Abstract
Plasmodium vivax, a protozoan malaria parasite of humans, represents a major public health concern in the Republic of Korea (= South Korea). However, little is known about the genetic properties and population structures of the P. vivax isolates circulating in South Korea. This article reviews known polymorphic genetic markers in South Korean isolates of P. vivax and briefly summarizes the current issues surrounding the gene and population structures of this parasite. The critical genetic characteristics of major antigens of the parasite, such as circumsporozoite protein (CSP), merozoite surface protein 1 (MSP-1) and MSP-3, Duffy binding protein (DBP), apical membrane antigen 1 (AMA-1), and GAM-1, are also discussed.
Keywords: Plasmodium vivax, antigenic protein, genetic polymorphism, South Korean strain
INTRODUCTION
Four different human malarial parasites are widely disseminated around the world: Plasmodium vivax, P. falciparum, P. ovale, and P. malariae [1]. While P. falciparum is the most deadly of these species, all are capable of causing human infections. In the mid-1950s, the World Health Organization (WHO) began a program to eradicate and halt the global spread of malaria [2]. After its inception, the total number of malaria cases reported in the temperate endemic regions of Europe, Asia, and the Americas decreased [3]. However, infection by P. vivax, which is the most prevalent type of human malaria infection, temporarily decreased and has been increasing lately to 70-80 million cases per year [3,4]. This is of concern, because it indicates that the threat of P. vivax present to the human population has continued to increase despite preventive efforts. In the late 1970s, it appeared that the vivax malaria threat would be successfully eradicated from South Korea, but in 1993 the parasite reemerged near the demilitarized zone between South and North Korea [5,6]. Since then, the number of malaria cases has increased exponentially in endemic regions of South Korea, and currently 1,000-2,000 cases are reported per year, presenting a problem to public medical care in South Korea [7].
A prerequisite to the design of effective vaccines for malaria is analysis of the polymorphisms contained in the genome of P. vivax. Of particular interest are those unique antigens that may be under selection by the host immune system. Subsequent studies on microsatellites, single nucleotide polymorphisms (SNPs) or repetitive regions may provide the basis for the strategic development of mono- or polyvalent vaccines, as well as aid in answering the fundamental molecular and epidemiological questions surrounding P. vivax. The ability to distinguish the subtle interplay of genetic transmission among different populations of malaria is necessary to fully understand the local and global epidemiology of a parasitic species. In this article, we will review the characteristics of the known polymorphisms in the genes of antigenic proteins from the South Korean isolates of P. vivax, and discuss the current issues surrounding the gene and population structures of this parasite.
POLYMORPHIC MOLECULAR MARKERS IN SOUTH KOREAN P. VIVAX
The high levels of antigenic polymorphism found in most P. vivax parasites indicate the development of sophisticated evasion of the human immune system [8]. Detailed knowledge of this genetic variation and comparisons of inter- and intra-speciesspecific levels of gene variation will both resolve epidemiological questions about parasite origin and provide tools with which to design an effective vaccine. Polymorphic repetitive sequences, particularly microsatellites and SNPs, have rarely been exploited as vaccine candidate for P.vivax infection. The determination of complete sequences and positional similarity mining with other interspecies sequences will accelerate the search for useful genetic markers [9]. In particular, a genome-wide next-generation sequencing technique and a gene-specific microarray system are needed [10,11].
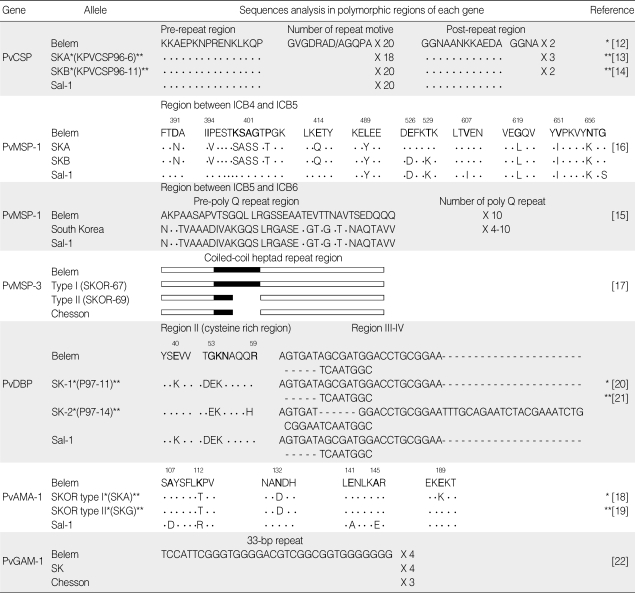
Elucidation of the genetic diversity of Korean isolates will enhance knowledge of the biology and epidemiology of P. vivax. To date, the following antigenic markers have been tested and reported for Korean P. vivax (Table 1): circumsporozoite protein (CSP), merozoite surface protein 1 (MSP-1) and MSP-3, Duffy binding protein (DBP), apical membrane antigen 1 (AMA-1), and GAM-1 [12-22].
