Abstract
The aromathecin topoisomerase I (top1) inhibitors offer promising scaffolds for the development of novel cancer chemotherapeutics. They are “composites” of the camptothecin and indenoisoquinoline top1 inhibitors. Interestingly, some structure-activity-relationship (SAR) overlap between the aromathecins and the indenoisoquinolines has been observed. For both classes, placement of certain polar groups in similar regions of the heteroaromatic system improves top1 inhibitory and antiproliferative activities. A series of water-soluble aromathecins substituted at position 14 with diaminoalkanes of various lengths has been prepared. These compounds all possess similar antiproliferative potency, but a general trend is observed: aromathecins with longer diaminoalkane substituents (> 6 carbons) possess lower anti-top1 activity than their smaller counterparts (2–4 carbons), presumably as a result of unfavorable hydrophobic interactions. This trend is also noted with the indenoisoquinolines, revealing additional SAR overlap that supports the hypothesis that there is a “universal” top1 inhibitor SAR.
Keywords: topoisomerase I, aromathecins, anticancer, polyamine
1. Introduction
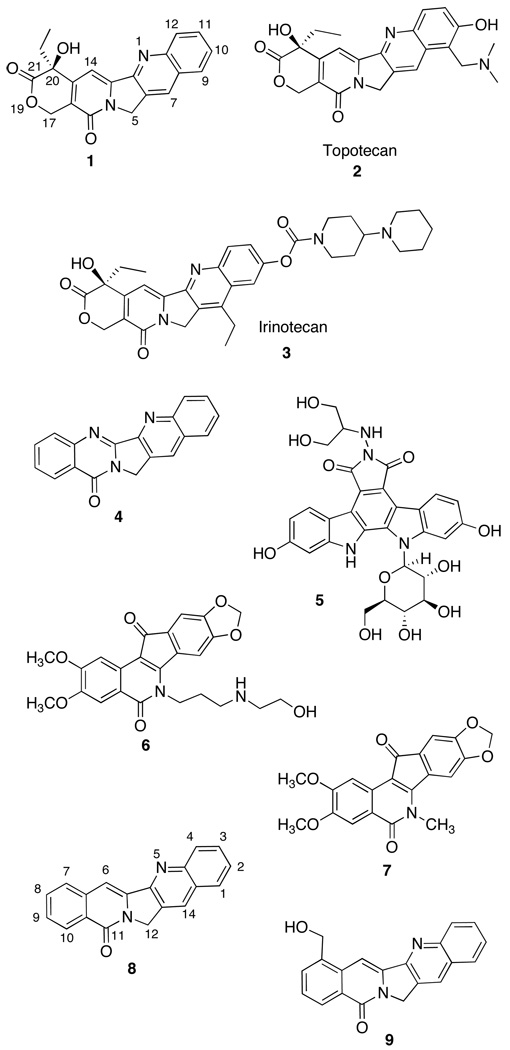
Topoisomerase I (top1) breaks one strand of double-stranded DNA. The cleaved strand then rotates around the uncleaved strand, resulting in relaxation of DNA supercoils necessary for replication and transcription. The broken DNA strand then re-ligates and relaxed DNA is released.1,2 Top1 is overexpressed in human cancers,3,4 and interest in top1 as a therapeutic target was stimulated by the isolation of camptothecin (1) and the later development of topotecan (2) and irinotecan (3),5 which inhibit the DNA religation reaction by intercalating at the cleavage site, eventually leading to apoptosis.6–9
Topotecan and irinotecan are potent anticancer drugs, but are limited by reversibility of their ternary drug-DNA-enzyme complexes, which necessitates long infusion times for maximum therapeutic benefit. Drug efflux pumps10 and resistance mutations11–12 also limit their efficacy. Side effects include diarrhea, immune suppression, and hemorrhagic cystitis.1,13–14 The E-ring hydroxylactone exists in equilibrium with an open hydroxyacid form that binds to human serum albumin, thus compromising bioavailability.1,15–18
Alternative top1 inhibitors have been based on natural products such as luotonin A (4)19 and the indolocarbazoles.20 Optimization of the latter class has provided edotecarin (5).13,21 The indenoisoquinolines, such as 6, are synthetic compounds based on the lead NSC 314622 (7).22–25 Two indenoisoquinolines are slated to begin clinical trials at the NIH.26
The 12H-5,11a-diazadibenzo[b,h]fluoren-11-one system, called “rosettacin” (8)27 when unsubstituted and “aromathecin” when substituted, was first discovered in the natural product 22-hydroxyacuminatine (9).28 This system is an analogue of camptothecin, with the E-ring lactone replaced by a benzene ring. As such, the system is a “hybrid” of the camptothecin and indenoisoquinoline systems. Although initial attempts to develop aromathecins were less than fruitful,29 subsequent efforts led to the synthesis of the more promising 14-substituted aromathecins.30 These compounds, substituted with amino alcohols and nitrogen heterocycles, possess both greater anti-top1 and antiproliferative activity than rosettacin and a greater ability to inhibit top1 than 22-hydroxyacuminatine.
Interestingly, indenoisoquinoline top1 inhibitory activities are increased by the same substituents (ethanolamine, morpholine, imidazole, etc.)23–24 that increase the activity of the aromathecins.23–24 Molecular modeling and crystallography8 indicate that the lactam region of the indenoisoquinoline system (where these substituents are located) overlaps spatially with both the 7-position of camptothecin and the 14-position of the aromathecin system in their respective ternary cleavage complexes. These substituents likely project into the DNA major groove in the cleavage complex.30 This spatial overlap suggests a significant degree of structure-activity relationship overlap between these two systems.8 The similar substituent effects suggest that other aspects of the indenoisoquinoline SAR might be “translated” to the aromathecin system.
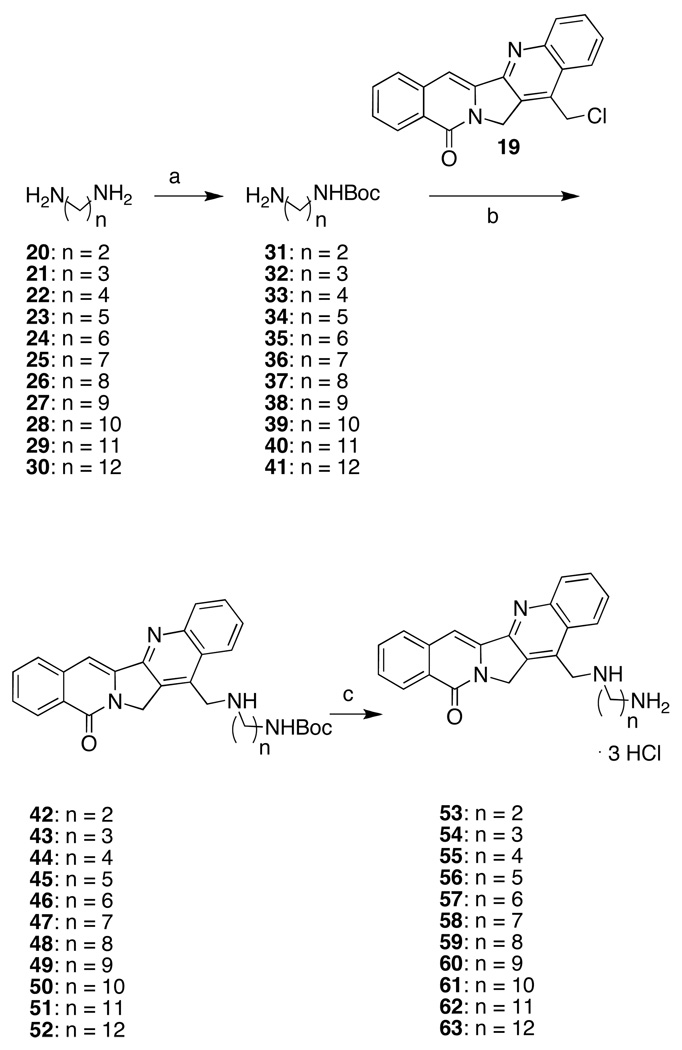
Indenoisoquinolines substituted with di- and polyamines are potent top1 inhibitors.31–32 It is known in general that di- and polyamine conjugates are effective “DNA-targeting” moieties for many classes of intercalating and anti-top1 compounds.31–35 In 2007, Morrell et al. determined that diamines with two- to four-carbon spacers were optimal substituents for bioactivity when placed on indenoisoquinolines.31 The question as to whether this SAR trend could also be applied to aromathecins served as a rationale for the synthesis of 14-[(aminoalkyl)-aminomethyl)aromathecins 53–63.
2. Chemistry
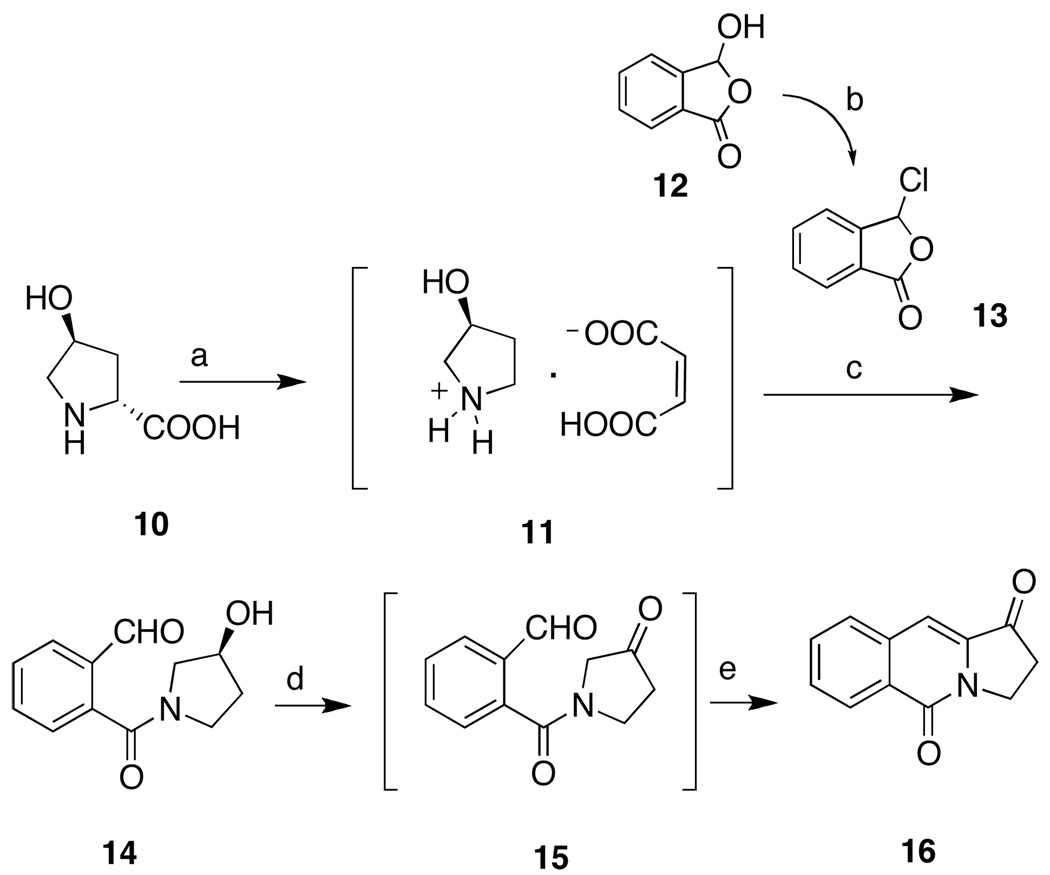
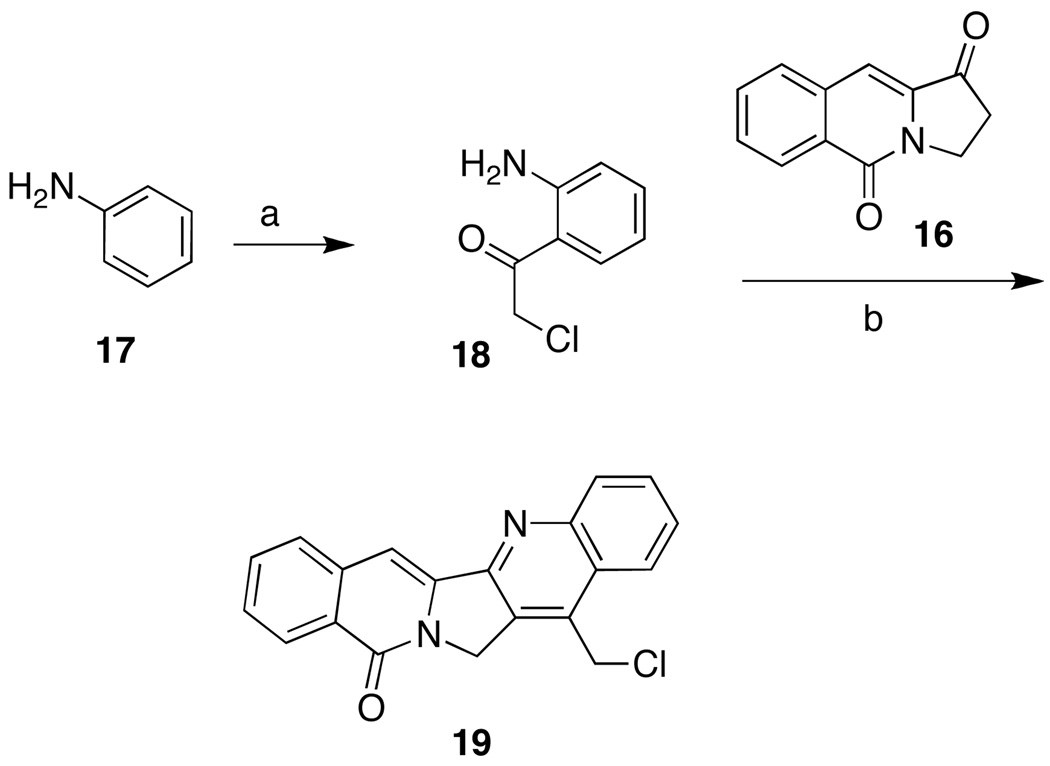
There are several routes known to the aromathecin system, including the condensation of pyrroloquinoline with phthalides,28–29 pyridone benzannulation–Heck coupling,36 and variants of the Friedlander condensation.30,37 Our synthesis of 14-substituted aromathecins proceeds through pyrroloquinolinedione 16 (Scheme 1), first prepared by Shamma and Novak in 1968.38 Intermediate 11 was prepared by the facile decarboxylation of commercially available trans-4-hydroxy-l-proline (10).39 Condensation of 11 with 3-chlorophthalide 13 (easily prepared from 2-carboxybenzaldehyde 12)40 yielded the hydroxyamide 14. Previously, this compound was oxidized and cyclized to 16 via oxidation with pyridinium dichromate and subsequent treatment with polyphosphoric acid.30 An improved variant of this oxidation-cyclization has been discovered. Subjecting 14 to Swern conditions and a brief reflux period affords 16 rapidly (via intermediate 15). These new conditions furnish the key intermediate in improved yield (50–60%) without recourse to heavy metal oxidants and extensive purification. 14-Chloromethylaromathecin (19)30 was prepared by Friedlander condensation of ketone 16 with aminoacetophenone 18 (Scheme 2). Compound 18 was in turn prepared from aniline (17) and chloroacetonitrile using the Sugasawa modification of the Friedel-Crafts acylation.41–42
Scheme 1.
