi: 10.1016/s0306-4522(00)00287-6. [DOI] [PubMed] [Google Scholar]
- [48].Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82(1):267–81. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- [49].Magnusson JE, Fleming AS. Rat pups are reinforcing to the maternal rat: Role of sensory cues. Psychobiology. 1995;23:69–75. [Google Scholar]
- [50].Malpeli JG, Schiller PH. A method of reversible inactivation of small regions of brain tissue. J Neurosci Methods. 1979;1(2):143–51. doi: 10.1016/0165-0270(79)90011-6. [DOI] [PubMed] [Google Scholar]
- [51].Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat Neurosci Lett. 1991;127(2):160–4. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- [52].Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86(2):145–59. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- [53].Miller SM, Lonstein JS. Dopamine D1 and D2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119:1072–83. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- [54].Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(23):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- [55].Moltz H, Levin R, Leon M. Differential effects of progesterone on the maternal behavior of primiparous and multiparous rats. J Comp Physiol Psychol. 1969;67(1):36–40. doi: 10.1037/h0026654. [DOI] [PubMed] [Google Scholar]
- [56].Moltz H, Robbins D, Parks M. Caesarean delivery and maternal behavior of primiparous and multiparous rats. J Comp Physiol Psychol. 1966;61(3):455–60. doi: 10.1037/h0023263. [DOI] [PubMed] [Google Scholar]
- [57].Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Phsychol. 1979;93:677–84. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- [58].Morgan HD, Fleming AS, Stern JM. Somatosensory control of the onset and retention of maternal responsiveness in primiparous Sprague-Dawley rats. Physiol Behav. 1992;51(3):549–55. doi: 10.1016/0031-9384(92)90178-5. [DOI] [PubMed] [Google Scholar]
- [59].Noonan M, Kristal MB. Effects of medial preoptic lesions on placentophagia and on the onset of maternal behavior in the rat. Physiol Behav. 1979;22(6):1197–202. doi: 10.1016/0031-9384(79)90276-2. [DOI] [PubMed] [Google Scholar]
- [60].Numan M. Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol. 1974;87(4):746–59. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- [61].Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5(4):163–90. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- [62].Numan M, Insel TR. The neurobiology of parental behavior. Springer—Verlag; New York: 2003. [Google Scholar]
- [63].Numan M, Callahan EC. The connections of the medial preoptic region and maternal behavior in the rat. Physiol Behav. 1980;25(5):653–65. doi: 10.1016/0031-9384(80)90367-4. [DOI] [PubMed] [Google Scholar]
- [64].Numan M, Corodimas KP. The effects of paraventricular hypothalamic lesions on maternal behavior in rats. Physiol Behav. 1985;35(3):417–25. doi: 10.1016/0031-9384(85)90318-x. [DOI] [PubMed] [Google Scholar]
- [65].Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD. Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behav Neurosci. 1988;102(3):381–96. doi: 10.1037//0735-7044.102.3.381. [DOI] [PubMed] [Google Scholar]
- [66].Numan M, Morrell JI, Pfaff DW. Anatomical identification of neurons in selected brain regions associated with maternal behavior deficits induced by knife cuts of the lateral hypothalamus in rats. J Comp Neurol. 1985;237(4):552–64. doi: 10.1002/cne.902370411. [DOI] [PubMed] [Google Scholar]
- [67].Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav Neurosci. 1994;108(2):379–94. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- [68].Numan M, Numan MJ. Importance of pup-related sensory inputs and maternal performance for the expression of Fos-like immunoreactivity in the preoptic area and ventral bed nucleus of the stria terminalis of postpartum rats. Behav Neurosci. 1995;109(1):135–49. doi: 10.1037//0735-7044.109.1.135. [DOI] [PubMed] [Google Scholar]
- [69].Numan M, Numan MJ. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [70].Numan M, Numan MJ. Projection sites of medial preoptic and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–84. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- [71].Numan M, Numan MJ, Marzella SR, Palumbo A. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain Res. 1998;792(2):348–52. doi: 10.1016/s0006-8993(98)00257-1. [DOI] [PubMed] [Google Scholar]
- [72].Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- [73].Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977;91(1):146–64. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- [74].Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–27. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- [75].Olypher AV, Klement D, Fenton AA. Cognitive disorganization in hippocampus: a physiological model of the disorganization in psychosis. J Neurosci. 2006;26(1):158–68. doi: 10.1523/JNEUROSCI.2064-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Partsalis AM, Zhang Y, Highstein SM. Dorsal Y group in the squirrel monkey. II. Contribution of the cerebellar flocculus to neuronal responses in normal and adapted animals. J Neurophysiol. 1995;73(2):632–50. doi: 10.1152/jn.1995.73.2.632. [DOI] [PubMed] [Google Scholar]
- [77].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd edition Academic Press; New York: 1997. [Google Scholar]
- [78].Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108(6):1163–71. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- [79].Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175(1):139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- [80].Pereira M, Morrell JI. Expression of maternal behavior during the late postpartum period: Effects of D1 and D2 dopamine receptor stimulation. (in preparation) [Google Scholar]
- [81].Pereira M, Seip KM, Morrell JI. Maternal Motivation and Its Neural Substrate Across the Postpartum Period. In: Bridges RS, editor. The Neurobiology of the Parental Mind. Elsevier Academic Press; San Diego, CA: 2008. pp. 39–59. [Google Scholar]