Table 1.
Genetic characteristics of alleles found in South Korean isolates
The letters indicate the polymorphic variation sites. The dots and dashes represent identical residues and deletions, respectively.
CSP
The CSP is abundantly expressed on the surface of the sporozoite and is one of the candidate antigens for malaria vaccine. The gene encoding P. vivax CSP (PvCSP) was first cloned and sequenced in 1985 [23]. PvCSP comprises 3 distinct domains; a central repetitive (CR) domain with a variable number of tandem repeats, and 2 highly conserved non-repetitive terminals. The CR domain varies in length and in sequence content among the strains of P. vivax and belongs to 1 of 2 genotypes of nonapeptide repeat units (GDRAD/AGPQA or ANGAGNQPG), named respectively VK210 or VK247 [23,24]. Investigation of genetic differences (i.e., polymorphisms) in the repeat region of CSP provides useful information on the geographical distribution of malaria isolates in endemic regions. CSP is also a prime candidate antigen for vaccines being developed for malaria, and an anti-CSP vaccine has successfully completed Phase III clinical trials [25].
South Korean P. vivax isolates contain unique PvCSP polymorphisms in the CR domain, and in the pre- and post-repeat regions [12-14]. Two predominant allelic variants with minor variations in nucleotide sequences in the VK210 genotype have been identified in all South Korean isolates; the nonapeptide sequences are repeated 18 times in South Korean type A (SKA) (or KPVCSP96-6) and 20 times in South Korean type B (SKB) (or KPVCSP96-11) [12-14]. No cases of VK247 or of the mixed type have been found in South Korean P. vivax [12]. Similarly, the VK210 type variant is predominant in Azerbaijan strains, as in the South Korean isolates [26]. The allelic frequency of VK210 in Thailand was found to be 77%, whereas 62% of Colombian isolates were VK247 [27,28]. We therefore postulate that this specific type predominance phenomenon could be attributed to an adaptive response to selection pressure on a particular genotype [29] or to a preferential production of sporozoites carrying a more efficient gene for certain mosquito species [30]. In addition, the effects of geographic origin or regional temporal fluctuations of genotypes could influence type predominance [29].
PvCSP SKA (or KPVCSP96-6) and SKB (or KPVCSP96-11) alleles from current South Korean isolates are similar to those from Chinese (CH-5) and North Korean isolates, respectively [12-14]. CH-5 and North Korean isolates are classified as the East Asian group and the apparent genetic similarities are explained as due to regional proximity [12]. Therefore, the data strongly suggest that the reemerged P. vivax in South Korea originated from East Asia. All isolates carry the same pre-repeat sequences [14] and harbor a 36-bp post-repeat insert (GGNAANKKAEDA) [12-14]. Both of these characteristics were previously observed in P. vivax isolates from North Korea and China (CH2-7) [31,32]. In the post-repeat region, the only difference between 2 Korean genotypes is the presence of 3 additional tandem copies of a 4 amino-acid sequences (GGNA) found in the SKA (or KPVCSP96-6) allele and 2 copies in the SKB (or KPVCSP96-11) allele.
MSP-1
Merozoite surface proteins, including MSP-1, MSP-2, and MSP-3, play an important role during the invasion phase of the erythrocyte's life cycle. These proteins have previously been studied as potential targets for vaccine development [25,33]. The P. vivax MSP-1 (PvMSP-1) is expressed as a 200 kDa protein on the merozoite surface, and has been cloned and sequenced [34]. The primary structure of PvMSP-1 was originally characterized from the Belem and Salvador-1 (Sal-1) isolates, and consists of conserved, semi-conserved, and polymorphic regions [34,35]. Sequence comparisons indicate that 10 regions are relatively conserved among the species of Plasmodium [34]. These interspecies conserved blocks (ICBs) show an overall sequence similarity of 48% in pair-wise alignments. This highly polymorphic nature may indicate an additional use for MSP-1 as a potential alternative molecular epidemiologic marker for genotyping P. vivax [36]. In malaria parasites isolated from Brazil [37], Sri Lanka [38], Colombia [39], Papua New Guinea [40], and Thailand [41], the intervening region between ICB5 and ICB6 demonstrates a dimorphic nature. Relatively frequent inter-allelic recombination types have also been observed, indicating the possibility of a new third type of PvMSP-1 in addition to types 1 (Belem) and 2 (Sal-1) [42].