Reagents and Conditions: (a) i. cat. 2-cyclohexen-1-one, cyclohexanol, reflux, ii. maleic acid, EtOAc, r.t.; (b) FeCl3, SOCl2, reflux; (c) MeOH, Et3N, r.t.; (d) i. DMSO, (COCl)2, CH2Cl2, −78 °C, ii. Et3N, −78 °C–r.t.; (e) reflux.
Scheme 2.
Reagents and Conditions: (a) i. BCl3·Me2S, 1,2,-dichloroethane, 0 °C, ii. chloroacetonitrile, AlCl3, reflux. iii. 2 M HCl, reflux; (b) p-TsOH, toluene, reflux.
Compound 19 undergoes Sn2 displacement easily at its benzylic chloride (Scheme 3). The diamine moieties were installed as their mono-Boc-protected carbamates to prevent polymerization under the displacement conditions and to aid in purification. Following Morrell et al.’s procedure, diamines 20–30 were readily protected using Boc2O to afford the carbamates 31–41.31 Treatment of 19 with excess protected diamine in DMSO at room temperature afforded the aromathecin carbamates 42–52. Compounds 42–52 were readily deprotected using methanolic HCl either in chloroform, or in the case of diaminoheptane analogue 47, refluxing benzene, to yield the water-soluble aromathecin salts 53–63. Elemental analysis proved these analogues were trihydrochloride salts, protonated on both amines and the quinoline nitrogen.
Scheme 3.
Reagents and Conditions: (a) Boc2O, CHCl3, r.t.; (b) DMSO, r.t.; (c) 3 M HCl, MeOH, CHCl3, r.t. or 3 M HCl, MeOH, benzene, reflux (47).
3. Results and Discussion
Aromathecin analogues were assayed for cytotoxic activity in the National Cancer Institute’s Developmental Therapeutics screen.43–44 Although some highly potent Boc-polyaminocamptothecins have been reported,33 indenoisoquinoline carbamates are largely inactive. 31,45 Therefore, the intermediate carbamates 42–52 were not tested.
Compounds accepted for testing were assayed against approximately 60 cell lines originating from various human tumors.43–44 Following an initial one-dose assay (at 10−5 molar), selected compounds were tested at five concentrations ranging from 10−8 to 10−4 molar. Cytotoxicity results are reported as GI50 values for selected cell lines from each subpanel, and overall antiproliferative potency is quantified as a mean-graph midpoint (MGM) in Table 1. The MGM is a measure of the average GI50 against all cell lines tested, where compounds whose GI50 values fall outside the concentration range tested (10−8 to 10−4 M) are assigned GI50 values of either 10−8 M or 10−4 M. For comparative purposes, the activities of camptothecin (1), the indenoisoquinolines 6 (MJ-III-65),1,23,25 and NSC314622 (7),22 and rosettacin (8) are reported.
Table 1.
Cytotoxicities and Topoisomerase I Inhibitory Activity of 14-Diamine-Substituted Aromathecin Analogues
| Cytotoxicity (GI50 in µM)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compd | Lung HOP-62 |
colon HCT-116 |
CNS SF-539 |
melanoma UACC-62 |
Ovarian OVCAR-3 |
renal SN12C |
prostate DU-145 |
breast MDA-MB-435 |
MGMb | Top 1 Cleavagec |
| 1 | 0.01 | 0.03 | 0.01 | 0.01 | 0.22 | 0.02 | 0.01 | 0.04 | 0.040 ± 0.019 | ++++ |
| 6 | 0.02 | 0.10 | 0.04 | 0.03 | 0.5 | <0.01 | <0.01 | 0.79 | 0.11 | ++++ |
| 7 | 1.3 | 35 | 41 | 4.2 | 73 | 68 | 37 | 96 | 20.0 | ++ |
| 8 | 68.2 | 32.7 | 66.7 | 97.2 | 39.8 | >100 | >100 | 41.8 | 58.9 | ++ |
| 53 | 1.4 | 1.5 | 8.3 | 12.6 | 1.7 | 1.5 | 1.4 | 11.0 | 2.8 ± 0.11 | +++ |
| 54 | 9.8 | 1.5 | 4.9 | 4.8 | 1.4 | 2.1 | 1.1 | 6.8 | 2.1 ± 0.38 | ++(+) |
| 55e | - | - | - | - | - | - | - | - | - | +++ |
| 56 | 2.6 | 3.2 | 7.2 | 1.9 | 8.1 | 3.2 | 1.1 | 12.6 | 2.6 ± 1.65 | + |
| 57 | 1.2 | 0.63 | 0.74 | 2.0 | 1.4 | 0.51 | 0.33 | 1.5 | 1.0 | ++(+) |
| 58e | - | - | - | - | - | - | - | - | - | ++ |
| 59 | 2.8 | 1.8 | 1.9 | 1.8 | 2.5 | 1.8 | 1.1 | 3.1 | 1.9 | + |
| 60e | - | - | - | - | - | - | - | - | - | + |
| 61d | - | - | - | - | - | - | - | - | - | 0 |
| 62e | - | - | - | - | - | - | - | - | - | 0 |
| 63 | 6.5 | 2.0 | 1.8 | 1.9 | 1.9 | 2.5 | 2.0 | 1.7 | 2.6 | 0 |
The cytotoxicity GI50 values are the concentrations corresponding to 50% growth inhibition.
Mean graph midpoint for growth inhibition of all human cancer cell lines successfully tested, ranging from 10−8 to 10−4 molar.
Compound-induced DNA cleavage due to top1 inhibition is graded by the following rubric relative to 1 µM camptothecin: 0, no inhibitory activity; +, between 20 and 50% activity; ++, between 50 and 75% activity; +++, between 75 and 100% of activity; ++++, equipotent.
Not selected for further testing; refer to text for details.
Declined for one-dose cytotoxicity testing by the NCI.
Top1 inhibition was evaluated by measurement of top1-dependent DNA cleavage at four concentrations, and inhibition data are expressed semiquantitatively using the following rubric: 0, no inhibitory activity; +, between 20 and 50% the activity of 1 µM camptothecin (1); ++, between 50 and 75% the activity of 1 µM camptothecin; +++, between 75–100% the activity of 1 µM camptothecin; and ++++, equipotent to or more potent than 1 µM camptothecin. Top1 inhibitory data for 1 and 6–8 are also included in Table 1.
Compounds 53–54, 56–57, 59, 61, and 63 were assayed for antiproliferative activity. These aromathecins, on average, display higher antiproliferative potency than many of the 14-substituted aromathecins previously investigated.30 These water-soluble salts are more easily formulated than many of the free bases previously tested. In addition, it is well known that positively charged substituents improve DNA targeting, binding, and intercalative potential through simple electrostatic complementarity to negatively charged DNA. This effect has been observed for camptothecins, indenoisoquinolines, acridines, and other polyaromatic hydrocarbon-based systems when substituted with di- and polyamines, amidines, and guanidines.31–35, 45–47
Interestingly, with the exception of compound 61, little variance in antiproliferative activity is observed in the compounds tested, regardless of side chain length. Anomalously, compound 61 actually induced cell growth by approximately 30% at a concentration of 10 µM in the initial one-concentration assay, although both the large observed variance between individual cell lines and the general trend among the rest of this series indicate that this result may be an artifact of testing. For indenoisoquinolines and other compounds, it was observed that an increasing C logP correlated well with decreasing cytotoxicity, possibly as a result of poor solubility, inefficient DNA-targeting, or other hydrophobic effects.31 There is no correlation for aromathecins, however; compounds 53 and 63 are equipotent, and compound 57, more hydrophobic than 53, is more active.
