Abstract
Chronic inflammation promotes the formation of ectopic lymphoid tissue morphologically resembling secondary lymphoid tissues, though it is unclear whether this is a location where antigen-specific immune responses develop or merely a site of lymphocyte accumulation. Ectopic lymphoid tissue formation is associated with many humoral autoimmune diseases, including lupus induced by tetramethylpecadentane (TMPD) in mice. We examined whether an immune response to NP-KLH and NP-OVA develops within ectopic lymphoid tissue (“lipogranulomas”) induced by TMPD in C57BL/6 mice. Following primary immunization, NP-specific B cells bearing V186.2 and related heavy chains as well as λ-light chains accumulated within ectopic lymphoid tissue. The number of anti-NP secreting B cells in the ectopic lymphoid tissue was greatly enhanced by immunization with NP-KLH Remarkably, the H-chain sequences isolated from individual lipogranulomas from these mice were diverse before immunization, whereas individual lipogranulomas from single immunized mice had unique oligo- or monoclonal populations of presumptive NP-specific B cells. H-chain CDR sequences bore numerous replacement mutations, consistent with an antigen-driven and T cell-mediated response. In mice adoptively transferred with OT-II or DO11 T cells, there was a striking accumulation of OVA-specific T cells in lipogranulomas after subcutaneous immunization with NP-OVA. The selective co-localization of proliferating, antigen-specific T and B lymphocytes in lipogranulomas from TMPD-treated mice undergoing primary immunization implicates ectopic lymphoid tissue as a site where antigen-specific humoral immune responses can develop. This has implications for understanding the strong association of humoral autoimmunity with lymphoid neogenesis, which may be associated with deficient censoring of autoreactive cells.
Keywords: B cells, T cells, lymphoid neogenesis, tetramethylpentadecane, chronic inflammation
INTRODUCTION
Lymphoid neogenesis, the formation of ectopic (tertiary) lymphoid tissue in response to inflammation (1,2), is associated with the production of autoantibodies in several diseases including Sjogren’s syndrome, rheumatoid arthritis, and myasthenia gravis (3). Whether ectopic lymphoid tissue participates directly in generating autoreactive B cells (e.g. by allowing the autoreactive cells to escape self-tolerance) or indirectly as a site where mature antibody-secreting cells can persist (4) is unknown. Intraperitoneal injection of non-autoimmune-prone mice such as BALB/c with the hydrocarbon oil 2, 6, 10, 14-tetramethylpentadecane (TMPD) triggers the formation of ectopic lymphoid tissue (“lipogranulomas”) and the development of lupus-like autoimmune disease with autoantibodies against small nuclear ribonucleoproteins (snRNPs) and dsDNA, immune complex-mediated glomerulonephritis, arthritis, and vasculitis (5–8). Within the ectopic lymphoid tissue induced by TMPD are CD11c+ dendritic cells (DCs) expressing the co-stimulatory molecule CD86. Expression of the lymphoid chemokines CXCL13 (BLC), CCL19 (ELC), and CCL21 (SLC) is likely to play a role in the accumulation of B cells, T cells, and DCs in TMPD-induced tertiary lymphoid tissue (9). Although individual TMPD-induced lipogranulomas bear some resemblance histologically to germinal centers, there are differences, including the absence of peanut agglutinin+ B cells and FDC-M1+ follicular dendritic cells (7). Nevertheless, proliferating (Ki-67+) lymphocytes can be demonstrated in these structures. Moreover, the B cells bear somatically mutated and isotype-switched immunoglobulin heavy chains (D. Nacionales, et al., Submitted), suggesting that lipogranulomas may support antigen-specific immune responses and may be a location where tolerance to autoantigens can be overcome.
The germinal center reaction regulates antigen-specific clonal evolution during the development of B cell memory (10). B cells in newly formed germinal centers may be oligoclonal (11), whereas those in TMPD-induced lipogranulomas are usually more diverse (D. Nacionales, et al., Submitted). The existence in TMPD-treated mice of occasional lipogranulomas containing oligoclonal B cells is currently unexplained.
Previous studies have shown that following immunization with NP-KLH, two anatomically and phenotypically distinct populations of antibody-forming cells arise in the spleen. As early as 2 days after immunization, primary foci consisting of antigen-binding B cells are seen along the periphery of the periarteriolar lymphoid sheaths (12). Initially these foci expand, but by day 14, they disappear, giving rise to a second responding population in the follicle, germinal center B cells, which appear on day 8–10 and persist at least until day 30 post- immunization. The primary foci are sites of interclonal competition for antigen among unmutated B cells, whereas germinal centers are sites of intraclonal competition between mutated sister lymphocytes (12) as well as interclonal competition between pre-existing germinal center B cells and follicular “visitors” that can join the germinal center reaction if they have a sufficiently high antigen-binding affinity (13).
In the present study, we analyzed antigen-specific B and T cells in TMPD-induced ectopic lymphoid tissue at 12 days after primary subcutaneous immunization with a test antigen, NP-KLH. We show that oligoclonal populations of NP-specific B cells develop in TMPD-induced ectopic lymphoid tissue and may displace or overgrow more diverse populations of non-NP specific B cells present prior to immunization. Along with the hapten-specific B cells, the TMPD-induced lipogranulomas contain carrier-specific T cells, strongly suggesting that ectopic lymphoid tissue can participate directly in the generation of antigen-specific B cell responses.
MATERIALS AND METHODS
Mice
Six-week-old female C57BL/6, BALBC/J, T cell transgenic C.Cg-Tg(DO11.10)10Dlo/J (DO11.10), and C57BL/6-Tg(TcraTcrb)425Cbn/J (OT-II) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in barrier cages. At 2 months of age, C57BL/6 and DO11.10 mice received 0.5 ml of 2, 6, 10, 14 tetramethylpentadecane (TMPD, Sigma-Aldrich, St. Louis, MO) i.p. or left untreated. Three months later, the mice were injected subcutaneously in the lower abdomen with 100 µg of 4-hydroxy-3-nitrophenyl acetyl (NP)17–19-conjugated keyhole limpet hemocyanin (KLH) (NP-KLH, Biosearch Technologies, novato, ca) precipitated in alum (Pierce, Rockford, IL). Lipogranulomas, spleen, and blood were harvested 4 to12 days later. These studies were approved by the Institutional Animal Care and Use Committee.
Anti-NP IgM and IgG ELISA
Pre-immune sera and sera obtained at the time of euthanasia were tested for IgM and IgG anti-NP antibodies (ELISA). Microtiter plates were coated with NP19-, NP30-, or NP3- conjugated BSA (Biosearch Technologies). Serially diluted serum samples were added for 1 hour at room temperature. Anti-NP IgM and IgG antibodies were detected using alkaline phosphatase conjugated goat anti-mouse IgM or IgG antibodies (1:1000 dilution, BD Biosciences, San Jose, CA) followed by phosphatase substrate (Sigma-Aldrich). Optical density was converted to concentration based on standard curves with sera from C57BL/6 mice immunized with NP-KLH using a four-parameter logistic equation (Softmax Pro 3.1 software, Molecular Devices Corporation, Sunnyvale, CA).