Alignment of 31 district alleles, including 2 South Korean allelic types, showed that PvMSP-1 has the mosaic pattern, consisting of the 7 inter-allele conserved blocks flanked by 6 variable blocks [43]. Sequence comparison and mapping of MSP-1 polymorphic regions between ICB5 and ICB6 obtained from 25 South Korean isolates showed very similar gene structures, which would belong to inter-allelic recombination types of MSP-1 [15]. The pre-poly Q repeat region of Korean isolates are identical to that of Sal-1 strain, whereas poly Q repeat region is similar to that of Belem strain. Comparison of sequences between ICB 5 and 6 with those of other regional isolates indicated that the gene structures of Korean isolates are similar to those of Thai isolates (TD424 and TD525) [15].
Investigation of genetic variability in the region between ICB4 and ICB5 of South Korean isolates using sequence analysis and a type-specific PCR showed the co-existence of 2 different genotypes, SKA and SKB [16]. Non-synonymous nucleotide point mutations were identified at 13 sites, and synonymous nucleotide substitutions were found at 16 sites. However, 12 missense mutations for SKA and 14 missense mutations for SKB of ICB4-5 were identified in comparison with the Belem strain (1081-2025 sites). Eleven point mutations appeared at the same sites in both SKA and SKB, whereas 2 missense mutations at the residues 526 (Glu→Asp) and 529 (Thr→Lys) were found only in SKB. A type-specific PCR study showed that, of the 100 samples collected in 1999, 52 vivax malaria patients had SKA, 28 had SKB, and 20 had both SKA and SKB [16]. When compared with genotypes of isolates from other global regions, the SKA and SKB of PvMSP-1 ICB4-5 were closely related to Solomon Island (Solo-83) and Philippine (Ph-49, Ph-52-2 and Ph-79) isolates [16]. However, the 2 alleles of South Korean isolates were clearly distinguishable from previously reported genotypes [16]. The low genetic diversity of Korean isolates may be due to the low transmission intensity in domestic areas with limited seasonal prevalence of malaria or to suboptimal conditions for the transmission of the mosquito [44]. In other global endemic areas, commonly known PvMSP-1 diversities, such as ICB2-ICB4 and ICB8-ICB10, have been indexed [45,46]. However, information on these regions has not been studied for PvMSP-1 from South Korean isolates.
MSP-3
The P. vivax MSP-3 (PvMSP-3) is a 150 kDa protein comprised of 3 characteristic domains; an alanine-rich central domain having a series of heptad repeats that are predicted to form a coiled-coil tertiary peptide structure, and a highly conserved C- and N-terminal region [47]. The PvMSP-3 gene family consists of 3 intraspecies genes, PvMSP-3α, PvMSP-3β, and PvMSP-3γ, and it exhibits a high degree of genetic diversity [48]. PvMSP-3 is a useful polymorphic marker in endemic areas [49]. The antigenic nature of PvMSP-3 made it a candidate for a potential malaria vaccine [25,50].
The genetic characteristics of PvMSP-3 from South Korean isolates were determined in 2004 [17]. The genetic properties of PvMSP-3 from the Korean isolates can be divided into 2 distinct allelic types: types I (SKOR-67) and II (SKOR-69). Type I is similar to that of the Belem strain, except for the degree of amino acid sequence variation. However, type II is more similar to the Chesson strain, with differences in amino acid sequences at positions 558-563 and 605-612. Both type I and type II isolates appear to differ from the North Korean strain, particularly in the 13 sequences that comprise the coiled-coil heptad repeats, although the sequence similarity between the 2 types of South Korean isolates and the North Korean strain is 87-90%. When the genotypes of PvMSP-3 in the Korean isolates were compared with those of PvMSP-1, a close correlation was found between the 2 genetic loci [17]. Type I of PvMSP-3 is associated with type A of PvMSP-1, and type II with type B. The 2 isolates also exhibit crossing combinations with type II for PvMSP-3 and type A (SKA) for PvMSP-1.
DBP
The P. vivax Duffy binding protein (PvDBP), one of the erythrocyte- binding proteins, is a 140 kDa protein that contains 2 functionally conserved cysteine-rich regions, regions II and IV [51,52]. The polymorphic nature of the antigen, particularly in region II, which contains the central binding domain necessary for the adherence to erythrocyte, is a major impediment to the successful design of a protective vaccine against vivax malaria [53]. Therefore, a precise sequence classification method that offers a high degree of accuracy will strengthen the potential use of a PvDBP malaria vaccine candidate.