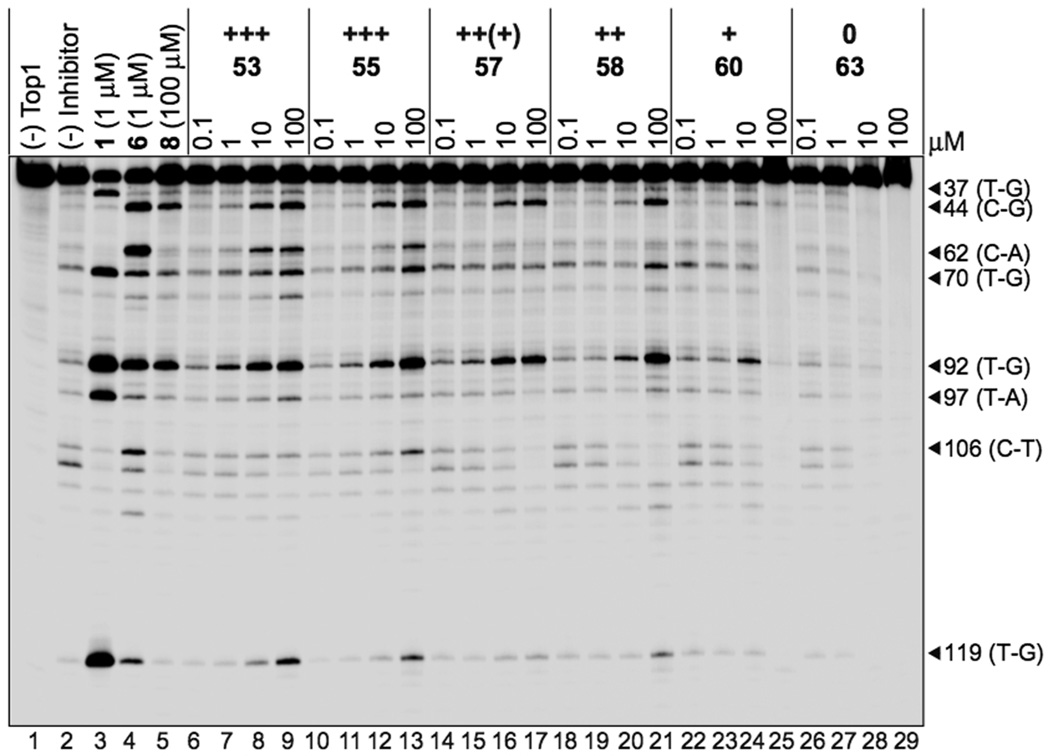
For top1 inhibition, the shorter diaminoalkanes confer good anti-top1 activity upon the aromathecin core, inducing top1-mediated DNA breakage as shown in Figure 2. These compounds induce DNA cleavage patterns resembling those caused by both camptothecins and indenoisoquinolines. Activity generally decreases with increasing side-chain length, however. With the exception of 56, up to six atoms between proximal and distal amines are tolerated readily for aromathecins. Activity decreases beyond this length, and compounds with ten or more carbons are completely inactive. An identical trend is noted for indenoisoquinolines, complete with compounds derived from 1,5-pentanediamine possessing significantly less anti-top1 activity than those derived from 1,6-hexanediamine.31 It is unknown why the pentanediamino compounds possess such low anti-top1 activity.
Figure 2.
Top1-mediated DNA cleavage induced by aromathecins 53, 55, 57, 58, 60, and 63. Lane 1: DNA alone; lane 2: Top1 alone; lane 3: 1, 1 µM; lane 4: 6, 1 µM; lane 5: 8, 100 µM; lanes 6–29: 53, 55, 57, 58, 60, and 63 at 0.1, 1, 10, and 100 µM, respectively, from left to right. Numbers and arrows on right indicate arbitrary cleavage site positions.
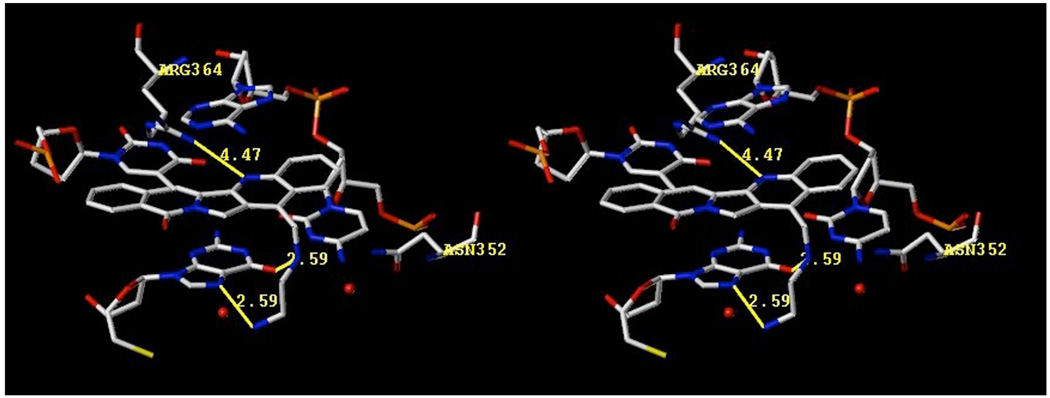
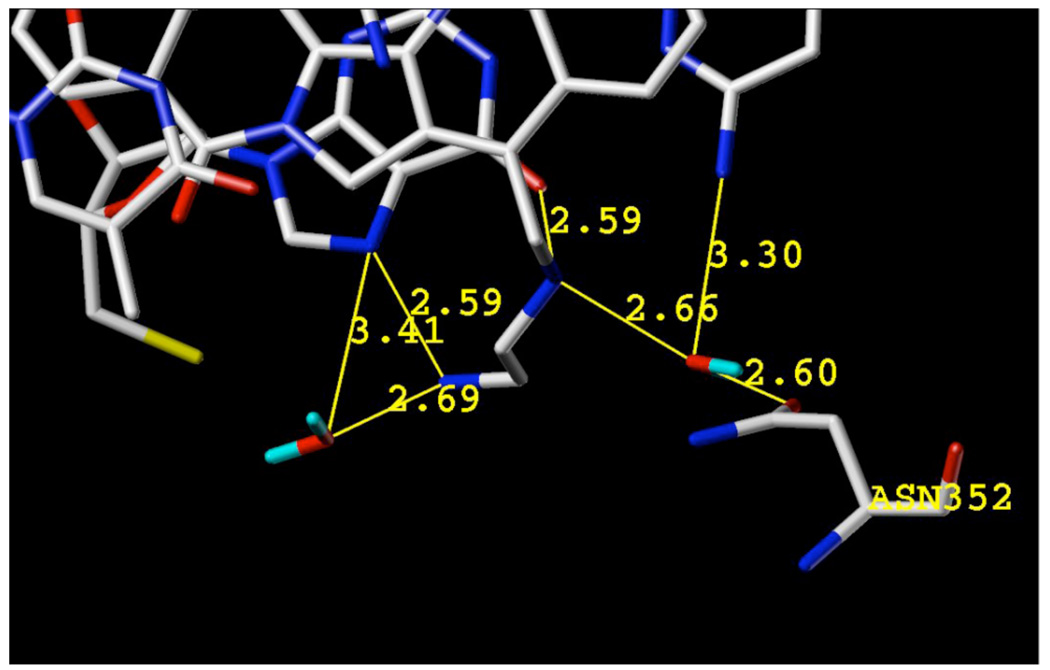
It has been confirmed by X-ray crystallography that the lactam substituents of indenoisoquinolines project into the major groove of the ternary complex.8 Hypothetical models predict that the 14-position substituents of aromathecins project into the same region, where they likely interact favorably with water and amino acids such as Asn352.48–49 Figure 3 shows a hypothetical model of compound 53, the most potent top1 inhibitor from this series, in ternary complex with top1 and DNA. The aromathecin core is proposed to intercalate between the base pairs flanking the top1-induced cleavage site, where the quinoline nitrogen faces toward the minor groove and may interact with Arg364. Although it is out of hydrogen-bonding distance (4.47 Å) in this model, this contact is closer in other aromathecin models,30 as well as in camptothecin and topotecan crystal structures,7–8 and hydrogen bonding might still be possible in the present case with induced fit. The diamine side chain projects into the major groove, where it is proposed to hydrogen bond with a flanking nucleobase. Water molecules likely participate in these interactions, and this hypothetical network of water-mediated H-bonds and polar contacts with the positively charged amines is shown in Figure 4. In this well-solvated environment, it can be seen how increasingly hydrophobic side-chains (such as those over eight carbons as in 60–63) would yield unfavorable interactions by possibly disturbing this intricate network of water molecules. Larger side chains may also be hindered by steric or entropic factors.
Figure 3.
Hypothetical model of aromathecin 53 in ternary complex with top1 and DNA showing proposed hydrogen bonds. Water molecules are red spheres; distances are between heavy atoms. The stereoview is programmed for wall-eyed viewing.
Figure 4.
Hypothetical model of water-mediated hydrogen bonds and polar contacts between diaminoethyl side chain of aromathecin 53, Asn352, and DNA base pairs. Water molecules are indicated in red, other structures are colored by atom type. Distances are between heavy atoms.
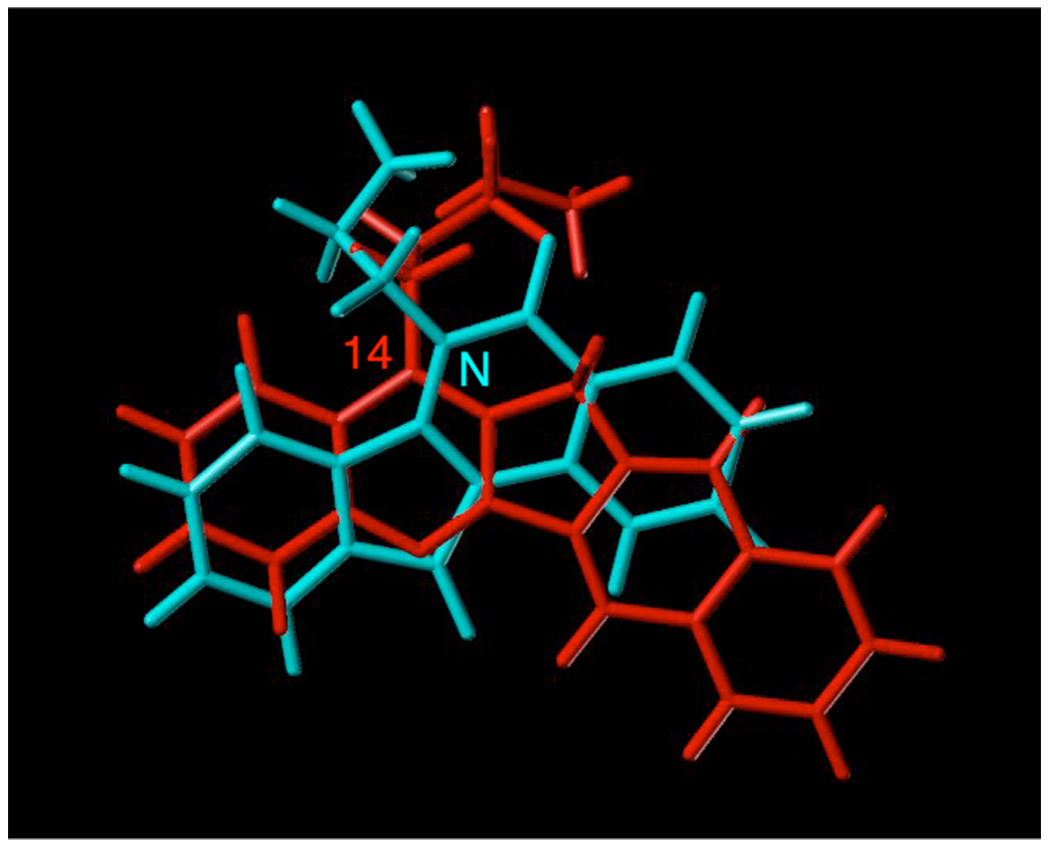
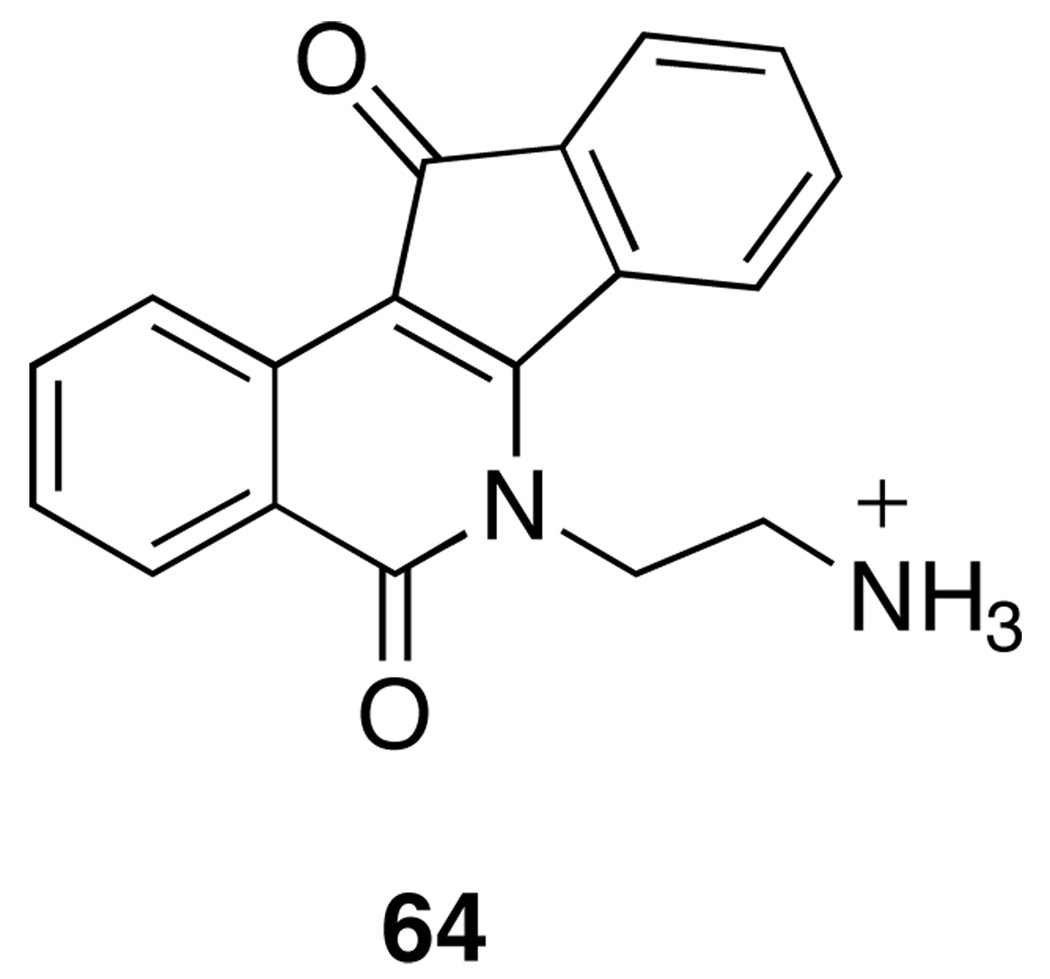
The fact that aromathecin diamines behave in a manner that is identical to analogous indenoisoquinolines with regard to top1 inhibition lends additional support to the proposed binding mode (where the 14-position faces the major groove). This behavior can be rationalized through molecular modeling. Figure 5 depicts the ligands 53 and 64 (Figure 6) in an overlay of the hypothetical models of their ternary complexes.8,31 The aromatic regions sit approximately in the same position, the side chains occupy identical regions spatially, and both interact with Asn352 and water molecules (not shown).