Bromodeoxyuridine (BrdU) labeling of B and T cells
BrdU was administered to BALB/cJ mice (0.2 mg BrdU i.p. every 4 hours for 3 doses) and again one day before euthanasia. Single cell suspensions of lipogranuloma or spleen tissue were made by collagenase treatment (7). The isolated cells were incubated with APC-conjugated anti-BrdU antibodies plus anti-CD3-FITC and anti-CD19-PE antibodies (BD Biosciences) and analyzed by flow cytometry. Isotype controls were employed to evaluate background fluorescence. Isolated lipogranuloma cells were washed in staining buffer (PBS supplemented with 0.1% NaN3 and 1% bovine serum albumin) and pre-incubated for 20 minutes with 1 µg of anti-CD16 (BD Biosciences) and 0.5 µl rat serum (Sigma Aldrich) at 4°C in 20 µl of staining buffer to block Fc binding. Primary antibodies were then added at pre-titrated amounts and incubated for 20 minutes at 4°C, followed by washing in staining buffer. Intracellular BrdU labeling was performed using the APC BrdU flow kit following the manufacturer’s instructions (BD Biosciences). Data were acquired on a CyAn ADP flow cytometer (Dako, Fort Collins, Colorado) and analyzed with FCS Express Version 3 (DeNovo Software, Thornhill, Ontario, Canada). At least 50,000 events per sample were acquired and analyzed using size gating to exclude dead cells.
κ/λ light chain staining
Lipogranulomas and spleen were fixed and embedded as previously described (7). Sections (4 µm) were stained with horse radish peroxidase (HRP)-conjugated goat anti-mouse κ- or -λ light chain antibodies (Southern Biotechnology, Birmingham, AL), developed with DAB (Vector Laboratories, Burlingame, CA), and viewed under a light microscope.
Anti-NP ELISPOT assay
Lipogranulomas and spleen (104 cells/well) from NP-KLH/ TMPD treated mice were collagenase treated as described (7) and single cell suspensions were plated on Multiscreen HTS plates (Millipore, Billerica, MA) coated with NP19-BSA. Lipogranuloma and spleen cells from non-immunized TMPD treated mice were used as the negative control. The cells were incubated overnight before adding alkaline phosphatase-conjugated goat anti-mouse IgM antibodies. Spots were developed with BCIP/NBT (Pierce) and incubated overnight before counting using a dissecting microscope.
VH gene sequences
Lipogranulomas and spleen were harvested at day 12 and mRNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, California). The pellets were washed with cold 75% (v/v) ethanol and resuspended in diethyl pyrocarbonate (DEPC)-treated water. One µg of RNA was treated with DNase I (Invitrogen) to remove genomic DNA and reverse transcribed to cDNA using Superscript First-Strand Synthesis System for RT-PCR (Invitrogen). PCR amplification of immunoglobulin H-chain cDNA was performed using a mixture of 8 forward primers (VHF1-8) and a consensus reverse primer (VHR2) as described (14) (D Nacionales, et al. Submitted). The PCR products were cloned into a TOPO vector (pCR4, Invitrogen) and the VDJ heavy chain sequences were determined by dideoxy sequencing and analyzed using the MacVector V 7.2.3 program (Accelrys Inc., San Diego CA).
Transfer of antigen-specific T cells
5 × 106 CD4+ T cells from OT-II or DO11.10 mice were transferred i.v. to C57BL/6J or BALB/cJ TMPD-treated recipients. Three days after T cell transfer, the mice were immunized s.c. with 200 µg NP17-OVA precipitated in alum. Seven days after immunization single cell suspensions of the draining lymph nodes, spleen, and lipogranulomas were prepared as above. Lipogranuloma cell suspensions from C57BL/6J recipients of OT-II T cells were analyzed by FACS using anti-CD3-FITC, anti-CD4-APC, anti-Vα2-PE, and anti-Vβ5-Biotin-Av/Pac Blue antibodies (BD Biosciences). BALB/cJ recipients of DO11.10 T cells were analyzed using anti-DO11.10 (KJI-26)-APC antibodies (Invitrogen, Caltag Laboratories, Carlsbad, CA).
T cell proliferation assay
Three months after TMPD or saline treatment, DO11.10 mice were immunized with 50 µg of specific peptide corresponding to amino acids 323–339 of ovalbumin (Ova323–339; Genscript Corporation, Piscataway, NJ) in alum. Five days later, CD4+ T cells were sorted using MACS anti-CD4 beads (Miltenyi Biotec, Auburn, CA). 2.5 × 104 CD4+ T cells were cultured with irradiated (3000 R), CD4-depleted APCs (2.5 × 105 cells/well) in quadruplicate. 2.5 µg/ml of soluble anti-CD3 or 10 µg/ml of OVA323–339 was added for 48–72 hours in a total volume of 200 µl of complete RPMI medium. One µCi [3H]-thymidine (Amersham Biosciences, Piscataway, NJ) was added for the final 16 h of culture and proliferation was determined using a liquid scintillation counter.
PCR analysis of T cell cytokines
Lipogranulomas and spleen were harvested 10 days after immunization with NP-KLH followed by isolation of mRNA and cDNA synthesis as described above. PCR amplification of β-actin, IL-4, IFNγ, and IL-21 was performed using the following primers: β-actin forward: (TGGAATCCTGTGGCATCCATGAAAC); β-actin reverse (TAAAACGCAGCTCAGTAACAGTCCG); (IL-4 forward: (CGAAGAACACCACAGAGAGTGAGCT); IL-4 reverse: (GACTCATTCATGGTGCAGCTTATCG); IFNγ forward: (AGCGGCTGACTGAACTCAGATTGTAG); IFNγ reverse: (GTCACAGTTTTCAGCTGTATAGGG); IL-21 forward: (ATGGAGAGGACCCTTGTCTG); IL-21 reverse: (GCTTGAGTTTGGCCTTCTGA). One µl of cDNA was added to a mixture containing 1X PCR buffer, 2.5 mM MgCl2, 400 µM dNTPs, 0.025 U of Taq DNA polymerase (Invitrogen), 1 µM each of forward and reverse primers, and DEPC-water in a 20 µl volume. Amplification was carried out for 5 min at 94°C, followed by 35 cycles of denaturation at 94°C for 2 min, annealing at 55°C for 30 sec, extension at 72°C for 45 sec and a final extension of 72°C for 10 min in a PTC-100 Programmable Thermal Controller (MJ Research, Inc., Waltham, MA). PCR products were visualized on 1% agarose gel.