Most of the predicted amino acid sequences in region II of PvDBP are quite conserved and show a high degree of sequence similarity to the Belem and Sal-1 strains [20,21]. Kho et al. [20] reported a 2.2% substitution rate in a total of 225 amino acids within region II of the Belem strain. Suh et al. also found a 3% substitution rate among the 606 amino acids of region II [21]. Phylogenetic analysis using a neighbor-joining method has also revealed that region II (amino acid 291-515) of DBP is divided into 2 distinct cluster groups [53]. The amino acid substitutions were commonly found to carry a few non-synonymous mutations in the middle of a cysteine-rich region. Although the consequences of mutations in region II are unknown, these variations are not predicted to affect the binding affinity of PvDBP to erythrocytes [54].
PvDBP regions III (or IV) from South Korean isolates can be classified into 2 alleles on the basis of the grouping of mutations in the nucleotides or the corresponding amino acids [20,21]. Tsuboi et al. classified PvDBP alleles into 3 groups according to the nucleotides inserted in region III (or IV) [55]. The first group has only a 6 bp insert, the second group has only a 30 bp insert, and the third group has both of these inserts. Sequence analysis of region III (or IV) has revealed that the SK-1 (or P97-11) allele has a tandem repeat of a 6 bp insert, which is identical to region III (or IV) of the Papua New Guinea isolates. The SK-2 (or P97-14) allele contains the same 30 bp insert and is also identical to the Sal-1 gene.
AMA-1
The P. vivax apical membrane antigen 1 (PvAMA-1) is thought to play an essential role in erythrocyte invasion since it shows a high degree of interspecies conservation [56,57]. This gene has only a few predominant haplotypes and thus limited genetic diversity has been shown in various geographic regions [52]. However, SNPs have been identified in some gene regions of PvAMA-1 [57]. Thus, identification of SNPs may provide an opportunity to use this gene as a marker for typing parasite populations [58].
Sequence analyses of the polymorphic region corresponding to nucleotides 324-735 (amino acids 108-245) of the PvAMA-1 gene have indicated on the basis of an amino acid residue substitution that 2 genotypes are present in South Korean isolates [18,19]. The Korean isolates contain non-synonymous sequence variations at 3 different positions as compared to the Belem strain. Variation at the position of amino acid residue 189 (Glu→Lys) has been found only in SKOR type I (or SKA). Comparison of 219 sequences of isolates collected from 9 countries revealed that the PvAMA-1 polymorphism in Korean isolates is similar to that in 2 Chinese isolates [18,19]. SKOR type I (or SKA) is identical to AAP, and SKOR type II (or SKG) is identical to an AAN haplotype of Chinese isolates. It was concluded that these 2 genotypes of AMA-1 are common in East Asian countries, such as China and South Korea [19]. However, due to a lack of detailed information on the molecular specificity, the overall geographical characteristics of PvAMA-1 are not yet known. To elucidate the geographical distribution of PvAMA-1, more information and genetic investigations of isolates from Southeast Asia, China, North Korea and their neighboring countries are necessary.
GAM-1
The P. vivax GAM-1 (PvGAM-1) is expressed during the gametocyte stage of the malaria life cycle. PvGAM-1 has a potential as a transmission-blocking vaccine candidate that could completely stop the development of parasites in the mosquito [59]. Although PvGAM-1 has been used as a new polymorphic marker for field studies of P. vivax population, frequent failures during gene amplification limits its suitability for this purpose [60]. PvGAM-1 displays polymorphic deletions of a repetitive 33 bp motif near the 3' end in a limited number of Sri Lankan and Indian isolates [61,62]. Sequence analysis of the polymorphic region of PvGAM-1 (Belem strain nt 3792-4029) in South Korean isolates revealed only 1 genotype containing four 33 bp motifs [22]. The predominance of a single genotype in South Korean isolates may imply that the intensity of malaria transmission in the region is very low, or it may indicate that the polymorphic region of PvGAM-1 is not a real epitope that is associated with transmission blocking [61,63]. In conclusion, PvGAM-1 is not a good polymorphic genetic marker in Korean P. vivax isolates.
POPULATION GENETICS AND TRANSMISSION INTENSITY
In endemic areas, the level of genetic diversity, or the extent of mixed type population of P. vivax, is expected to differ by the transmission efficiency, since the only chance for genetic recombination is during meiotic periods [57]. Plasmodium parasites are haploid cells throughout their life cycle, except in the sexual stage. Malaria parasites in hyperendemic zones, such as Papua New Guinea and Thailand, exhibit higher transmission rates and higher degrees of recombination in the genes encoding malaria vaccine antigens, including CSP, MSP-1, and MSP-3α [27,40,64]. In P. vivax malaria, the estimated proportion of genetically mixed type infections in Papua New Guinea, Thailand, and India ranges from 30% to 65% [27,40,64]. On the other hand, the frequency of the mixed type infection (observed by type-specific MSP-1 PCR) in South Korean isolates is nearly 20% [16]. Overall, South Korean P. vivax usually shows relatively low genetic diversity. This is probably due to the low rate of malaria transmission intensity and the few strains involved.