Figure 5.
Ligand overlay of hypothetical ternary complex models of aromathecin 53 (green) and indenoisoquinoline 64 (cyan). The positions of the lactam nitrogen and 14-position are also shown in their respective colors.
Figure 6.
Structure of Indenoisoquinoline 64.
It is worth noting the disparities between cytotoxicity and top1 inhibition across this analogue series. Such a disparity is not unprecedented and has been previously observed in indenoisoquinolines,23,31 anthracenes,46 camptothecins,33 and aromathecins30 alike. Dallavalle et al. propose that beyond Coulombic attraction of the drugs to DNA, the cellular pharmacology of positively-charged “polyamine” conjugates is an exceedingly complex interplay of intrinsic molecular structure, hydrophobicity, and subcellular localization.33 This is consistent with Morrell et al.’s hypotheses that differential ADME properties are responsible for these discrepancies.31
If such a disparity exists between cytotoxicity and top1 inhibition, then what is the macromolecular target of compounds such as 56 and 63 that possess little anti-top1 activity? A COMPARE analysis was performed on data from compound 56, which, with its low anti-top1 activity, is effectively a “targetless” aromathecin. The NCI’s COMPARE algorithm attempts to correlate the antiproliferative potency of a compound with a possible target or mechanism of action.44,50–51 When the data for 56 were used to seed a COMPARE search, the program returned compounds such as amsacrine47 and teniposide with a moderately high (> 0.75) correlation. These compounds act predominantly through inhibition of topoisomerase II (top2). When compounds 56, 57, 59, and 63 were assayed for the ability to induce top2-mediated DNA cleavage, however, little inhibitory activity was found. It is also worth noting that at high concentrations, longer diamines like 60 and 63 suppress the formation of top1-DNA cleavage complexes (as shown in Figure 3) 31, although the shorter diamines do not. This could indicate that the antiproliferative activity of compounds such as 63 may involve topoisomerase binding or non-specific DNA intercalation. Also, the role of cellular polyamine transporters in the pharmacokinetics of these molecules cannot be discounted.52
The high degree of overlap in the SAR at the indenoisoquinoline lactam nitrogen and the aromathecin 14-position, down to specific substituents, is significant, and supports the hypothesis that there is a “common” or “universal” structure-activity relationship among different classes of top1 inhibitors. Different substituents have been “translated” effectively between camptothecins and indenoisoquinolines in optimization of the latter class’ indenone ring.53 This SAR overlap is also seen between camptothecins and aromathecins in translation of active substituents from the former’s 7-position (such as the N-methylpiperazine of lurtotecan and camptothecin analogues)1,17 to the 14-position of aromathecins.30 There is also overlap between the SARs of indenoisoquinolines, isoquinocinnolinones, and dibenzonaphthyridones in the usage of common lactam and ring substituents.54–55 Indeed, Staker et al. have described certain structural features common to all effective top1 inhibitors, including major groove substituents, a planar ring that faces the nonscissile strand, and a hydrogen-bond acceptor facing the minor groove.8 These new results could thus support the translation of other substituents to the aromathecin class to produce novel potent top1 inhibitors.
4. Conclusions
In conclusion, a new series of water-soluble 14-substituted aromathecins bearing diaminoalkanes of various lengths has been synthesized by a facile route beginning with commercially available starting materials. An improved preparation of the key intermediate in aromathecin synthesis is also reported. The analogues were assayed against top1 and various human cancer cell lines. This series contains the most cytotoxic aromathecins synthesized thus far, their improved antiproliferative potency likely due to increased solubility and multiple DNA-targeting positive charges. In general, top1 inhibition decreases with increasing side chain length, likely reflecting the effect of unfavorable hydrophobic clashes in the charged and well-solvated major groove of the ternary complex. Hypothetical models seem to support this assumption, as well as the hypothesis that hydrogen bonding may play a significant role in ternary complex stabilization. No correlation is observed between cytotoxicity and top1 inhibition, however, indicating top1 is not the sole determinant of antiproliferative potency. Interestingly, the top1 inhibition trend is identical to that observed for indenoisoquinolines. In addition to supporting our proposed binding mode, these data indicate additional SAR overlap with this related class of top1 inhibitors, and possibly lend support to the hypothesis that there may be features common to all effective top1 poisons.
5. Experimental
5.1. General Procedures
Reagents and solvents were purchased from commercial vendors and were used without further purification. Analytical thin-layer chromatography was performed on Baker-flex silica gel IB2-F plastic-backed TLC plates. Preparative thin-layer chromatography was performed on Analtech silica gel G1200 µM glass plates. Compounds were visualized with both short and long-wavelength UV light. Silica gel flash chromatography was performed using 40–63 µm flash silica gel. Melting points were determined in capillary tubes using a Mel-Temp apparatus and are uncorrected. Infrared spectra were obtained as films on salt plates using CHCl3, CDCl3, or CHCl3/MeOH as the solvent unless otherwise specified, using a Perkin-Elmer Spectrum One FT-IR spectrometer. All spectra are baseline-corrected. 1H NMR spectra were obtained at 300 or 500 MHz, using a Bruker ARX300 and Bruker Avance 500 (QNP and TXI 5 mm probe), respectively. Mass spectral analyses were performed at the Purdue University Campus-Wide Mass Spectrometry Center. ESIMS (electrospray ionization mass spectrometry) was performed using a FinniganMAT LCQ Classic mass spectrometer system. Combustion microanalyses were performed at the Purdue University Microanalysis Laboratory using a Perkin-Elmer Series II CHNS/O model 2400 analyzer and all reported values are within 0.4% of calculated values. Precursor compounds 11,39 13,49 14,30 and 1841–42 were prepared as previously described and as depicted in Scheme 1 and Scheme 2.
5.2 Synthesis of Precursors
5.2.1. 2,3-Dihydropyrrolo[1,2-b]isoquinoline-1,5-dione (16).38
Anhydrous CH2Cl2 (170 mL) was cooled, under an argon atmosphere, to −78 °C. DMSO (2.96 g, 37.9 mmol) was added, followed, dropwise, by oxalyl chloride (2.40 g, 18.9 mmol). Upon cessation of gas evolution (10 min), compound 1430 (3.78 g, 17.2 mmol), as a solution in CH2Cl2 (20 mL), was added slowly, and the resultant opaque off-white solution was stirred for 15 min. Triethylamine (8.69 g, 86 mmol) was added, and the mixture became orange. After stirring for 5 min at −78 °C, the mixture was warmed to room temperature (TLC indicated the presence of 15), and darkened as cyclization began. The mixture was stirred for 30 min and heated at reflux for 2 h. The black solution was cooled and washed with H2O (2 × 200 mL). The aqueous layer was extracted with CHCl3 (150 mL). The combined organic layers were washed with H2O (2 × 200 mL), saturated NaCl (200 mL), dried over anhydrous sodium sulfate, treated with decolorizing carbon (1.00 g), and filtered. The filtrate was concentrated to yield a pale-orange microcrystalline solid (2.01 g, 59%) after washing with MeOH (40 mL) and ether (20 mL): mp 180–183 °C (lit38 mp 191–192 °C). 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.1 Hz, 1 H), 7.80-7.65 (m, 3 H), 7.29 (s, 1 H), 4.42 (t, J = 7.0 Hz, 2 H), 2.99 (t, J = 7.3 Hz, 2 H).
5.2.2. 14-Chloromethyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (19).30
Compound 16 (0.284 g, 1.43 mmol) and compound 1849 (0.291 g, 1.72 mmol) were diluted with toluene (35 mL) and p-TsOH (0.272 g, 1.43 mmol) was added. The mixture was heated at reflux (using a Dean-Stark trap to collect azeotroped water) for 17 h. The bright-orange suspension was cooled, concentrated, and diluted with CHCl3 (100 mL). The organic phase was washed with sat. NaHCO3 (200 mL) and the aqueous layer was extracted with CHCl3 (100 mL), and the combined organic layers were washed with 5% NaHCO3 and sat. NaCl (100 mL each). The solution was dried over anhydrous sodium sulfate and concentrated to yield a yellow amorphous solid (0.425 g, 89%) after washing with MeOH (50 mL): mp 274–280 °C [(dec), lit30 mp 270 °C (dec)]. 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.1 Hz, 1 H), 8.27 (d, J = 7.9 Hz, 1 H), 8.19 (d, J = 8.2, 1 H), 7.84-7.59 (m, 6 H), 5.44 (s, 2 H), 5.04 (s, 2 H).
5.3. General Procedure for Synthesis of Mono-Boc Diaminoalkanes 31–41.31
Boc2O (0.5 g, 2.29 mmol) was dissolved in CHCl3 (10 mL) and added dropwise to the diamine (11.4 mmol), dissolved in CHCl3 (50 mL). The reaction mixture was allowed to stir overnight at room temperature. The solution was concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 10% MeOH-1% Et3N in CHCl3 to afford the mono-Boc-protected diamines in acceptable purity after drying in vacuo.
5.3.1. Mono-Boc-1,2-diaminoethane (31)
From 20, the general procedure afforded the desired product as a clear, pale-yellow viscous oil, (0.382 g, 100% with minor impurities): 1H NMR (300 MHz, CDCl3) δ 4.9 (bs, 1 H), 3.19 (q, J = 5.8 Hz, 2 H), 2.80 (t, J = 6.0 Hz, 2 H), 1.43 (s, 11 H).
5.3.2. Mono-Boc-1,3-diaminopropane (32)
From 21, the general procedure afforded the desired product as a clear, pale-yellow viscous oil (0.330 g, 83%): 1H NMR (300 MHz, CDCl3) δ 4.97 (bs, 1 H), 3.21 (q, J = 6.2 Hz, 2 H), 2.75 (t, J = 6.6 Hz, 2 H), 1.62-1.53 (m, 2 H), 1.41 (s, 11 H).