RESULTS
Antigen-specific B cell responses in ectopic lymphoid tissue
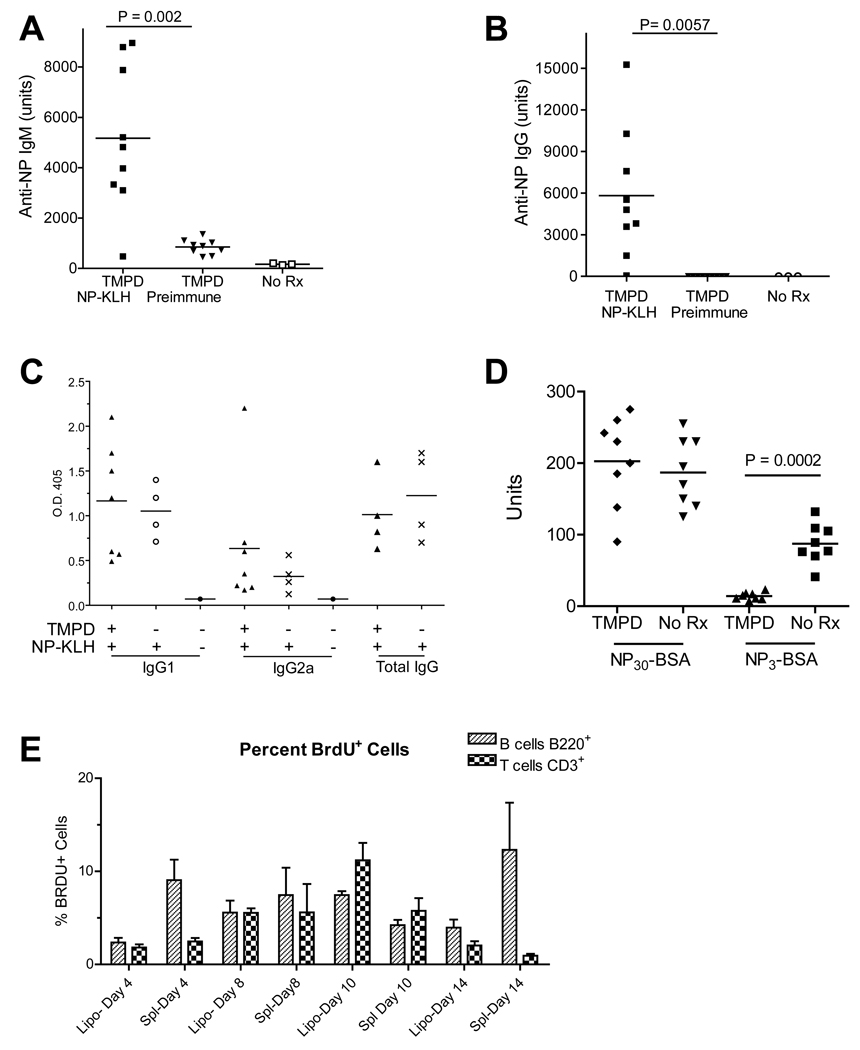
Intraperitoneal injection of TMPD leads to the formation of ectopic lymphoid tissue containing B and T lymphocytes as well as activated DCs (7). We asked whether lipogranulomas are a site where specific T cell-dependent antibody responses develop. TMPD-treated B6 mice were immunized s.c. with NP-KLH, which induces a highly restricted antibody response dominated by B cells bearing V186.2 H-chains and λ1 light chain specific for the NP hapten (11,15). Compared with controls, NP-KLH immunized mice displayed significantly higher levels of serum anti-NP IgM and IgG 12 days post-immunization (Fig. 1A–B). Both TMPD-treated and untreated mice developed a typical IgG1-dominated response following immunization with NP-KLH in alum (Fig. 1C). NP-specific IgG1, IgG2a, and total IgG levels were unaffected by TMPD treatment (Fig. 1C).
Figure 1. Serum anti-NP response after immunization with NP-KLH.
A and B, B6 mice injected with TMPD 3 months earlier were immunized with NP-KLH. At day 12, sera were tested for IgM (A) and IgG (B) anti-NP antibodies by ELISA using NP-BSA. There was a significant increase in both IgM and IgG anti-NP from pre-immune sera to day 12 immune sera. C, Isotypes of anti-NP antibodies (ELISA) from mice either pre-treated or not pre-treated with TMPD prior to immunization with NP-KLH. D, Affinity of anti-NP antibodies developing in TMPD-treated mice vs. controls (no Rx) immunized with NP-KLH. Serum binding activity (measured in arbitrary units using a standard curve) was measured using NP30-BSA (low and high avidity/affinity antibodies) and NP3-BSA (high avidity/affinity antibodies). E, In vivo BrdU labeling of T and B cells in the lipogranulomas and spleens of TMPD-treated and NP-KLH immunized mice (n = 3). Single cell suspensions were stained with anti-CD45R (B220) and anti-CD3 antibodies and with an anti-BrdU antibody. Data are expressed as the % of BrdU+ B cells or T cells, respectively, at 4, 8, 10, or 14 days after immunization with NP-KLH.
To evaluate the affinity of the antibody responses, reactivity with NP30-BSA and NP3-BSA was determined by ELISA. Interestingly, although the total serum levels of antibodies reactive with NP30-BSA in mice pre-treated with TMPD were similar to those in untreated mice, reactivity of sera from the TMPD-pre-treated mice with NP3-BSA was significantly less than the reactivity of sera from untreated mice (Fig. 1D). Thus, the affinity of the anti-NP antibodies induced by immunization with NP-KLH was lower in mice with TMPD-induced ectopic lymphoid tissue than in controls.
Because one of the characteristics of secondary lymphoid tissue is the proliferation of antigen-specific B and T lymphocytes, it was of interest to examine T and B cell proliferation in the ectopic lymphoid tissue induced by TMPD. Mice were fed BrdU in the drinking water and immunized with NP-KLH. Following immunization, there was a progressive increase in the number of B220+ cells and CD3+ cells labeled with anti-BrdU antibodies both in the spleen and in the lipogranulomas, which was apparent as early as day 4–8 after immunization and peaked around day 10 (Fig. 1E). These data indicate that primary immunization with an exogenous antigen stimulated lymphocyte proliferation within ectopic lymphoid tissue and that the magnitude of this proliferative response was comparable to that in a secondary lymphoid organ, the spleen.