- [82].de Vasconcelos A Pereira, Klur S, Muller C, Cosquer B, Lopez J, Certa U, Cassel JC. Reversible inactivation of the dorsal hippocampus by tetrodotoxin or lidocaine: a comparative study on cerebral functional activity and motor coordination in the rat. Neuroscience. 2006;141(4):1649–63. doi: 10.1016/j.neuroscience.2006.05.023. [DOI] [PubMed] [Google Scholar]
- [83].Perrin G, Meurisse M, Lévy F. Inactivation of the medial preoptic area or the bed nucleus of the stria terminalis differentially disrupts maternal behavior in sheep. Horm Behav. 2007;52(4):461–73. doi: 10.1016/j.yhbeh.2007.06.010. [DOI] [PubMed] [Google Scholar]
- [84].Popeski N, Woodside B. Central nitric oxide synthase inhibition disrupts maternal behavior in the rat. Behav Neurosci. 2004;118(6):1305–16. doi: 10.1037/0735-7044.118.6.1305. [DOI] [PubMed] [Google Scholar]
- [85].Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953(12):205–14. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- [86].Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89(7):722–732. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- [87].Rosenblatt JS. Prepartum and postpartum regulation of maternal behavior in the rat. Ciba Found Symp. 1975;33:17–32. doi: 10.1002/9780470720158.ch3. [DOI] [PubMed] [Google Scholar]
- [88].Rosenblatt JS, Hazelwood S, Poole J. Maternal behavior in male rats: effects of medial preoptic area lesions and presence of maternal aggression. Horm Behav. 1996;30(3):201–15. doi: 10.1006/hbeh.1996.0025. [DOI] [PubMed] [Google Scholar]
- [89].Rosenblatt JS, Mayer AD, Siegel HI. Maternal behavior among nonprimate mammals. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology. vol. 7. Plenum Press; New York: 1985. pp. 229–298. [Google Scholar]
- [90].Sandkühler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305(1):77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- [91].Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res. 1987;68(1):168–78. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- [92].Siegel S. Nonparametric statistics for the behavioral sciences. McGraw-Hill; New York: 1956. [Google Scholar]
- [93].Silva MRP, Bernardi MM, Cruz-Casallas PE, Felicio LF. Pimozide injections into the nucleus accumbens disrupt maternal behaviour in lactating rats. Pharmacol Toxicol. 2003;93:42–7. doi: 10.1034/j.1600-0773.2003.930106.x. [DOI] [PubMed] [Google Scholar]
- [94].Silva MRP, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav. 2001;68:461–8. doi: 10.1016/s0091-3057(01)00471-3. 2001. [DOI] [PubMed] [Google Scholar]
- [95].Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131(12):17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- [96].Stern JM. Multisensory regulation of maternal behavior and masculine sexual behavior: a revised view. Neurosci Biobehav Rev. 1990;14(2):183–200. doi: 10.1016/s0149-7634(05)80219-2. [DOI] [PubMed] [Google Scholar]
- [97].Stern JM. Somatosensation and maternal care in Norway rats. In: Rosenblatt JS, Snowdon CT, editors. Advances in the Study of Behavior. Parental care—Evolution, Mechanisms, and Adaptive Significance. vol. 25. Academic Press; New York: 1996. pp. 243–294. [Google Scholar]
- [98].Stern JM, Johnson SK. Perioral somatosensory determinants of nursing behavior in Norway rats (Rattus norvegicus) J Comp Psychol. 1989;103(3):269–80. doi: 10.1037/0735-7036.103.3.269. [DOI] [PubMed] [Google Scholar]
- [99].Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47(5):993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- [100].Stern JM, Keer SE. Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res. 1999;99:231–9. doi: 10.1016/s0166-4328(98)00108-9. [DOI] [PubMed] [Google Scholar]
- [101].Stern JM, Taylor LA. Haloperidol inhibits maternal retrieval and licking, but facilitates nursing behavior and milk ejection in lactating rats. J Neuroendocrinol. 1991;3:591–6. doi: 10.1111/j.1365-2826.1991.tb00323.x. [DOI] [PubMed] [Google Scholar]
- [102].Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74(1):17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- [103].Terkel J, Bridges RS, Sawyer CH. Effects of transecting lateral neural connections of the medial preoptic area on maternal behavior in the rat: nest building, pup retrieval and prolactin secretion. Brain Res. 1979;169(2):369–80. doi: 10.1016/0006-8993(79)91037-0. [DOI] [PubMed] [Google Scholar]
- [104].Uriarte N, Ferreira A, Rosa XF, Sebben V, Lucion AB. Overlapping litters in rats: effects on maternal behavior and offspring emotionality. Physiol Behav. 2008;93(45):1061–70. doi: 10.1016/j.physbeh.2008.02.004. [DOI] [PubMed] [Google Scholar]
- [105].Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113(2):377–90. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- [106].Walsh CJ, Fleming AS, Lee A, Magnusson JE. The effects of olfactory and somatosensory desensitization on Fos-like immunoreactivity in the brains of pup-exposed postpartum rats. Behav Neurosci. 1996;110(1):134–53. doi: 10.1037//0735-7044.110.1.134. [DOI] [PubMed] [Google Scholar]
- [107].Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81(4):967–88. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- [108].Waraczynski M, Perkins M. Temporary inactivation of the retrorubral fields decreases the rewarding effect of medial forebrain bundle stimulation. Brain Res. 2000;885(2):154–65. doi: 10.1016/s0006-8993(00)02908-5. [DOI] [PubMed] [Google Scholar]
- [109].Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–9. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- [110].Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Annals of the New York Academy of Sciences. 1999;877:113–28. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- [111].Zhuravin IA, Bures J. Extent of the tetrodotoxin induced blockade examined by pupillary paralysis elicited by intracerebral injection of the drug. Exp Brain Res. 1991;83(3):687–90. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]