5.3.3. Mono-Boc-1,4-diaminobutane (33)
From 22, the general procedure afforded the desired product as a clear yellow viscous oil (0.443 g, 100% with minor impurities): 1H NMR (300 MHz, CDCl3) δ 4.74 (bs, 1 H), 3.10 (q, J = 6.1 Hz, 2 H), 2.70 (t, J = 6.7 Hz, 2 H), 1.5-1.43 (m, 4 H), 1.41 (s, 11 H).
5.3.4. Mono-Boc-1,5-diaminopentane (34)
From 23, the general procedure afforded the desired product as a viscous yellow oil (0.418 g, 90%): 1H NMR (300 MHz, CDCl3) δ 4.56 (bs, 1 H), 3.12 (q, J = 6.3 Hz, 2 H), 2.71 (t, J = 6.7 Hz, 2 H), 1.67-1.28 (m, 17 H).
5.3.5. Mono-Boc-1,5-diaminohexane (35)
From 24, the general procedure afforded the desired product as a yellow semisolid (0.428 g, 86%): 1H NMR (300 MHz, CDCl3) δ 4.54 (bs, 1 H), 3.13 (q, J = 6.5 Hz, 2 H), 2.70 (t, J = 6.6 Hz, 2 H), 1.43 (s, 11 H), 1.34-1.30 (m, 8 H).
5.3.6. Mono-Boc-1,7-diaminoheptane (36)
From 25, the general procedure afforded the desired product as a clear, pale-yellow viscous oil, (0.387 g, 73%): 1H NMR (500 MHz, CDCl3) δ 4.54 (bs, 1 H), 3.0-3.2 (m, 2 H), 2.68 (t, J = 7.0 Hz, 2 H), 1.62-1.55 (m, 4 H), 1.42 (s, 9 H), 1.1-1.3 (m, 8 H).
5.3.7. Mono-Boc-1,8-diaminooctane (37)
From 26, the general procedure afforded the product as a colorless semisolid (0.493 g, 91%): 1H NMR (300 MHz, CDCl3) δ 4.50 (bs, 1 H), 3.11 (q, J = 6.8 Hz, 2 H), 2.70 (t, J = 6.8 Hz, 2 H), 1.60-1.50 (m, 4 H), 1.44 (s, 11 H), 1.40-1.30 (b, 8 H).
5.3.8. Mono-Boc-1,9-diaminononane (38)
From 27, the general procedure afforded the product as a viscous, colorless oil which solidified upon standing (0.437 g, 74%): 1H NMR (300 MHz, CDCl3) δ 4.50 (bs, 1 H), 3.13 (q, J = 6.5 Hz, 2 H). 2.69 (t, J = 6.8 Hz, 2 H), 1.40-1.30 (m, 13 H), 1.30-1.20 (m, 12 H).
5.3.9. Mono-Boc 1,10-diaminodecane (39)
From 28, the general procedure afforded the product as a colorless semisolid (0.598 g, 96%): 1H NMR (300 MHz, CDCl3) δ 4.51 (bs, 1 H), 3.13 (q, J = 6.5 Hz, 2 H), 2.69 (t, J = 6.8 Hz, 2 H), 1.50-1.30 (m, 15 H), 1.30-1.20 (m, 12 H).
5.3.10. Mono-Boc-1,11-diaminoundecane (40)
From 29, the general procedure afforded the product as a colorless semisolid (0.588 g, 90%): 1H NMR (300 MHz, CDCl3) δ 4.49 (bs, 1 H), 3.13 (q, J = 6.7 Hz, 2 H), 2.70 (t, J = 6.8 Hz, 2 H), 1.60-1.40 (m, 17 H), 1.30-1.20 (m, 12 H).
5.3.11. Mono-Boc-1,12-diaminododecane (41)
From 30, the general procedure afforded the product as a colorless semisolid (0.547 g, 79%): 1H NMR (300 MHz, CDCl3) δ 4.50 (bs, 1 H), 3.11 (q, J = 5.7 Hz, 2 H), 2.70 (t, J = 6.9 Hz, 2 H), 1.60-1.20 (m, 31 H).
5.4. Preparation of Aromathecins
5.4.1. 14-(2'-tert-Boc-Aminoethyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (42)
Compound 19 (0.070 g, 0.210 mmol) was diluted in DMSO (20 mL) and 31 (0.101 g, 0.631 mmol) was dissolved in DMSO (1 mL) and added to the suspension. The mixture was sonicated briefly to break up the starting material and stirred at room temperature for 19 h, poured into H2O (100 mL), and extracted with CHCl3 (1 × 100 mL, 1 × 60 mL). The organic layers were washed with H2O (2 × 140 mL, 2 × 280 mL), dried over anhydrous sodium sulfate, and concentrated. The residue was adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.4% Et3N in CHCl3. The obtained solid was further purified by preparative TLC (SiO2, 1.5% MeOH, and a few drops of Et3N in CHCl3) to yield a yellow amorphous solid (0.059 g, 62%) after washing with ether: mp 170–173 °C. IR (film) 3436, 2975, 1712, 1655, 1617, 1598, 1502, 1456, 1364, 1171, 753, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.1 Hz, 1 H), 8.25 (dd, J = 12.2 Hz, 8.3 Hz, 2 H), 7.79-7.56 (m, 6 H), 5.43 (s, 2 H), 4.92 (bs, 1 H), 4.37 (s, 2 H), 3.31 (q, J = 5.6 Hz, 2 H), 2.91 (t, J = 5.8 Hz, 2 H), 1.68 (s, 1 H, under residual solvent peak), 1.42 (s, 9 H); ESIMS m/z (rel intensity) 457 (MH+, 65), 357, (MH+ – Boc, 100). Anal. Calcd for C27H28N4O3·0.75 H2O: C, 68.99; H, 6.33; N, 11.92.Found: 68.76; H, 6.51; N, 11.89.
5.4.2. 14-(3'-tert-Boc-Aminopropyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (43)
Compound 19 (0.070 g, 0.210 mmol) was diluted in DMSO (25 mL) and compound 32 (0.110 g, 0.631 mmol) was dissolved in DMSO (1 mL) and added, and the mixture was sonicated briefly to break up the starting material. The mixture was stirred at room temperature for 16 h, poured into H2O (100 mL), and extracted with CHCl3 (2 × 70 mL, 2 × 40 mL). The organic layers were washed with H2O (4 × 140 mL), dried over anhydrous sodium sulfate, and concentrated. The residue was adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.6% Et3N in CHCl3. The obtained solid was further purified by preparative TLC (SiO2, 1.5% MeOH, few drops Et3N in CHCl3) to yield a yellow amorphous solid (0.061 g, 62%) after washing with ether: mp 145–158 °C. IR (film) 3307, 2975, 1696, 1659, 1622, 1602, 1512, 1365, 1345, 1170, 754, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 7.9 Hz, 1 H), 8.30 (d, J = 8.5 Hz, 1 H), 8.24 (d, J = 8.2 Hz, 1 H), 7.79-7.57 (m, 6 H), 5.47 (s, 2 H), 4.87 (bs, 1 H), 4.36 (s, 2 H), 3.22 (q, J = 6.4 Hz, 2 H), 2.85 (t, J = 6.5 Hz, 2 H), 1.75-1.70 (m, 3 H), 1.39 (s, 9 H); ESIMS m/z (rel intensity) 471 (MH+, 35), 371, (MH+ – Boc, 100). Anal. Calcd for C28H30N4O3: C, 71.47; H, 6.43; N, 11.91. Found: C, 71.12; H, 6.64; N, 11.81.
5.4.3. 14-(4'-tert-Boc-Aminobutyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (44)
Compound 19 (0.070 g, 0.210 mmol) was diluted in DMSO (20 mL) and compound 33 (0.119 g, 0.630 mmol) was dissolved in DMSO (2 mL) and added. The mixture was stirred at room temperature for 17 h, poured into H2O (100 mL), and extracted with CHCl3 (60 mL); additionally, MeOH (70 mL) was added to the organic phase to aid solubility. The organic layer was washed with H2O (4 × 150 mL), dried over anhydrous sodium sulfate, and concentrated. The obtained residue was adsorbed onto SiO2 and purified by flash column chromatography (SiO2), eluting with 0.6% Et3N in CHCl3, to yield a yellow amorphous solid (0.069 g, 68%) after washing with ether: mp 168–175 °C (dec). IR (film) 3351, 2930, 1693, 1657, 1621, 1601, 1509, 1365, 1250, 1173, 753, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.0 Hz, 1 H), 8.26 (t, J = 8.4 Hz, 1 H), 7.79-7.57 (m, 6 H), 5.47 (s, 2 H), 4.67 (bs, 1 H), 4.37 (s, 2 H), 3.14 (q, J = 6.0 Hz, 2 H), 2.80 (t, J = 6.2 Hz, 2 H), 1.59-1.55 (m, 5 H), 1.42 (s, 9 H); ESIMS m/z (rel intensity) 485 (MH+, 27), 385, (MH+ – Boc, 100).
5.4.4. 14-(5'-tert-Boc-Aminopentyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (45)
Compound 19 (0.081 g, 0.243 mmol) and compound 34 (0.147 g, 0.729 mmol) were diluted with DMSO (25 mL), and the mixture was stirred at room temperature for 19 h. The reaction mixture was diluted with H2O (100 mL), and extracted with CHCl3, (2 × 100 mL), with additional H2O (100 mL) being added to break up the resultant emulsion. The organic layers were washed with H2O (3 × 100 mL), dried over anhydrous sodium sulfate, concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.4% Et3N in CHCl3 to yield a pale-yellow amorphous solid (0.079 g, 66%) after washing with ether: mp 186–189 °C. IR (film) 3368, 2928, 2856, 1693, 1656, 1619, 1600, 1512, 1453, 1365, 1250, 1172, 835, 752, 686 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.0 Hz, 1 H), 8.26 (t, J = 8.8 Hz, 2 H), 7.78-7.57 (m, 6 H), 5.47 (s, 2 H), 4.66 (bs, 1 H), 4.67 (s, 2 H), 3.12 (q, J = 6.2 Hz, 2 H), 2.78 (t, J = 7.0 Hz, 2 H), 1.60-1.35 (m, 16 H); ESIMS m/z (rel intensity) 499 (MH+, 57), 997, (2MH+ 100).
5.4.5. 14-(6'-tert-Boc-Aminohexyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (46)
Compound 19 (0.070 g, 0.210 mmol) was diluted with DMSO (20 mL) and compound 35 (0.136 g, 0.631 mmol) was dissolved in DMSO (3 mL) and pipetted into the suspension. The mixture was stirred at room temperature for 16 h, poured into H2O (120 mL) and extracted with CHCl3 (2 × 50 mL, 1 × 30 mL). The organic layers were washed with H2O (1 × 120 mL, 4 × 100 mL), dried over anhydrous sodium sulfate, concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.4% Et3N in CHCl3. The obtained residue was further purified by preparative TLC (SiO2, 1% MeOH, and a few drops of Et3N in CHCl3), to yield a pale-yellow amorphous solid (0.055 g, 51%) after washing with ether: mp 99–104 °C. IR (film) 3437, 3360, 3285, 2928, 1705, 1655, 1619, 1601, 1452, 1365, 1172, 835, 752, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 8.1 Hz, 1 H), 8.25 (t, J = 8.1 Hz, 2 H), 7.80-7.54 (m, 6 H), 5.46 (s, 2 H), 4.63 (bs, 1 H), 4.35 (s, 2 H), 3.10 (q, J = 6.3 Hz, 2 H), 2.77 (t, J = 7.0 Hz, 2 H), 1.60-1.30 (m, 18 H); ESIMS m/z (rel intensity), 513, (MH+, 80), 413 (MH+ – Boc, 100).