Knowledge of the genetic population structure of malaria parasites helps to reveal the distribution of genetic differences within a limited population and to elucidate how variations change over time. Moreover, quantification provides useful information, including the level of genetic drift, how alleles are spatially distributed, and how the genetic diversity is organized. There is considerable population diversity of Plasmodium sp. in endemic regions [52,57]. However, little is known about the genetic diversity and population dynamics of P. vivax in the Korean Peninsula, although malaria has been prevalent for many centuries and is a current public health problem.
CONCLUSION
Genetic studies of several antigenic proteins, including CSP, MSP-1, MSP-3, DBP, and AMA-1 isolated from strains of P. vivax malaria that reemerged in South Korea, have shown the co-existence of 2 distinct haplotypes. The genetic diversity of the genes of interest is very low in South Korean isolates. This may be due to the effect of the limited seasonal prevalence and the transmission abilities of the mosquito vector. In comparison with the polymorphism seen in genetic markers in other endemic regions, South Korean isolates are most similar to those of East Asia, including China and North Korea. Very few studies have investigated the genetic population structure of P. vivax in Korean isolates. Studies remain inconclusive regarding both the genetic characteristics of antigens, including mutations that lead to antigenic variation and population structures of P. vivax. Future studies may provide further information that may lead to the development of an effective P. vivax vaccine.
References
- 1.Warrell DA, Gilles HM. Essential Malariology. 4th ed. London: A Hodder Arnold Publication; 2002. pp. 8–34. [Google Scholar]
- 2.Bruce-Chwatt LJ. Malaria and its control: present situation and future prospects. Ann Rev Public Health. 1987;8:75–110. doi: 10.1146/annurev.pu.08.050187.000451. [DOI] [PubMed] [Google Scholar]
- 3.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 5.Paik YH, Rhee HI, Shim JC. Malaria in Korea. Jpn J Exp Med. 1988;58:55–66. [PubMed] [Google Scholar]
- 6.Chai IH, Lim GI, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who has never been abroad. Korean J Parasitol. 1994;32:195–200. doi: 10.3347/kjp.1994.32.3.195. [DOI] [PubMed] [Google Scholar]
- 7.Shin EH, Guk SM, Kim HJ, Lee SH, Chai JY. Trends in parasitic diseases in the Republic of Korea. Trends Parasitol. 2008;24:143–150. doi: 10.1016/j.pt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Hisaeda H, Yasutomo K, Himeno K. Malaria: immune evasion by parasites. Int J Biochem Cell Biol. 2005;37:700–706. doi: 10.1016/j.biocel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Carlton J. The Plasmodium vivax genome sequencing project. Trends Parasitol. 2003;19:227–231. doi: 10.1016/s1471-4922(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 10.Verra F, Mangano VD, Modiano D. Genetics of susceptibility to Plasmodium falciparum: from classical malaria resistance genes towards genome-wide association studies. Parasite Immunol. 2009;31:234–253. doi: 10.1111/j.1365-3024.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- 11.Carret CK, Horrocks P, Konfortov B, Winzeler E, Qureshi M, Newbold C, Ivens A. Microarray-based comparative genomic analyses of the human malaria parasite Plasmodium falciparum using Affymetrix arrays. Mol Biochem Parasitol. 2005;144:177–186. doi: 10.1016/j.molbiopara.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Kho WG, Park YH, Chung JY, Kim JP, Hong ST, Lee WJ, Kim TS, Lee JS. Two new genotypes of Plasmodium vivax circumsporozoite protein found in the Republic of Korea. Korean J Parasitol. 1999;37:265–270. doi: 10.3347/kjp.1999.37.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim CS, Kim YK, Lee KN, Kim SH, Hoffman KJ, Song KJ, Song JW. The analysis of circumsporozoite-protein gene sequences from South Korean isolates of Plasmodium vivax. Ann Trop Med Parasitol. 2001;95:229–235. doi: 10.1080/00034980120053997. [DOI] [PubMed] [Google Scholar]