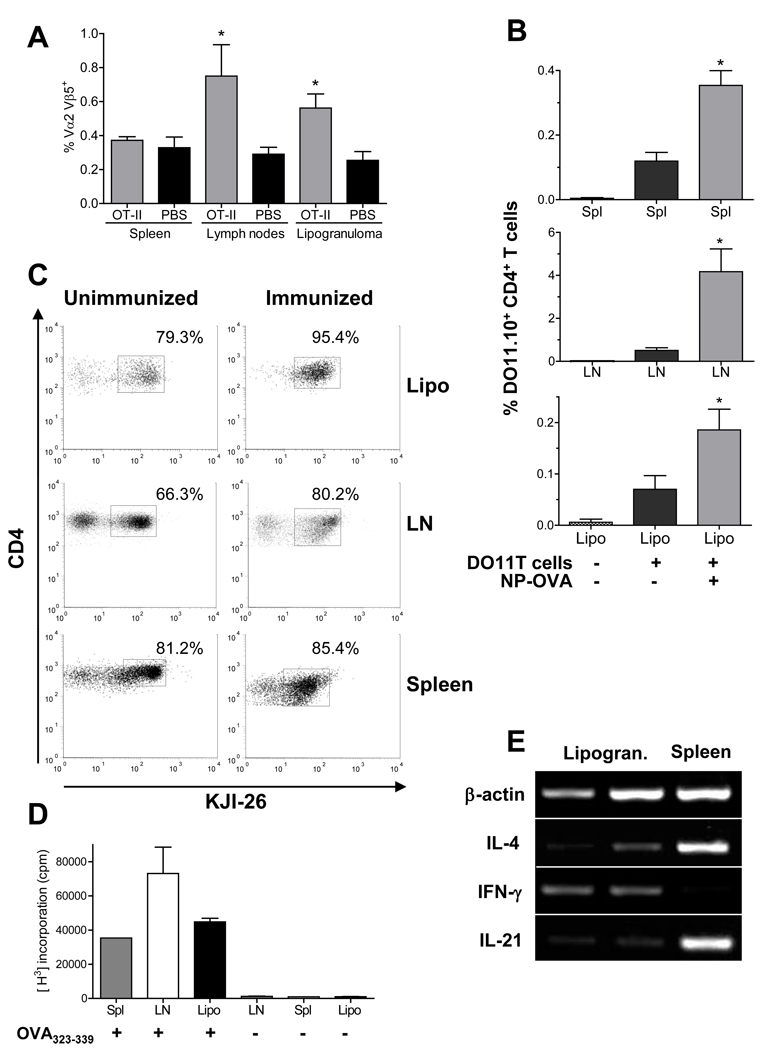
OVA-specific T cells localize and expand in ectopic lymphoid tissue
To further address whether ectopic tissue facilitates the development of de novo immune responses, we analyzed antigen-specific CD4+ T cell responses within the lipogranulomas following immunization. CD4+ ovalbumin (OVA) 323–339 peptide-specific T cells were transferred from either OT-II or DO11.10 mice into TMPD treated recipients. Either PBS or CD4+ OVA-specific T cells from OT-II mice were injected into TMPD-treated B6 mice, followed by immunization with NP-OVA 3 days later. Seven days after immunization, we identified the OVA transgenic T cells (Vα2+Vβ5+CD4+ cells) (16) in various lymphoid tissues using flow cytometry. There was a significant increase of Vα2+Vβ5+CD4+ T cells in the draining lymph nodes and lipogranulomas from mice injected with OT-II T cells compared to the control PBS injection (Fig. 2A). As expected, the DO11.10 antigen-specific T cells also were present at increased frequency in the lipogranulomas of BALB/c mice after immunization (Fig. 2B). Transfer of DO11.10 T cells to non-immunized mice verified that the increased numbers of antigen-specific T cells in the lipogranulomas were not merely related to the transfer of CD4+ T cells (Fig. 2B). We also treated DO11.10 mice with TMPD and 3 months later immunized with OVA323–339. Seven days after immunization, the lipogranulomas contained almost exclusively antigen-specific KJI-26+ CD4+ T cells, whereas both spleen and draining lymph nodes contained a population of KJI-26− CD4+ T cells (Fig. 2C). In contrast, lipogranulomas from non-immunized mice contained a population of non-transgenic (KJI-26−) T cells. We further assessed the presence of OVA-specific T cells in the lipogranulomas by isolating CD4+ T cells from TMPD treated DO11.0 mice and stimulating in vitro with OVA323–339. Antigen-specific CD4+ T cells from lipogranulomas, spleen, and draining lymph nodes all proliferated similarly (Fig. 2D). The expansion of antigen-specific CD4+ T cells in the lipogranulomas upon immunization coupled with their ability to proliferate when stimulated in vitro provides evidence that ectopic lymphoid tissue is a site of antigen-specific T cell activation and proliferation.
Figure 2. OVA-specific T cells in lipogranulomas.
A, Single cell suspensions from spleen, lymph nodes, or lipogranulomas of mice injected i.v. with 5 × 106 OT-II T cells (n = 6) or PBS (n = 5) were analyzed by flow cytometry 7 days after immunization with NP-OVA. The Vα2+Vβ5+ values represent the total percentage of CD3+CD4+ T cells. (* Lymph nodes, P = 0.009; Lipogranuloma, P = 0.02, Mann Whitney test) B, Mice were injected i.v. with DO11.10 T cells (n = 12) or PBS (n = 5) were analyzed by flow cytometry. Some mice that received DO11.10 T cells were immunized with NP-OVA (n = 7) and others were not (n = 5). (* Spleen, P = 0.0006; Lymph node, P = 0.01, Lipogranuloma, P = 0.02, Mann Whitney test) C, Single cell suspensions of lipogranulomas, spleen and draining lymph nodes from immunized or non-immunized mice were gated on live CD3+CD4+ cells (cytox blue) and then the % of KJI-26+ (DO11.10 T cells) was determined from CD3+ CD4+ cells (representative of three independent experiments). D, Isolated CD4+ T cells were cultured with or without OVA323–339 for 72 h. T cell proliferation was measured by [3H] incorporation (representative of three independent experiments). E, cDNA was synthesized from two lipogranulomas (Lipo) or spleen from a TMPD treated mice immunized with NP-KLH. IL-4, IFNγ, and IL-21 expression was determined by RT-PCR and normalized to β-actin expression (representative of four different mice).
To further demonstrate that the T cells in the lipogranuloma are active participants in an antigen-specific immune response, we looked at the production of T cell inflammatory cytokines in lipogranulomas and spleen after immunization with the test antigen NP-KLH. Compared to the spleen, lipogranulomas from the same mouse expressed higher levels of IFNγ mRNA but lower levels of IL-4 and IL-21 (Fig. 2E). Thus, following immunization, T cells in the lipogranulomas were proliferating (Fig. 1E), there was an increased number of antigen-specific CD4+ cells (Fig. 2B–C), and there was production of T cell cytokines, particularly IFNγ (Fig. 2E), consistent with the presence of effector T cells.