5.4.6. 14-(7'-tert-Boc-Aminoheptyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (47)
Compound 19 (0.055 g, 0.165 mmol) was diluted with DMSO (25 mL). Compound 36 (0.114 g, 0.495 mmol) was dissolved in a small amount of DMSO and added to the suspension. The mixture was stirred at room temperature for 22 h, diluted with CHCl3 (40 mL), and washed with H2O (4 × 50 mL). The organic phase was dried over anhydrous sodium sulfate, concentrated, and the residue was washed with ether and filtered. The filtrate was diluted with CHCl3 (30 mL) and washed again with H2O (3 × 20 mL) to remove residual DMSO, before it was dried over anhydrous sodium sulfate, combined with the filtered residue, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with a gradient of CHCl3 to 0.5% MeOH-0.5% Et3N to yield a yellow amorphous solid that was further purified by preparative TLC (SiO2, 1.2% MeOH in CHCl3), to yield a pale-yellow amorphous solid (0.039 g, 45%) after washing with ether: mp 97–105 °C. IR (film) 3436, 2926, 2855, 1691, 1655, 1619, 1601, 1512, 1453, 1365, 1252, 1174, 836, 753, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.58 (d, J = 7.8 Hz, 1 H), 8.27 (t, J = 9.4 Hz, 2 H), 7.80-7.58 (m, 6 H), 5.48 (s, 2 H), 4.69 (bs, 1 H), 4.37 (s, 2 H), 3.10 (q, J = 6.3 Hz, 2 H), 2.77 (t, J = 7.0 Hz, 2 H), 1.60-1.20 (m, 20 H); ESIMS m/z (rel intensity), 527, (MH+, 78), 427 (MH+ – Boc, 100).
5.4.7. 14-(8'-tert-Boc-Aminooctyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (48)
Compound 19 (0.071 g, 0.213 mmol) was diluted with DMSO (25 mL), and compound 37 was dissolved in DMSO (2 mL) and added to the suspension. The mixture was stirred at room temperature for 16 h, diluted with CHCl3 (40 mL) and washed with H2O (3 × 50 mL, 1 × 80 mL). The organic phase was dried over anhydrous sodium sulfate, concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with a gradient of CHCl3-0.5% MeOH in CHCl3. The resultant residue was purified further by preparative TLC (1% MeOH in CHCl3) to yield a yellow amorphous solid (0.048 g, 42%) after washing with ether and hexanes: mp 96–101 °C. IR (film) 3436, 3361, 3287, 2924, 2854, 1684, 1655, 1618, 1600, 1452, 1364, 1250, 1173, 834, 752, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.0 Hz, 1 H), 8.26 (t, J = 9.1 Hz, 2 H), 7.78-7.56 (m, 6 H), 5.46 (s, 2 H), 4.61 (bs, 1 H), 4.36 (s, 2 H), 3.12 (q, J = 6.5 Hz, 2 H), 2.76 (t, J = 7.1 Hz, 2 H), 1.70-1.20 (m, 22 H); ESIMS m/z (rel intensity) 541 (MH+, 32), 441 (MH+ – Boc, 100), 563 (MNa+, 37).
5.4.8. 14-(9'-tert-Boc-Aminononyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (49)
Compound 19 (0.070 g, 0.210 mmol) was diluted with DMSO (20 mL) and compound 38 (0.163 g, 0.631 mmol) was added as a suspension in DMSO (5 mL). The mixture was stirred overnight at 20 h, diluted with H2O (100 mL) and extracted with CHCl3 (2 × 50 mL, 1 × 30 mL). The organic layers were washed with H2O (1 × 120 mL, 4 × 100 mL), dried over anhydrous sodium sulfate, concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.2% Et3N in CHCl3. The obtained residue was further purified by preparative TLC (SiO2, 1% MeOH, and few drops of Et3N in CHCl3), to afford the desired product as a yellow amorphous solid (0.054 g, 47%) after washing with ether: mp 90–98 °C. IR (film) 3438, 3360, 2925, 2854, 1693, 1655, 1618, 1600, 1511, 1452, 1365, 1250, 1173, 836, 752, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (J = 7.8 Hz, 1 H), 8.27 (dd, J = 13.4 Hz, 8.2 Hz), 7.79-7.56 (m, 6 H), 5.47 (s, 2 H), 4.56 (bs, 1 H), 4.38 (s, 2 H), 3.10 (q, J = 6.7 Hz, 2 H), 2.78 (t, J = 7.1 Hz, 2 H), 1.74-1.26 (m, 24 H); ESIMS m/z (rel intensity), 555 (MH+, 100).
5.4.9. 14-(10'-tert-Boc-Aminodecyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (50)
Compound 19 (0.070 g, 0.210 mmol) was diluted with DMSO (20 mL) and compound 39 (0.172 g, 0.630 mmol) was added as a suspension in DMSO (5 mL). The reaction mixture was stirred at room temperature for 17 h, diluted with H2O (120 mL), and extracted with CHCl3 (2 × 50 mL, 2 × 80 mL, 1 × 30 mL). The organic layers were washed with H2O (5 × 200 mL), dried over anhydrous sodium sulfate, concentrated, and the resultant residue was adsorbed onto SiO2 and purified by flash column chromatography (SiO2) eluting with 0.2% Et3N in CHCl3. The obtained material was further purified by preparative TLC (0.7% MeOH, and a few drops of Et3N in CHCl3), to yield a bright pale-yellow amorphous solid (0.062 g, 52%) after washing with ether: mp 88–95 °C. IR (film) 3437, 3285, 2924, 2853, 1703, 1655, 1618, 1600, 1453, 1364, 1172, 834, 752, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.0 Hz, 1 H), 8.27 (dd, J = 13.6 Hz, 8.4 Hz, 2 H), 7.80-7.55 (m, 6 H), 5.47 (s, 2 H), 4.53 (bs, 1 H), 4.37 (s, 2 H), 3.10 (q, J = 5.8 Hz, 2 H), 2.77 (t, J = 7.0 Hz, 2 H), 1.64-1.25 (m, 26 H); ESIMS m/z (rel intensity), 569 (MH+, 46), 1137 (2MH+, 100).
5.4.10. 14-(11'-tert-Boc-Aminoundecyl-1'-aminomethyl)-12H-5,11a diazadibenzo[b,h]fluoren-11-one (51)
Compound 19 (0.070 g, 0.210 mmol) and compound 40 (0.180 g, 0.630 mmol) were diluted with DMSO (25 mL) and the mixture was stirred at room temperature for 16 h. The mixture was diluted with H2O (100 mL), extracted with CHCl3 (2 × 50 mL, 2 × 90 mL, 1× 50 mL, 1× 30 mL), and the organic layers were washed with H2O (5 × 200 mL), before they were dried over anhydrous sodium sulfate, concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.2% Et3N in CHCl3, to yield a yellow amorphous solid (0.067 g, 55%) after washing with ether and hexanes: mp 88–100 °C. IR (film) 3436, 3368, 2923, 2853, 1704, 1655, 1618, 1600, 1453, 1365, 1169, 835, 752, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.58 (d, J = 8.2 Hz, 1 H), 8.28 (dd, J = 13.0 Hz, 8.4 Hz, 2 H), 7.82-7.58 (m, 6 H), 5.49 (s, 2 H), 4.52 (bs, 1 H), 4.38 (s, 2 H), 3.10 (q, J = 6.6 Hz, 2 H), 2.77 (t, J = 7.1 Hz, 2 H), 1.59-1.24 (m, 28 H); ESIMS m/z (rel intensity), 583 (MH+, 100).
5.4.11. 14-(12'-tert-Boc-Aminododecyl-1'-aminomethyl)-12H-5,11a diazadibenzo[b,h]fluoren-11-one (52)
Compound 19 (0.070 g, 0.210 mmol) and compound 41 (0.190 g, 0.631 mmol) were diluted with DMSO (25 mL) and the mixture was stirred at room temperature for 17 h. The mixture was diluted with H2O (100 mL), extracted with CHCl3 (1 × 50 mL, 3 × 80 mL, 1 × 50 mL) and the organic layers were washed with H2O (5 × 200 mL), before they were dried over anhydrous sodium sulfate, concentrated, adsorbed onto SiO2, and purified by flash column chromatography (SiO2), eluting with 0.2% Et3N in CHCl3, to yield a pale-yellow amorphous solid (0.088g, 70%) after partially dissolving in ether and precipitating out with hexanes: mp 81–90 °C. IR (film) 3435, 3369, 2922, 2852, 1689, 1655, 1618, 1600, 1453, 1365, 1168, 634, 752, 685 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.58 (d, J = 7.7 Hz, 1 H), 8.29 (dd, J = 12.6, 7.9 Hz, 2 H), 7.81-7.59 (m, 6 H), 5.49 (s, 2 H), 4.50 (bs, 1 H), 4.38 (s, 2 H), 3.10 (q, J = 6.7 Hz, 2 H), 2.76 (t, J = 7.1 Hz, 2 H), 1.57-1.23 (m, 30 H); ESIMS m/z (rel intensity), 597 (MH+, 100). Anal. Calcd for C37H48N4O3 · 0.5 H2O: C, 73.36; H, 8.15; N, 9.25. Found: C, 73.09; H, 8.25; N, 9.23.
5.4.12 14-(2'-Aminoethyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (53)
Compound 42 (0.050 g, 0.110 mmol) was dissolved in CHCl3 (30 mL). Methanolic HCl (3 M, 10 mL) was added dropwise. The addition was slightly exothermic. The mixture was stirred at room temperature for 2 h and concentrated to yield a red-orange solid after washing with ether and drying in vacuo. The powder was then washed with a solution of CHCl3 (containing several drops of MeOH) to remove residual Et3N·HCl, yielding the desired product as a red powder (0.035 g, 69%) after washing with ether: mp 228–235 °C (dec). IR (KBr) 3400, 2991, 2725, 1651, 1595, 1337, 1156, 770, 684 cm−1; 1H NMR (300 MHz, D2O) δ 7.78 (d, J = 8.6 Hz, 1 H), 7.65 (s, 2 H), 7.52-7.39 (m, 4 H), 7.08-7.03 (t, J = 6.6 Hz, 1 H), 7.00 (s, 1 H), 5.12 (s, 2 H), 4.64 (s, 2 H), 3.65 (t, J = 6.8 Hz, 2 H), 3.41 (t, J = 7.0 Hz, 2 H); ESIMS m/z (rel intensity) 357 (MH+, 100). Anal. Calcd for C22H23Cl3N4O: C, 56.73; H, 4.98; N, 12.03. Found: C, 56.90; H, 4.75; N, 11.83.
5.4.13. 14-(3'-Aminopropyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (54)
Compound 43 (0.052 g, 0.111 mmol) was dissolved in CHCl3 (30 mL). Methanolic HCl (3 M, 10 mL) was added dropwise. The mixture was stirred at room temperature for 2 h and concentrated to yield a red-orange solid (0.043 g, 82%) after washing with ether and drying in vacuo: mp 232–240 °C (dec). IR (KBr) 3435, 2922, 1659, 1623, 1601, 1479, 1334, 762, 686 cm−1; 1H NMR (300 MHz, D2O): δ 7.75 (d, J = 8.3 Hz, 1 H), 7.61 (s, 2 H), 7.52-7.41 (m, 4 H), 7.04-6.96 (m, 2 H), 5.10 (s, 2 H), 4.60 (s, 2 H), 3.38 (t, J = 7.9 Hz, 2 H), 3.10 (t, J = 7.4 Hz, 2 H), 2.20-2.00 (m, 2 H); ESIMS m/z (rel intensity) 371(MH+, 100). Anal. Calcd for C23H25Cl3N4O: C, 57.57; H, 5.25; N, 11.68. Found: C, 57.91; H, 5.24; N, 11.72.
5.4.14. 14-(4'-Aminobutyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (55)
Compound 44 (0.055 g, 0.113 mmol) was dissolved in CHCl3 (30 mL). Methanolic HCl (3 M, 10 mL) was added dropwise. The mixture was stirred at room temperature for 2 h and concentrated to yield a red-orange solid (0.050 g, 90%) after washing with ether and drying in vacuo: mp 222–228 °C (dec). IR (KBr) 3401, 2928, 1657, 1596, 1476, 1334, 762, 681 cm−1; 1H NMR (300 MHz, D2O) δ 7.72 (d, J = 8.2 Hz, 1 H), 7.60-7.45 (m, 3 H), 7.36-7.32 (m, 3 H), 7.10-7.00 (m, 1 H), 6.86 (s, 1 H). 5.05 (s, 2 H), 4.55 (s, 2 H), 3.33 (t, J = 6.8 Hz, 2 H), 3.05 (t, J = 7.6 Hz, 2 H), 1.90-1.70 (m, 4 H); ESIMS m/z (rel intensity) 385 (MH+, 100). Anal. Calcd for C24H27Cl3N4O·H2O: C, 56.31; H, 5.71; N, 10.95. Found: C, 56.07; H, 5.44; N, 10.58.