- 14.Kim T, Kim YJ, Song KJ, Song JW, Cha SH, Kim YK, Shin YK, Suh IB, Lim CS. The molecular characteristics of circumsporozoite protein gene subtypes from Plasmodium vivax isolates in Republic of Korea. Parasitol Res. 2002;88:1051–1054. doi: 10.1007/s00436-002-0699-z. [DOI] [PubMed] [Google Scholar]
- 15.Lim CS, Kim SH, Kwon SI, Song JW, Song KJ, Lee KN. Analysis of Plasmodium vivax merozoite surface protein-1 gene sequences from resurgent Korean isolates. Am J Trop Med Hyg. 2000;62:261–265. doi: 10.4269/ajtmh.2000.62.261. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Hwang SY, Shin JH, Moon CS, Kim DW, Kho WG. Molecular genetic characterization of the merozoite surface protein 1 Gene of Plasmodium vivax from reemerging Korean isolates. Clin Vaccine Immunol. 2009;16:733–738. doi: 10.1128/CVI.00493-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han ET, Song TE, Park JH, Shin EH, Guk SM, Kim TY, Chai JY. Allelic dimorphism in the merozoite surface protein-3α in Korean isolates of Plasmodium vivax. Am J Trop Med Hyg. 2004;71:745–749. [PubMed] [Google Scholar]
- 18.Han ET, Park JH, Shin EH, Choi MH, Oh MD, Chai JY. Apical membrane antigen-1 (AMA-1) gene sequences of re-emerging Plasmodium vivax in South Korea. Korean J Parasitol. 2002;40:157–162. doi: 10.3347/kjp.2002.40.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung JY, Chun EH, Chun JH, Kho WG. Analysis of the Plasmodium vivax apical membrane antigen-1 gene from re-emerging Korean isolates. Parasitol Res. 2003;90:325–329. doi: 10.1007/s00436-002-0777-2. [DOI] [PubMed] [Google Scholar]
- 20.Kho WG, Chung JY, Sim EJ, Kim DW, Chung WC. Analysis of polymorphic regions of Plasmodium vivax Duffy binding protein of Korean isolates. Korean J Parasitol. 2001;39:143–150. doi: 10.3347/kjp.2001.39.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh IB, Hoffman KJ, Kim SH, Song KJ, Song JW, Lee JS, Lim CS. The analysis of Plasmodium vivax Duffy receptor binding domain gene sequence from resurgent Korea isolates. Parasitol Res. 2001;87:1007–1010. doi: 10.1007/s004360100478. [DOI] [PubMed] [Google Scholar]
- 22.Kho WG, Chung JY, Hwang UW, Chun JH, Park YH, Chung WC. Analysis of polymorphic region of GAM-1 gene in Plasmodium vivax Korean isolates. Korean J Parasitol. 2001;39:313–318. doi: 10.3347/kjp.2001.39.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985;230:815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg R, Wirtz RA, Lanar DE, Sattabongkot J, Hall T, Waters AP, Prasittisuk C. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science. 1989;245:973–976. doi: 10.1126/science.2672336. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Pathak S. Malaria vaccine: a current perspective. J Vector Borne Dis. 2008;45:1–20. [PubMed] [Google Scholar]
- 26.Leclerc MC, Menegon M, Cligny A, Noyer JL, Mammadov S, Aliyev N, Gasimov E, Majori G, Severini C. Genetic diversity of Plasmodium vivax isolates from Azerbaijan. Malar J. 2004;3:40. doi: 10.1186/1475-2875-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui L, Mascorro CN, Fan Q, Rzomp KA, Khuntirat B, Zhou G, Chen H, Yan G, Sattabongkot J. Genetic diversity and multiple infections of Plasmodium vivax malaria in Western Thailand. Am J Trop Med Hyg. 2003;68:613–619. doi: 10.4269/ajtmh.2003.68.613. [DOI] [PubMed] [Google Scholar]
- 28.González JM, Hurtado S, Arévalo-Herrera M, Herrera S. Variants of the Plasmodium vivax circumsporozoite protein (VK210 and VK247) in Colombian isolates. Mem Inst Oswaldo Cruz. 2001;96:709–712. doi: 10.1590/s0074-02762001000500023. [DOI] [PubMed] [Google Scholar]
- 29.Kim JR, Imwong M, Nandy A, Chotivanich K, Nontprasert A, Tonomsing N, Maji A, Addy M, Day NP, White NJ, Pukrittayakamee S. Genetic diversity of Plasmodium vivax in Kolkata, India. Malar J. 2006;5:71. doi: 10.1186/1475-2875-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez MH, Gonzalez-Ceron L, Hernandez JE, Nettel JA, Villarreal C, Kain KC, Wirtz RA. Different prevalences of Plasmodium vivax phenotypes VK210 and VK247 associated with the distribution of Anopheles albimanus and Anopheles pseudopunctipennis in Mexico. Am J Trop Med Hyg. 2000;62:122–127. doi: 10.4269/ajtmh.2000.62.122. [DOI] [PubMed] [Google Scholar]