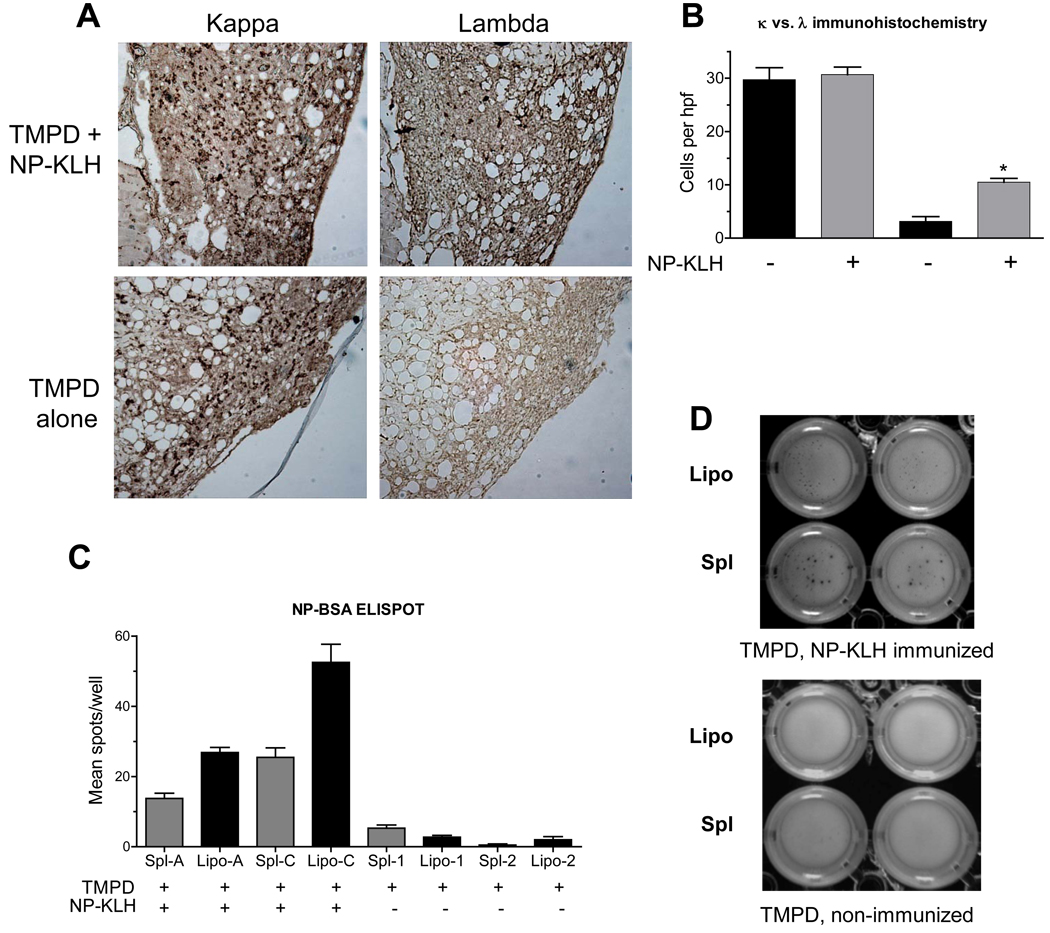
NP-specific B cells and anti-NP antibody production in ectopic lymphoid tissue
In view of the preferential pairing of λ1 L-chains with V186.2 H chains, paraffin-embedded tissue was stained for κ and λ light chains (Fig. 3A). Lipogranulomas from both immunized and nonimmunized mice contained large numbers κ light chain bearing B cells, showing that immunization does not substantially affect the total number of B cells in the lipogranulomas. The lipogranulomas from mice immunized with NP-KLH had discrete areas of strong λ light chain staining, whereas non-immunized mice had very weak λ staining (Fig. 3A). Immunized mice had significantly more λ light chain bearing cells in the lipogranulomas than controls, an expected response to NP-KLH (Fig. 3B). To determine whether these B cells secreted anti-NP antibodies, ELISPOT assays were performed using NP-BSA as antigen. The lipogranuloma cells from NP-KLH immunized, TMPD-treated mice displayed more spots than those from TMPD-treated, non-immunized mice (Fig. 3C). Interestingly the spots from the spleen, although less numerous than those from lipogranulomas, were larger (Fig. 3D), suggesting that the antigen-specific cells in the spleen secreted more immunoglobulin per cell than those from lipogranulomas and/or that the antibody affinity was lower in the lipogranulomas, as suggested earlier (Fig. 1D).
Figure 3. Anti-NP B cells in ectopic lymphoid tissue.
A, Light chain staining of B cells in lipogranulomas from B6 mice treated with TMPD alone or treated with TMPD and then immunized with NP-KLH. Paraformaldehyde-fixed tissue was analyzed 12 days after NP-KLH immunization. Paraffin sections were stained with anti-κ and anti-λ light chain antibodies. B, Number of κ and λ positive cells per high power field in mice treated with TMPD + NP-KLH immunization or with TMPD alone (* P = 0.02, Mann Whitney test; representative of five independent experiments). C, ELISPOT assay for anti-NP B cells. MultiScreen HTS IP plates containing a 0.45 µm Immobilon-P membrane were coated with 1 µg/mL NP-BSA. Lipogranuloma and spleen cells from TMPD treated mice (n = 2) or TMPD-treated and NP-KLH immunized mice (n = 2) were added to triplicate wells for 24 h before adding biotinylated goat anti-mouse IgM antibodies, streptavidin-peroxidase, and BCIP-NBT substrate. Number of spots per well was determined (* P = 0.03 for both mouse A and mouse B, Mann Whitney test; representative of three independent experiments). D, Relative sizes of individual spots in ELISPOT assays using lipogranuloma (Lipo) or spleen (Spl) cells from TMPD treated mice either with or without NP-KLH immunization.
H-chain sequences from spleen and ectopic lymphoid tissue of NP-KLH immunized mice
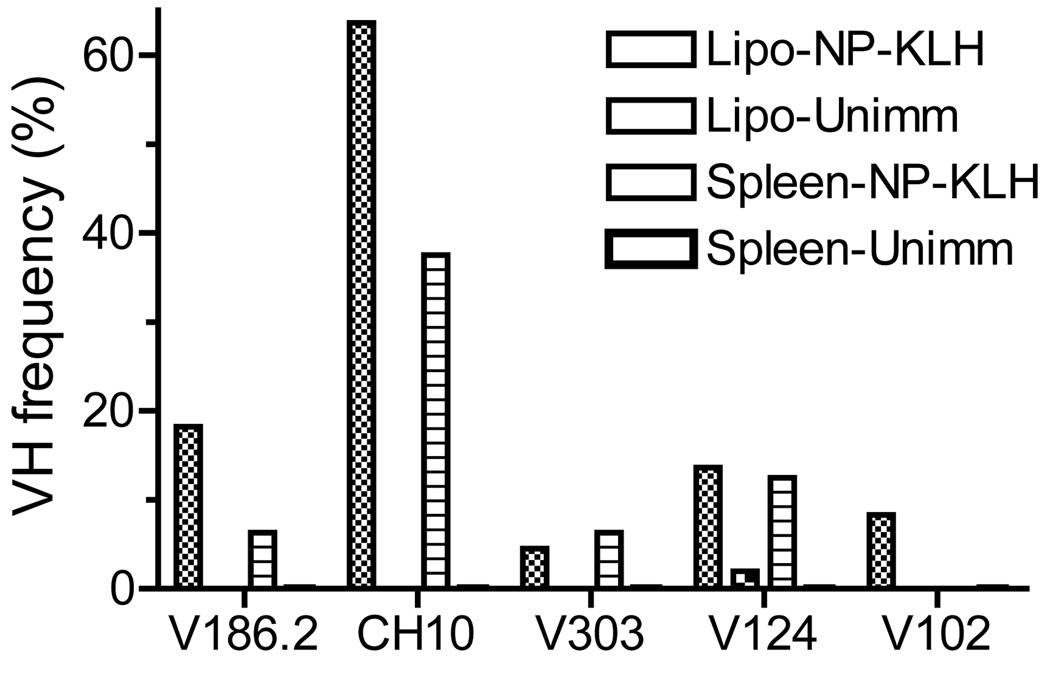
To further verify that the lipogranulomas contained NP-specific B cells, we amplified and sequenced VH genes from lipogranulomas and spleens. A preponderance of V186.2 and other VH genes (CH10, V303, V102) implicated in the formation of anti-NP antibodies (15) was found in the lipogranulomas and spleens of immunized mice (Fig. 4). B cells from the lipogranulomas expressed almost exclusively V186.2 or other VH genes implicated in the anti-NP response, representing >90% of the total sequences (including 20% V186.2). In comparison, 60% of VH sequences from the spleen were V186.2, CH10, V303, or V102 (7% V186.2). V186.2 and other genes encoding presumptive NP-specific antibodies were infrequent in the lipogranulomas from un-immunized mice. The higher percentage of likely NP-specific VH genes in lipogranulomas vs. spleen from immunized mice is consistent with the possibility that T cell-dependent clonal expansion of antigen-specific B cells may be ongoing within the lipogranulomas.