5.4.15. 14-(5'-Aminopentyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (56)
Compound 45 (0.065 g, 0.130 mmol) was diluted in CHCl3 (40 mL) and filtered to remove particulate matter. Methanolic HCl (3 M, 10 mL) was added dropwise, and the dark red mixture was stirred for 2 h at room temperature and concentrated to provide a bright red amorphous solid (0.056 g, 86%) after washing with ether and drying in vacuo: mp 245–251 °C (dec). IR (KBr) 3399, 2929, 1654, 1474, 1336, 1217, 766, 683 cm−1; 1H NMR (300 MHz, D2O) δ 7.75-7.45 (m, 7 H), 7.20-7.02 (m, 2 H), 5.12 (s, 2 H), 4.57 (s, 2 H), 3.27 (t, J = 6.9 Hz, 2 H), 2.98 (t, J = 6.4 Hz, 2 H), 1.80-1.60 (m, 4 H), 1.50-1.40 (m, 2 H); ESIMS m/z (rel intensity). 399 (MH+, 100). Anal. Calcd for C25H29Cl3N4O·2 H2O: C, 55.21; H, 6.12; N, 10.30. Found: C, 55.29; H, 5.97; N, 10.20.
5.4.16. 14-(6'-Aminohexyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (57)
Compound 46 (0.050 g, 0.097 mmol) was dissolved in CHCl3 (30 mL) and methanolic HCl (3 M, 10 mL) was added dropwise. The orange cloudy mixture was stirred at room temperature for 2 h and concentrated to afford a dark red amorphous solid (0.043 g, 85%) after washing with ether and drying in vacuo: mp 230–235 °C. IR (KBr) 3412, 2928, 1657, 1622, 1596, 1478, 1337, 765, 682 cm−1; 1H NMR (300 MHz, D2O) δ 7.72 (d, J = 8.4 Hz, 1 H), 7.60 (s, 2 H), 7.51-7.42 (m, 4 H), 7.06 (t, J = 5.4 Hz, 1 H), 6.95 (s, 1 H), 5.08 (s, 2 H), 4.54 (s, 2 H), 3.25 (t, J = 7.7 Hz, 2 H), 2.97 (t, J = 7.4 Hz, 2 H), 1.80-1.60 (m, 4 H), 1.30-1.40 (m, 4 H); ESIMS m/z (rel intensity) 413 (MH+, 100). Anal. Calcd for C26H31Cl3N4O·H2O: C, 57.84; H, 6.16; N, 10.38. Found: C, 57.53; H, 6.48; N, 10.03.
5.4.17. 14-(7'-Aminoheptyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (58)
Compound 47 (0.035 g, 0.066 mmol) was diluted with benzene (25 mL) and methanolic HCl (3 M, 10 mL) was added dropwise, upon which the solution became dark red. The mixture was heated at reflux for 3 h, cooled, and concentrated to yield a bright orange solid (0.030 g, 85%) after washing with ether: mp 208–215 °C (dec). IR (KBr) 3400, 2929, 1657, 1620, 1600, 1457, 1340, 1132, 764, 687 cm−1; 1H NMR (300 MHz, D2O) δ 7.75-7.69 (m, 3 H), 7.58-7.48 (m, 4 H), 7.20-7.08 (m, 2 H), 5.13 (s, 2 H), 4.56 (s, 2 H), 3.23 (t, J = 8.1 Hz, 2 H), 2.95 (t, J = 7.7 Hz, 2 H), 1.80-1.50 (m, 4 H), 1.30-1.20 (m, 6 H); ESIMS m/z (rel intensity) 427 (MH+, 100). Anal. Calcd for C27H33Cl3N4O·1.5 H2O: C, 57.60; H, 6.45; N, 9.95. Found: C, 57.47; H, 6.49; N, 9.64.
5.4.18. 14-(8'-Aminooctyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (59)
Compound 48 (0.042 g, 0.078 mmol) was dissolved in CHCl3 (30 mL) and methanolic HCl (3 M, 10 mL) was added dropwise, upon which the solution became dark orange-red. The mixture was stirred at room temperature for 2 h and concentrated to yield a dark red flaky solid (0.036, 85%) after washing with ether and drying in vacuo: mp 200–203 °C (dec). IR (KBr) 3401, 2929, 2856, 1758, 1656, 1597, 1619, 1479, 1338, 1135, 764, 688 cm−1; 1H NMR (300 MHz, D2O) 7.70 δ (d, J = 8.1 Hz, 1 H), 7.60-7.38 (m, 6 H), 7.04-7.02 (m, 1 H), 6.89 (s, 1 H), 5.04 (s, 2 H), 4.50 (s, 2 H), 3.22 (t, J = 7.6 Hz, 2 H), 2.94 (t, J = 7.3 Hz, 2 H), 1.80-1.50 (m, 4 H), 1.30-1.20 (m, 8 H); ESIMS m/z (rel intensity) 441 (MH+, 100). Anal. Calcd for C28H35Cl3N4O·H2O: C, 59.21; H, 6.57; N, 9.86. Found: C, 59.03; H, 6.81; N, 9.78.
5.4.19. 14-(9'-Aminononyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (60)
Compound 49 (0.046 g, 0.083 mmol) was dissolved in CHCl3 (20 mL) and methanolic HCl (3 M, 10 mL) was added dropwise. The mixture was stirred for 2 h 20 min at room temperature, concentrated, and azeotroped with benzene, to afford a bright red amorphous solid (0.038 g, 82%) after washing with ether and drying in vacuo: mp 192–196 °C (dec). IR (KBr) 3414, 2928, 2854, 1657, 1622, 1599, 1478, 1336, 761, 688 cm−1; 1H NMR (300 MHz, D2O) δ 7.73-7.65 (m, 3 H), 7.52-7.45 (m, 4 H), 7.20-7.00 (m, 2 H), 5.10 (s, 2 H), 4.53 (s, 2 H), 3.21 (t, J = 6.9 Hz, 2 H), 2.92 (J = 7.2 Hz, 2 H), 1.70-1.50 (m, 4 H), 1.40-1.20 (m, 10 H); ESIMS m/z (rel intensity) 455 (MH+, 100). Anal. Calcd for C29H37Cl3N4O: C, 61.76; H, 6.61; N, 9.93. Found: C, 61.50; H, 7.00; N, 9.71.
5.4.20. 14-(10'-Aminodecyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (61)
Compound 50 (0.056 g, 0.096 mmol) was dissolved in CHCl3 (30 mL) and methanolic HCl (3 M, 10 mL) was added dropwise. The addition was slightly exothermic. The mixture was stirred for 2 h at room temperature and concentrated to yield a red amorphous solid (0.049 g, 87%) after washing with ether and drying in vacuo: mp 185–190 °C (dec). IR (KBr) 3433, 2925, 2854, 1657, 1622, 1599, 1469, 1335, 764, 688 cm−1; 1H NMR (300 MHz, D2O) δ 7.75-7.69 (m, 3 H), 7.56-7.49 (m, 4 H), 7.15-7.08 (m, 2 H), 5.13 (s, 2 H), 4.56 (s, 2 H), 3.21 (t, J = 7.4 Hz, 2 H), 2.91 (t, J = 7.5 Hz, 2 H), 1.70-1.50 (m, 4 H), 1.40-1.20 (bm, 12 H); ESIMS m/z (rel intensity) 469 (MH+, 100). Anal. Calcd for C30H39Cl3N4O·0.6 H2O: C, 61.19; H, 6.88; N, 9.51. Found: C, 60.97; H, 7.16; N, 9.30.
5.4.21. 14-(11'-Aminoundecyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (62)
Compound 51 (0.056 g, 0.097 mmol) was dissolved in CHCl3 (30 mL) and methanolic HCl (3 M, 10 mL) was added dropwise. The reaction was slightly exothermic. The mixture was stirred for 2 h at room temperature and concentrated to yield a red-orange amorphous solid (0.052 g, 91%) after washing with ether and drying in vacuo: mp 183–188 °C (dec). IR (KBr) 3431, 2925, 2853, 1657, 1622, 1601, 1469, 1336, 762, 688 cm−1; 1H NMR (300 MHz, D2O) δ 7.75-7.52 (m, 7 H), 7.19-7.13 (m, 2 H), 5.16 (s, 2 H), 4.58 (s, 2 H), 3.19 (t, J = 6.2 Hz, 2 H), 2.91-2.85 (m, 2 H), 1.70-1.50 (m, 4 H), 1.40-1.10 (m, 14 H); ESIMS m/z (rel intensity) 483 (MH+, 100). Anal. Calcd for C31H41Cl3N4O·0.5 H2O: C, 61.95; H, 7.04; N, 9.32. Found: C, 61.88; H, 7.31; N, 9.10.
5.4.22. 14-(12'-Aminododecyl-1'-aminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (63)
Compound 52 (0.069 g, 0.115 mmol) was dissolved in CHCl3 (30 mL) and methanolic HCl (3 M, 10 mL) was added dropwise. The reaction mixture was stirred at room temperature for 2 h and concentrated to yield a bright red amorphous solid (0.064 g, 92%) after washing with ether and drying in vacuo: mp 200–204 °C (dec). IR (KBr) 3409, 2924, 2851, 1657, 1619, 1598, 1467, 1337, 764, 688 cm−1; 1H NMR (300 MHz, D2O, 60 °C) δ 7.98-7.81 (m, 4 H), 7.72 (t, J = 7.8 Hz, 3 H), 7.43-7.37 (m, 2 H), 5.29 (s, 2 H), 4.71 (s, 2 H), 3.23 (t, J = 7.6 Hz, 2 H), 3.02-2.92 (m, 2 H), 1.71-1.56 (m, 4 H), 1.30-1.15 (m, 16 H); ESIMS m/z (rel intensity), 497, (MH+, 100). Anal. Calcd for C32H43Cl3N4O·H2O: C, 63.42; H, 7.15; N, 9.24; Cl, 17.55. Found: C, 63.06; H, 7.48; N, 9.12; Cl, 17.17.
5.5. Topoisomerase I-Mediated DNA Cleavage Reactions
Human recombinant Top1 was purified from Baculovirus as previously described.56 DNA cleavage reactions were prepared as previously reported30 (for review see57) with the exception of the DNA substrate. Briefly, a 117-bp DNA oligonucleotide (Integrated DNA Technologies) encompassing the previous identified Top1 cleavage sites identified in the 161-bp fragment from pBluescript SK(−) phagemid DNA was employed. This 117-bp oligonucleotide contains a single 5’-cytosine overhang, which was 3’-end labeled by fill-in reaction with [α-32P]-dGTP in React 2 buffer (50 mM Tris-HCl, pH 8.0, 100 mM MgCl2, 50 mM NaCl) with 0.5 units of DNA polymerase I (Klenow fragment, New England BioLabs). Unincorporated 32P-dGTP was removed using mini Quick Spin DNA columns (Roche, Indianapolis, IN), and the eluate containing the 3'-end-labeled DNA substrate was collected. Approximately 2 nM of radiolabeled DNA substrate was incubated with recombinant Top1 in 20 µL of reaction buffer [10 mM Tris-HCl (pH 7.5), 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, and 15 µg/ml BSA] at 25 °C for 20 min in the presence of various concentrations of compounds. The reactions were terminated by adding SDS (0.5% final concentration) followed by the addition of two volumes of loading dye (80% formamide, 10 mM sodium hydroxide, 1 mM sodium EDTA, 0.1% xylene cyanol, and 0.1% bromphenol blue). Aliquots of each reaction were subjected to 20% denaturing PAGE. Gels were dried and visualized by using a Phosphoimager and ImageQuant software (Molecular Dynamics). For simplicity, cleavage sites were numbered as previously described in the 161-bp fragment.56
5.6. Modeling Studies
The crystal structure of topotecan in complex with top1 and a short DNA fragment were downloaded from the Protein Data Bank (PDB code 1K4T).8 An atom of Hg and one molecule of PEG were deleted. Hydrogens were added and minimized by the Powell method, using the MMFF94s force field and MMFF94 charges. Analogues of aromathecins and indenoisoquinolines were constructed in Sybyl 8.1 (Tripos, Inc.). Fixed positive charges were assigned to amines using Sybyl atom types, and the structures were energy minimized using a conjugate gradient method, MMFF94s force field, and MMFF94 charges. The structure of the ligand was aligned onto topotecan using the “fit atoms” function. In the case of 64, the alignment was based on both the overlay of the crystal structures 1K4T and 1SC7, and on earlier overlays of indenoisoquinolines and aromathecins.8,30 The aligned ligand was overlapped in the crystal structure, and the structure of topotecan was deleted. This new ternary complex was re-subjected to energy minimization using a standard Powell method, the MMFF94s force field, and MMFF94 charges, converging to termination at 0.05 kcal/mol*Å, with a distance-dependent dielectric function. The structure of the ligand and a sphere with a radius of 5 Å were allowed to move during the minimization, and the surrounding structures were frozen in an aggregate.