- 31.Mann VH, Huang T, Cheng Q, Saul A. Sequence variation in the circumsporozoite protein gene of Plasmodium vivax appears to be regionally biased. Mol Biochem Parasitol. 1994;68:45–52. doi: 10.1016/0166-6851(94)00148-0. [DOI] [PubMed] [Google Scholar]
- 32.Collins WE, Skinner JC, Krotoski WA, Cogswell FB, Gwadz RW, Broderson JR, Ma NS, Mehaffey P, Sutton BB. Studies on the North Korean strain of Plasmodium vivax in Aotus monkeys and different anophelines. J Parasitol. 1985;71:20–27. [PubMed] [Google Scholar]
- 33.Mahanty S, Saul A, Miller LH. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J Exp Biol. 2003;206:3781–3788. doi: 10.1242/jeb.00646. [DOI] [PubMed] [Google Scholar]
- 34.del Portillo HA, Longacre S, Khouri E, David PH. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci USA. 1991;88:4030–4034. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson HL, Tucker JE, Kaslow DC, Krettli AU, Collins WE, Kiefer MC, Bathurst IC, Barr PJ. Structure and expression of the gene for Pv200, a major blood-stage surface antigen of Plasmodium vivax. Mol Biochem Parasitol. 1992;50:325–333. doi: 10.1016/0166-6851(92)90230-h. [DOI] [PubMed] [Google Scholar]
- 36.Imwong M, Pukrittayakamee S, Grüner AC, Rénia L, Letourneur F, Looareesuwan S, White NJ, Snounou G. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar J. 2005;4:20. doi: 10.1186/1475-2875-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porto M, Ferreira MU, Camargo LM, Premawansa S, del Portillo HA. Second form in a segment of the merozoite surface protein 1 gene of Plasmodium vivax among isolates from Rondônia (Brazil) Mol Biochem Parasitol. 1992;54:121–124. doi: 10.1016/0166-6851(92)90104-r. [DOI] [PubMed] [Google Scholar]
- 38.Premawansa S, Snewin VA, Khouri E, Mendis KN, David PH. Plasmodium vivax: recombination between potential allelic types of the merozoite surface protein MSP1 in parasites isolated from patients. Exp Parasitol. 1993;76:192–199. doi: 10.1006/expr.1993.1022. [DOI] [PubMed] [Google Scholar]
- 39.Maestre A, Sunil S, Ahmad G, Mohmmed A, Echeverri M, Corredor M, Blair S, Chauhan VS, Malhotra P. Inter-allelic recombination in the Plasmodium vivax merozoite surface protein 1 gene among Indian and Colombian isolates. Malar J. 2004;3:4. doi: 10.1186/1475-2875-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolakovich KA, Ssengoba A, Wojcik K, Tsuboi T, al-Yaman F, Alpers M, Adams JH. Plasmodium vivax: favored gene frequencies of the merozoite surface protein-1 and the multiplicity of infection in a malaria endemic region. Exp Parasitol. 1996;83:11–19. doi: 10.1006/expr.1996.0044. [DOI] [PubMed] [Google Scholar]
- 41.Putaporntip C, Jongwutiwes S, Tanabe K, Thaithong S. Interallelic recombination in the merozoite surface protein 1 (MSP-1) gene of Plasmodium vivax from Thai isolates. Mol Biochem Parasitol. 1997;84:49–56. doi: 10.1016/s0166-6851(96)02786-7. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Q, Stowers A, Huang TY, Bustos D, Huang YM, Rzepczyk C, Saul A. Polymorphism in Plasmodium vivax MSA1 gene--the result of intragenic recombinations? Parasitology. 1993;106:335–345. doi: 10.1017/s003118200006707x. [DOI] [PubMed] [Google Scholar]
- 43.Putaporntip C, Jongwutiwes S, Sakihama N, Ferreira MU, Kho WG, Kaneko A, Kanbara H, Hattori T, Tanabe K. Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc Natl Acad Sci USA. 2002;99:16348–16353. doi: 10.1073/pnas.252348999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ree HI. Studies on Anopheles sinensis, the vector species of vivax malaria in Korea. Korean J Parasitol. 2005;43:75–92. doi: 10.3347/kjp.2005.43.3.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez A, Vicini J, Patarroyo ME, Murillo LA, Patarroyo MA. Plasmodium vivax: polymorphism in the merozoite surface protein 1 gene from wild Colombian isolates. Exp Parasitol. 2000;95:215–219. doi: 10.1006/expr.2000.4534. [DOI] [PubMed] [Google Scholar]
- 46.Putaporntip C, Jongwutiwes S, Seethamchai S, Kanbara H, Tanabe K. Intragenic recombination in the 3' portion of the merozoite surface protein 1 gene of Plasmodium vivax. Mol Biochem Parasitol. 2000;109:111–119. doi: 10.1016/s0166-6851(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 47.Galinski MR, Corredor-Medina C, Povoa M, Crosby J, Ingravallo P, Barnwell JW. Plasmodium vivax merozoite surface protein-3 contains coiled-coil motifs in an alanine-rich central domain. Mol Biochem Parasitol. 1999;101:131–147. doi: 10.1016/s0166-6851(99)00063-8. [DOI] [PubMed] [Google Scholar]