Figure 4. VH segment usage in lipogranulomas and spleen.
Lipogranulomas from a TMPD-treated mouse immunized with NP-KLH were pooled at day 12 and RNA was isolated. Immunoglobulin VH sequences were determined from lipogranulomas (n = 50) and spleen (n = 50) from the same mouse. All of the sequences recovered from lipogranulomas bore V186.2, CH10, V303, V102, or V124 vs. 62.6% of the sequences recovered from the spleen (representative of three independent experiments).
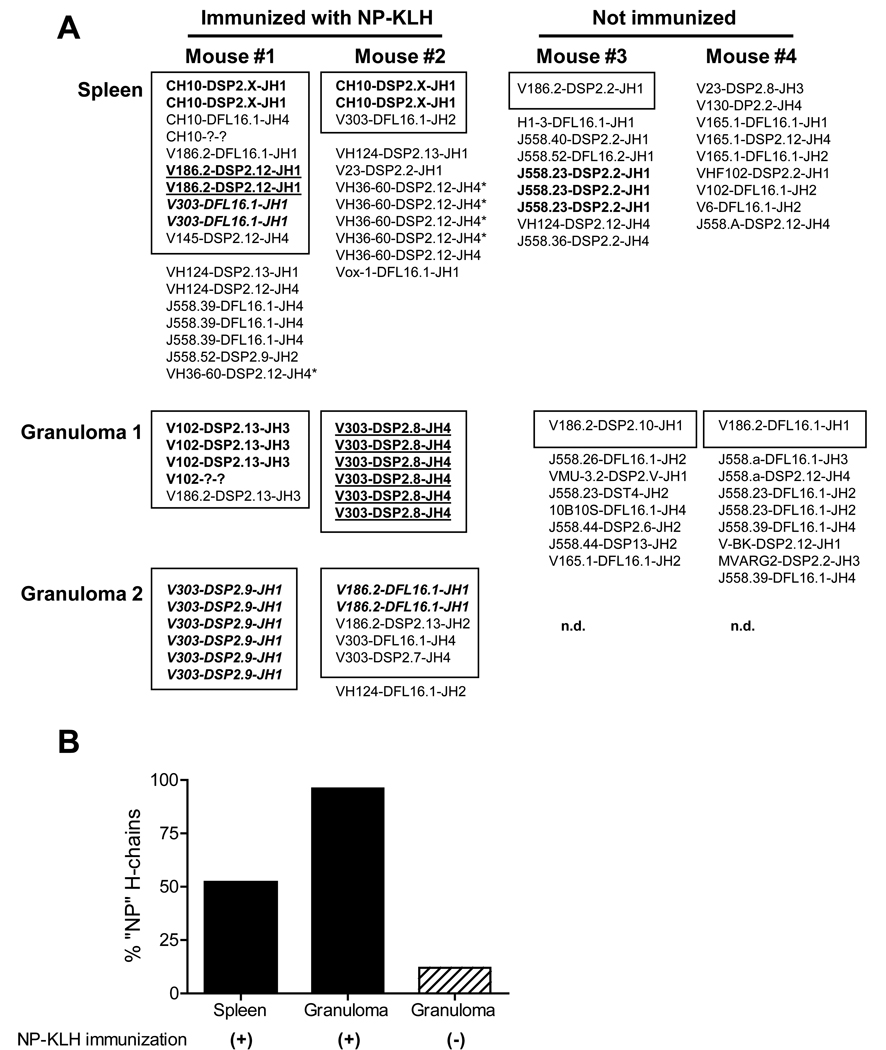
To assess the clonality of B cells in ectopic lymphoid tissue induced by TMPD following immunization with the test antigen, we sequenced the VH genes expressed by B cells in individual lipogranulomas and spleen. As shown in Fig. 5A, diverse VH sequences were recovered from the spleens of TMPD-treated mice immunized with NP-KLH. In some instances the same VH-D-JH combination was obtained from two individual clones from the same spleen. Interestingly, the combination CH10-DSP2.X-JH1 (CH10 is implicated in the formation of anti-NP antibodies) was recovered in duplicate clones from two different mice (Fig. 5A). In striking contrast to spleen, individual lipogranulomas from TMPD-treated mice, immunized 12 days earlier with NP-KLH, contained highly oligoclonal populations of B cells expressing VH sequences associated with anti-NP reactivity (V186.2 and related sequences) (Fig. 5A, Mouse #1 and #2, lipogranulomas 1 and 2). The lipogranulomas from immunized mice displayed almost exclusively (95%) V186.2 and analogous VH sequences vs. 50% of the VH genes recovered from the spleens of NP-KLH immunized mice (Fig. 5B). Lipogranulomas from TMPD-treated mice that were not immunized with NP-KLH contained more diverse populations of B cells with few V186.2 and related sequences (Fig. 5A–B). The “anti-NP” sequences found in individual lipogranulomas were distinct from those isolated in spleen and other lipogranulomas from the same mouse.
Figure 5. Oligoclonal VH sequences from lipogranulomas of immunized mice.
Heavy chain sequences are shown from spleen and lipogranulomas of two mice immunized with NP-KLH (day 12) and two pre-immune (not immunized) mice. V-D-J sequences were amplified from cDNA by PCR and sequenced to determine VH, D, and JH usage. Boxed sequences utilize VH sequences associated with anti-NP reactivity. Related sequences are shown in the same format.
Analysis of somatic mutation frequencies in V186.2 sequences revealed that 8/9 (88%) of the lipogranuloma V186.2 sequences were somatically mutated (Table I). Lipogranuloma and spleen sequences both had R/S ratios > 5.7, suggestive of the occurrence of somatic hypermutation during a primary response to NP (17). None of the somatically mutated sequences contained the prototypical 33 (W → L) mutation characteristic of affinity maturation (18), consistent with an early NP-specific response in the lipogranulomas from these mice that had received only a primary immunization.
Table I.