Figure 1.
Representative Top1 Inhibitors.
Acknowledgments
This work was made possible by the National Institutes of Health (NIH) through support with Research Grant UO1 CA89566. This work was also made possible by the Lynn and McKeehan Fellowships (Purdue University, M.A.C.), and by the CIC/SROP program (Purdue University, B.C.). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. M.A.C. wishes to thank Drs. Andrew Morrell, Karl Wood, and Huaping Mo for their assistance and insight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Pommier Y. Nature Reviews Cancer. 2006;6:789. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 2.Wang JC. Nature Rev. Mol. Cell Biol. 2002;3:430. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Husain I, Mohler JL, Seigler HF, Besterman JM. Cancer. Res. 1994;54(2):539. [PubMed] [Google Scholar]
- 4.Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M. Science. 1989;246:1046. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- 5.Wall ME, Wani MC, Cook CE, Palmer KH, A.T M, Sim GA. J. Am. Chem. Soc. 1966;88:3888. The. [Google Scholar]
- 6.Hsiang YH, Hertzberg R, Hecht S, Liu LF. J. Biol. Chem. 1985;260:14873. [PubMed] [Google Scholar]
- 7.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr, Stewart L. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15387. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staker BL, Feese MD, Cushman M, Pommier Y, Zembower D, Stewart L, Burgin A. J. Med. Chem. 2005;48:2336. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 9.Li TK, Liu LF. Annu. Rev. Pharmacol. Toxicol. 2001;41:53. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa H, Saito H, Ikegami Y, Aida-Hyugaji S, Sawada S, Ishikawa T. Cancer Letters. 2006;234:81. doi: 10.1016/j.canlet.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 11.Pommier Y, Pourquier P, Urasaki Y, Wu J, Laco G. Drug Resist. Updat. 1999;2:307. doi: 10.1054/drup.1999.0102. [DOI] [PubMed] [Google Scholar]
- 12.Chrencik JE, Staker BL, Burgin AB, Jr, Pourquier P, Pommier Y, Stewart L, Redinbo MR. J. Mol. Biol. 2004;339:773. doi: 10.1016/j.jmb.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 13.Teicher BA. Biochem. Pharmacol. 2008;75:1262. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Schaeppi U, Fleischman R, Cooney DW. Cancer Chemother. Rep. 1974;58:25. (NSC-100880) [PubMed] [Google Scholar]
- 15.Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y. Cancer Res. 1989;49:1465. [PubMed] [Google Scholar]
- 16.Hsiang Y-H, Liu LF, Wall ME, Wani MC, Nicholas AW, Manikumar G, Kirschenbaum S, Silber R, Potmesil M. Cancer Res. 1989;49:4385. [PubMed] [Google Scholar]
- 17.Luzzio MJ, Besterman JM, Emerson DL, Evans MG, Lackey K, Leitner PL, McIntyre G, Morton B, Myers PL, Peel M, Sisco JM, Sternbach DD, Tong W, Truesdale A, Uehling DE, Vuong A, Yates I. J. Med. Chem. 1995;38:395. doi: 10.1021/jm00003a001. [DOI] [PubMed] [Google Scholar]
- 18.Haas NB, LaCreta FP, Walczak J, Hudes GR, Brennan JM, Ozols RF, O’Dwyer PJ. Cancer Res. 1994;54(5):1220. [PubMed] [Google Scholar]
- 19.Cagir A, Jones SH, Gao R, Eisenhauer BM, Hecht SM. J. Am. Chem. Soc. 2003;125:13628. doi: 10.1021/ja0368857. [DOI] [PubMed] [Google Scholar]
- 20.Bailly C, Riou JF, Colson P, Houssier C, Rodrigues-Pereira E, et al. Biochemistry. 1997;36:3917. doi: 10.1021/bi9624898. [DOI] [PubMed] [Google Scholar]
- 21.Saif MW, Diasio RB. Clin. Colorectal Cancer. 2005;5(1):27. doi: 10.3816/ccc.2005.n.014. [DOI] [PubMed] [Google Scholar]
- 22.Kohlhagen G, Paull KD, Cushman M, Nagafuji P, Pommier Y. Mol. Pharm. 1998;54:50. doi: 10.1124/mol.54.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Nagarajan M, Morrell A, Fort BC, Meckley MR, Antony S, Kohlhagen G, Pommier Y, Cushman M. J. Med. Chem. 2004;47(23):5651. doi: 10.1021/jm040025z. [DOI] [PubMed] [Google Scholar]
- 24.Nagarajan M, Morrell A, Ioanoviciu A, Antony S, Kohlhagen G, Hollingshead M, Pommier Y, Cushman M. J. Med. Chem. 2006;49:6283. doi: 10.1021/jm060564z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antony S, Jayaraman M, Laco G, Kohlhagen G, Kohn KW, Cushman M, Pommier Y. Cancer Res. 2003;63:7428. [PubMed] [Google Scholar]
- 26.Antony S, Agama KK, Miao Z-H, Takagi K, Wright MH, Robles AI, Varticovski L, Nagarajan M, Morrell A, Cushman M, Pommier Y. Cancer Res. 2007;67(21):10397. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 27.Cheng K, Rahier NJ, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM. J. Am. Chem. Soc. 2005;127(3):838. doi: 10.1021/ja0442769. [DOI] [PubMed] [Google Scholar]
- 28.Xiao X, Antony S, Pommier Y, Cushman M. J. Med Chem. 2006;49:1408. doi: 10.1021/jm051116e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox BM, Xiao X, Antony S, Kohlhagen G, Pommier Y, Staker BL, Stewart L, Cushman M. J. Med Chem. 2003;46:3275. doi: 10.1021/jm0300476. [DOI] [PubMed] [Google Scholar]
- 30.Cinelli MA, Morrell A, Dexheimer T, Scher E, Pommier Y, Cushman M. J. Med. Chem. 2008;51(15):4609. doi: 10.1021/jm800259e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell A, Placzek MS, Steffen JD, Antony S, Agama K, Pommier Y, Cushman M. J. Med Chem. 2007;50:2040. doi: 10.1021/jm0613119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarajan M, Xiao X, Antony S, Kohlhagen G, Pommier Y, Cushman M. J. Med. Chem. 2003;46:5712. doi: 10.1021/jm030313f. [DOI] [PubMed] [Google Scholar]
- 33.Dallavalle S, Giannini G, Alloatti D, Casati A, Marastoni A, Musso L, Merlini L, Morini G, Penco C, Pisano C, Tinelli S, De Cesare M, Beretta GL, Zunino F. J. Med. Chem. 2006;49(17):5177. doi: 10.1021/jm060285b. [DOI] [PubMed] [Google Scholar]
- 34.Ohara K, Smietana M, Restouin A, Mollard S, Borg J-P, Collette Y, Vasseur J-J. J. Med. Chem. 2007;50:6465. doi: 10.1021/jm701207m. [DOI] [PubMed] [Google Scholar]
- 35.Delcros J-G, Tomasi S, Carrington S, Martin B, Renault J, Blagbrough IS, Uriac P. J. Med. Chem. 2002;45:5098. doi: 10.1021/jm020843w. [DOI] [PubMed] [Google Scholar]
- 36.Babjak M, Kanazawa A, Anderson RJ, Greene AE. Org. Biomol. Chem. 2006;4:407. doi: 10.1039/b516154a. [DOI] [PubMed] [Google Scholar]
- 37.Grillet F, Baumlová B, Prévost G, Constant J-F, Chaumeron S, Bigg CH, Greene AE, Kanazawa A. Bioorg. Med. Chem. Lett. 2008;18(6):2143. doi: 10.1016/j.bmcl.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 38.Shamma M, Novak L. Tetrahedron. 1969;25:2275. [Google Scholar]
- 39.Houghton PG, Humphrey GR, Kennedy DJ, Roberts DC, Wright SHB. J. Chem. Soc. Perkin Trans. 1993;1:1421. [Google Scholar]
- 40.Sloan KB, Koch SAM. J. Org. Chem. 1983;48:635. [Google Scholar]
- 41.Sugasawa T, Toyoda T, Adachi M, Sasakura K. J. Am. Chem. Soc. 1978;100:4842. [Google Scholar]
- 42.Sugasawa T, Adachi M, Sakasura K, Kitagawa A. J. Org. Chem. 1979;44(4):578. [Google Scholar]
- 43.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J. Natl. Cancer Inst. 1990;82(13):1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 44.Boyd MR, Paull KD. Drug Development Res. 1995;34:91. [Google Scholar]
- 45.Nagarajan M, Morrell A, Antony S, Kohlhagen G, Agama K, Pommier Y, Ragazzon P, Garbett NC, Chaires JB, Hollingshead M, Cushman M. J. Med Chem. 2006;49:5129. doi: 10.1021/jm060046o. [DOI] [PubMed] [Google Scholar]
- 46.Wunz TP, Craven, Karol MD, Hill C, Remers WA. J. Med. Chem. 1990;33:1459. doi: 10.1021/jm00168a005. [DOI] [PubMed] [Google Scholar]
- 47.Denny WA. Curr. Med. Chem. 2002;9:1655. doi: 10.2174/0929867023369277. [DOI] [PubMed] [Google Scholar]
- 48.Ioanoviciu A, Antony A, Pommier Y, Staker BL, Stewart L, Cushman M. J. Med. Chem. 2005;48:4803. doi: 10.1021/jm050076b. [DOI] [PubMed] [Google Scholar]
- 49.Marchand C, Antony S, Kohn KW, Cushman M, Ioanoviciu A, Staker BL, Burgin AB, Stewart L, Pommier Y. Mol. Cancer. Ther. 2006;5:287. doi: 10.1158/1535-7163.MCT-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. J. Natl. Cancer. Inst. 1989;81:1088. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 51.Paull KD, Hamel E, Malspeis L. In: Cancer Chemotherapeutic Agents. Foye WO, editor. Washington, DC: American Chemical Society; 1995. p. 9. [Google Scholar]
- 52.Porter CW, Miller J, Bergeron RJ. Cancer Res. 1984;44:126. [PubMed] [Google Scholar]
- 53.Morrell A, Placzek M, Parmley S, Grella B, Antony S, Pommier Y, Cushman M. J. Med. Chem. 2007;50:4388. doi: 10.1021/jm070307+. [DOI] [PubMed] [Google Scholar]
- 54.Sharma L, Tsai Y-C, Liu AA, Liu AF, LaVoie EJ. Eur. J. Med. Chem. 2009;44(4):1471. doi: 10.1016/j.ejmech.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 55.Satyanarayana M, Feng W, Cheng L, Liu AA, Tsai Y-C, Liu LF, LaVoie EJ. Bioorg. Med. Chem. 2008;16(16):7824. doi: 10.1016/j.bmc.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 56.Pourquier P, Ueng L-M, Fertala J, Wang D, Park H-K, Essigmann JM, Bjornsti M-A, Pommier Y. J. Biol. Chem. 1999;274:8516. doi: 10.1074/jbc.274.13.8516. [DOI] [PubMed] [Google Scholar]
- 57.Dexheimer TS, Pommier Y. Nature Protocols. 2008;3(11):1736. doi: 10.1038/nprot.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]