- 48.Bruce MC, Galinski MR, Barnwell JW, Snounou G, Day KP. Polymorphism at the merozoite surface protein-3 locus of Plasmodium vivax: global and local diversity. Am J Trop Med Hyg. 1999;61:518–525. doi: 10.4269/ajtmh.1999.61.518. [DOI] [PubMed] [Google Scholar]
- 49.Zakeri S, Barjesteh H, Djadid ND. Merozoite surface protein-3alpha is a reliable marker for population genetic analysis of Plasmodium vivax. Malar J. 2006;5:53. doi: 10.1186/1475-2875-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hisaeda H, Saul A, Reece JJ, Kennedy MC, Long CA, Miller LH, Stowers AW. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J Infect Dis. 2002;185:657–664. doi: 10.1086/339187. [DOI] [PubMed] [Google Scholar]
- 51.Fang XD, Kaslow DC, Adams JH, Miller LH. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44:125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- 52.de Souza-Neiras WC, de Melo LM, Machado RL. The genetic diversity of Plasmodium vivax. Mem Inst Oswaldo Cruz. 2007;102:245–254. doi: 10.1590/s0074-02762007000300002. [DOI] [PubMed] [Google Scholar]
- 53.Cole-Tobian J, King CL. Diversity and natural selection in Plasmodium vivax Duffy binding protein gene. Mol Biochem Parasitol. 2003;127:121–132. doi: 10.1016/s0166-6851(02)00327-4. [DOI] [PubMed] [Google Scholar]
- 54.Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180:497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuboi T, Kappe SH, al-Yaman F, Prickett MD, Alpers M, Adams JH. Natural variation within the principal adhesion domain of the Plasmodium vivax Duffy binding protein. Infect Immun. 1994;62:5581–5586. doi: 10.1128/iai.62.12.5581-5586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Q, Saul A. Sequence analysis of the apical membrane antigen I (AMA-1) of Plasmodium vivax. Mol Biochem Parasitol. 1994;65:183–187. doi: 10.1016/0166-6851(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 57.Cui L, Escalante AA, Imwong M, Snounou G. The genetic diversity of Plasmodium vivax population. Trends Parasitol. 2003;19:220–226. doi: 10.1016/s1471-4922(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 58.Figtree M, Pasay CJ, Slade R, Cheng Q, Cloonan N, Walker J, Saul A. Plasmodium vivax synonymous substitution frequencies, evolution and population structure deduced from diversity in AMA 1 and MSP 1 genes. Mol Biochem Parasitol. 2000;108:53–66. doi: 10.1016/s0166-6851(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 59.Snewin VA, Premawansa S, Kapilananda GM, Ratnayaka L, Udagama PV, Mattei DM, Khouri E, Del Giudice G, Peiris JS, Mendis KN, David PH. Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 1995;181:357–362. doi: 10.1084/jem.181.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imwong M, Pukrittakayamee S, Looareesuwan S, Poirriez J, Pasvol G, White NJ, Snounou G. Plasmodium vivax: polymerase chain reaction amplification artifacts limit the suitability of pvgam1 as a genetic marker. Exp Parasitol. 2001;99:175–179. doi: 10.1006/expr.2001.4646. [DOI] [PubMed] [Google Scholar]
- 61.Snewin VA, Khouri E, Wattavidanage J, Perera L, Premawansa S, Mendis KN, David PH. A new polymorphic marker for PCR typing of Plasmodium vivax parasites. Mol Biochem Parasitol. 1995;71:135–138. doi: 10.1016/0166-6851(94)00040-t. [DOI] [PubMed] [Google Scholar]
- 62.Prajapati SK, Verma A, Adak T, Yadav RS, Kumar A, Eapen A, Das MK, Singh N, Sharma SK, Rizvi MA, Dash AP, Joshi H. Allelic dimorphism of Plasmodium vivax gam-1 in the Indian subcontinent. Malar J. 2006;5:90. doi: 10.1186/1475-2875-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Udagama PV, David PH, Peiris JS, Ariyaratne YG, Perera KL, Mendis KN. Demonstration of antigenic polymorphism in Plasmodium vivax malaria with a panel of 30 monoclonal antibodies. Infect Immun. 1987;55:2604–2611. doi: 10.1128/iai.55.11.2604-2611.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joshi H. Markers for population genetic analysis of human plasmodia species, P. falciparum and P. vivax. J Vector Borne Dis. 2003;40:78–83. [PubMed] [Google Scholar]