V186.2 sequences from mice undergoing primary NP-KLH immunization
| TMPD/NP- KLH treated B6 mice (day 12) |
Total # of sequences |
# of V186.2 |
# of mutated V186.2 |
# of 33 (W → L) |
FR S mutations (V186.2) |
FR R mutations (V186.2) |
CDR S mutations (V186.2) |
CDR R mutations (V186.2) |
R/S FR* |
R/S CDR* |
|---|---|---|---|---|---|---|---|---|---|---|
| Lipogranuloma | 31 | 9 | 8 | 0 | 11 | 8 | 1 | 6 | 0.72 | 6 |
| Spleen | 31 | 7 | 5 | 0 | 13 | 17 | 2 | 15 | 1.3 | 7.5 |
ratio of replacement (R) to silent (S) mutations in V186.2 sequences
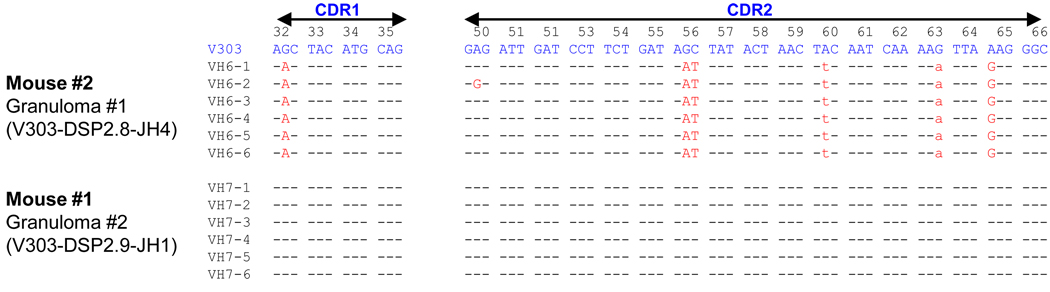
To help address the issue of whether the lipogranuloma B cells were undergoing a primary anti-NP response locally as opposed to migrating there from other sites, such as secondary lymphoid tissue, the CDRs of related sequences isolated from lipogranulomas were compared (Fig. 6). Sequences obtained from individual lipogranulomas displayed identical or closely related somatic mutations, as might be expected if they underwent expansion locally (e.g. Mouse #2, lipogranuloma 1, V303-DSP2.8-JH4 sequences) (Fig. 6, top). However, although occasional new somatic mutations were found (e.g. sequence VH6-2) extensive “clonal trees” were not seen. In other cases (e.g. Mouse #1, lipogranuloma 2, V303-DSP2.9-JH1) collections of identical germline sequences were found (Fig. 6, bottom). Individual somatic mutations seen in the CDR sequences recovered from lipogranulomas were not found in the spleen of the same mouse, providing further evidence that the splenic and lipogranuloma anti-NP responses developed independently of each other.
Figure 6. CDR1 and CDR2 sequences from H-chains isolated from lipogranulomas.
Sequences of H-chains from Mouse #1, lipogranuloma 2 (V303-DSP2.9-JH1) and Mouse #2, Lipogranuloma 1 (V303-DSP2.8-JH4) are aligned. Somatic mutations are indicated. Replacement mutations capitalized, silent mutations lower case.
DISCUSSION
There are many examples of structures resembling secondary lymphoid tissue arising at sites of chronic inflammation (2), but it remains unclear whether this ectopic lymphoid tissue is a site of cognate T-B interactions. Specifically, although autoantigen-specific B cells have been reported in ectopic lymphoid tissue (19,20), it is not known whether immune responses actually develop there or whether antigen-specific B cells arising in secondary lymphoid tissues subsequently colonize the ectopic lymphoid tissue. Here, we addressed this question by active subcutaneous immunization coupled with tracking of antigen-specific B and T lymphocytes to peritoneal ectopic lymphoid tissue induced by TMPD. To our knowledge, this is the first study to show that both hapten-specific B cells and proliferating, carrier-specific, T effector cells are present within ectopic lymphoid tissue.
Our data strongly suggest that cognate antigen-specific T-B interactions occur in ectopic lymphoid tissue. Following subcutaneous immunization with antigens such as NP-OVA, T and B-cell proliferation was seen in the TMPD-induced ectopic lymphoid tissue (Fig. 1E) and both OVA-specific T cells and NP hapten-specific B cells accumulated there (Fig. 2–Fig. 3). T cells in the ectopic lymphoid tissue also produced IFNγ and other cytokines (Fig. 2E). Heavy chain sequences isolated from ectopic lymphoid tissue were highly enriched for V186.2 and other H-chains known to generate NP-specific antibodies (Fig. 4–Fig. 5) and the proportion of such sequences was higher than in the spleen. Lipogranulomas also exhibited strong λ light chain staining consistent with an anti-NP response (Fig. 3). Moreover, anti-NP antibody secreting cells were enriched in ectopic lymphoid tissue in comparison with the spleen. Taken together, these data suggest that antigen-specific B cell and T cell responses may preferentially develop within the ectopic lymphoid tissue.
Individual lipogranulomas from pre-immune mice contain relatively diverse populations of B cells (Fig. 5; D Nacionales, et al. submitted). In contrast, following subcutaneous immunization, the B cells present in individual lipogranulomas were highly oligoclonal or even monoclonal (Fig. 5–6) and preferentially utilized H-chains previously reported in anti-NP responses. Oligoclonal B cell expansions also are seen in individual germinal centers microdissected form secondary lymphoid tissues (11), suggesting that individual lipogranulomas from TMPD-treated mice are in some respects analogous to single germinal centers. The oligoclonal B cell proliferation apparent in TMPD-induced ectopic lymphoid tissue is consistent with previous observations in ectopic lymphoid tissues from rheumatoid arthritis, Sjogren’s syndrome, and myasthenia gravis patients (19,21,22).
A key question is whether the B cells in ectopic lymphoid tissue develop in situ or migrate into the ectopic lymphoid tissue from secondary lymphoid organs, such as the lymph nodes or spleen. In view of the timing of immunization and the VH sequences obtained, it is unlikely that the B cells found in lipogranulomas 10–12 days after immunization originated from the germinal centers of secondary lymphoid tissues followed by migration to the tertiary lymphoid tissues. The fact that individual lipogranulomas contained non-overlapping and unrelated sets of clonal B cells also suggests they were not “seeded” with the products of previous germinal center reactions in the lymph nodes or spleen. Moreover, the sequences recovered from spleen did not overlap with the lipogranuloma sequences.
We did not find the extensive clonal trees reported previously from spleen of MRL mice (23). However, extensive clonal trees were not seen in individual germinal centers, either (11) and the sequences illustrated in Fig. 6 (Mouse #2, Granuloma #1) are not that dissimilar from those reported previously from individual germinal centers. The lack of clonal trees could reflect the relatively small number of sequences analyzed per lipogranuloma or a lower rate of somatic hypermutation in ectopic lymphoid tissue vs. secondary lymphoid tissues.
There are other important differences between the ectopic lymphoid tissue induced by TMPD and authentic germinal center reactions, notably the absence of well-developed FDC networks and PNA+ B cells (7). ELISPOTs were larger using spleen cells vs. cells from the ectopic lymphoid tissue, suggesting that the splenic anti-NP B cells secrete more antibody than those from ectopic lymphoid tissue or that affinity maturation of B cells in the lipogranuloma is less efficient than in the spleen, an interpretation consistent with the lower affinity of anti-NP antibodies in the sera of TMPD-treated mice vs. controls (Fig. 1D) and the paucity of clonal trees. Together, these data suggest that 1) a significant portion of the low affinity serum anti-NP response in TMPD-treated mice may derive from the ectopic lymphoid tissue and 2) affinity maturation may be defective in the ectopic lymphoid tissue- i.e. high affinity B cells may enjoy less of a competitive advantage over lower affinity cells in ectopic lymphoid tissue than in authentic secondary lymphoid tissues. We speculate that reduced affinity maturation in the lipogranulomas might reflect an absence of follicular dendritic cells in view of the lack of FDC-M1+ staining (7). Further studies will be necessary, however, to determine whether the low affinity of serum anti-NP antibodies in TMPD-treated mice is due to their production in ectopic lymphoid tissue or is a systemic effect of TMPD treatment.
The presence of oligoclonal B cell populations in individual lipogranulomas, lack of shared H-chain sequences between lipogranulomas and spleen and between individual lipogranulomas from the same mouse, expression of AID and the presence of circular DNA intermediates generated during active class switch recombination (D Nacionales, et al., Submitted), as well as the presence of proliferating B and T cells in these structures lead us to conclude that TMPD-induced ectopic lymphoid tissue is a site of germinal center-like cognate T-B interactions. However, the lipogranulomas may not be true germinal centers and instead could be more analogous to the previously reported extrafollicular sites of antigen-driven somatic hypermutation of rheumatoid factor B cells (24). Recently, ectopic lymphoid tissue also was found to express AID in the salivary glands of patients with Sjogren’s syndrome (25), supporting the idea that this may be a general feature of ectopic lymphoid tissue in a variety of locations.
The formation of ectopic lymphoid tissue is strongly associated with autoimmunity and autoantibody production in a variety of disorders (2), including the rheumatoid synovium (21,26), salivary glands in Sjogren’s syndrome (27), thymus in myasthenia gravis (19), and the thyroid in Hashimoto’s thyroiditis (28). In several examples of organ-specific autoimmune disease, autoreactive B cells have been found within ectopic lymphoid tissue in the target tissues. We have shown recently that anti-RNP autoantibody-producing B cells are enriched in ectopic lymphoid tissue of TMPD-treated mice, strongly suggesting that this may be a site where autoreactivity may develop preferentially (D Nacionales, et al., Submitted). It will be of interest to determine where the antigen presenting cells responsible for activating autoreactive T cells acquire self-antigens, as the present data indicate that APCs from remote (e.g. subcutaneous) locations are capable of homing to ectopic lymphoid tissue located within the peritoneum. The role of chemokines, such as CXCL19, CXCL21, and CXCL13, expressed at high levels in the ectopic lymphoid tissue (7) in establishing autoantibody production in ectopic sites remains to be determined. Finally, it will be of interest to see if therapy aimed at disrupting the formation of ectopic lymphoid tissue will prevent the development of lupus in TMPD-treated mice.
ACKNOWLEDGMENTS
This work was supported with resources and the use of facilities at the Malcolm Randall VA Medical Center, Gainesville, FL. We thank Dr. Laurence Morel for providing OT-II mice and the University of Florida DNA Sequencing Core and Flow Cytometry Core Lab for their assistance.
This work was supported by research grants R01-AR44731 and T32-AR007603 from the US Public Health Service and by generous gifts from Lupus Link, Inc. (Daytona Beach, FL) and Mr. Lewis M. Schott to the UF Center for Autoimmune Disease. Dr. Nacionales is the recipient of an Arthritis Foundation Postdoctoral Fellowship.
REFERENCES
- 1.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J.Exp.Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat.Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 3.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J.Leukoc.Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- 4.Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur.J.Immunol. 2001;31:2726–2732. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J.Exp.Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoh M, Kumar A, Kanwar YS, Reeves WH. Antinuclear antibody production and immune complex glomerulonephritis in BALB/c mice treated with pristane. Proc.Natl.Acad.Sci.USA. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nacionales DC, Kelly KM, Lee PY, Zhuang H, Weinstein JS, Sobel E, Kuroda Y, Akaogi J, Satoh M, Reeves WH. Type I interferon production by tertiary lymphoid tissue developing in response to 2, 6, 10, 14 tetramethylpentadecane (pristane) Am.J.Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhary VR, Grande JP, Luthra HS, David CS. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology.(Oxford) 2007 doi: 10.1093/rheumatology/kem117. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J.Exp.Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu.Rev.Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 11.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J.Exp.Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J.Exp.Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature (Lond.) 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 14.White HN. Restriction-PCR fingerprinting of the immunoglobulin VH repertoire: direct detection of an immune response and global analysis of B cell clonality. Eur.J.Immunol. 1998;28:3268–3279. doi: 10.1002/(SICI)1521-4141(199810)28:10<3268::AID-IMMU3268>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J.Exp.Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol.Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss U, Zoebelein R, Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur.J.Immunol. 1992;22:511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- 18.Allen D, Simon T, Sablitzky F, Rajewsky K, Cumano A. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J. 1988;7:1995–2001. doi: 10.1002/j.1460-2075.1988.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sims GP, Shiono H, Willcox N, Stott DI. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J.Immunol. 2001;167:1935–1944. doi: 10.4049/jimmunol.167.4.1935. [DOI] [PubMed] [Google Scholar]
- 20.Gause A, Gundlach K, Zdichavsky M, Jacobs G, Koch B, Hopf T, Pfreundschuh M. The B lymphocyte in rheumatoid arthritis: analysis of rearranged V kappa genes from B cells infiltrating the synovial membrane. Eur.J.Immunol. 1995;25:2775–2782. doi: 10.1002/eji.1830251010. [DOI] [PubMed] [Google Scholar]
- 21.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc.Natl.Acad.Sci.U.S.A. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. J.Clin.Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc.Natl.Acad.Sci.USA. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur.J.Immunol. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bombardieri M, Barone F, Humby F, Kelly S, McGurk M, Morgan P, Challacombe S, de Vita S, Valesini G, Spencer J, Pitzalis C. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren's syndrome. J.Immunol. 2007;179:4929–4938. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 26.Natvig JB, Randen I, Thompson K, Forre O, Mageed RA, Jefferis R, Carson DA, Tighe H, Pascual V, Victor KD. Probing of the rheumatoid factor (RF) V gene repertoire in rheumatoid arthritis (RA) by hybridoma clones. Clin.Exp.Rheumatol. 1990;8 Suppl 5:75–80. [PubMed] [Google Scholar]
- 27.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J.Clin.Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armengol MP, Juan M, Lucas-Martin A, Fernandez-Figueras MT, Jaraquemada D, Gallart T, Pujol-Borrell R. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokinecontaining active intrathyroidal germinal centers. Am.J.Pathol. 2001;159:861–873. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]