Abstract
Objective
To evaluate intensive care-related factors as predictors of depressive symptoms 6 months after acute lung injury (ALI)
Design
Prospective cohort study
Setting
Thirteen intensive care units (ICUs) in 4 hospitals in Baltimore, MD
Patients
Consecutive ALI survivors (n = 160; 71% from medical ICUs) screened for depressive symptoms at six months post-ALI
Interventions
None
Measurements and Main Results
We prospectively measured 12 features of critical illness and ICU care and used multivariable regression to evaluate associations with depressive symptoms as measured by the Hospital Anxiety and Depression (HAD) depression score. The prevalence of a positive screening for depression (score ≥8) at 6 months post-ALI was 26%. Depressive symptoms were significantly associated with surgical (versus medical or trauma) ICU admission (relative risk [RR] 2.2, 95% confidence interval [CI] 1.1 – 4.2), maximum daily Sequential Organ Failure Assessment score of >10 (RR 2.1, 95% CI 1.1 – 3.5), and mean daily ICU benzodiazepine dose of ≥75mg of midazolam-equivalent (RR 2.1, 95% CI 1.1 – 3.5).
Conclusions
Depressive symptoms at 6 months post-ALI are common and potentially associated with ICU-related factors. Mechanisms by which critical illness and intensive care management associate with depressive symptoms merit further investigation.
Keywords: depression, intensive care units, respiratory distress syndrome, adult, critical care, outcome assessment (health care)
Introduction
Survivors of acute lung injury (ALI) experience substantial long-term morbidity, including impairments in quality of life (1,2) and neuromuscular (3,4), cognitive (5), and psychological (6,7) function. Depression appears particularly common, with a point prevalence of clinically significant depressive symptoms ranging from 17% to 43% at 12–24 months after ALI (6). Prior studies of intensive care unit (ICU) survivors have demonstrated that factors related to patient recovery, including longer time since ALI (8) and improved post-ICU physical functioning (9), are associated with fewer depressive symptoms. However, little is known about how features of critical illness and ICU care themselves relate to post-ALI depression (6,10). Thus, we measured symptoms of depression at six months after ALI and evaluated associations of those symptoms with 12 intensive care-related factors measured prospectively in the ICU.
Materials and Methods
Study Population
Consecutive mechanically-ventilated patients with ALI were enrolled in a prospective cohort study conducted in 13 ICUs at 4 hospitals in Baltimore, MD, USA (11). To avoid inclusion of patients with primary neurological disease or trauma, neurological specialty ICUs at the participating hospitals were not included. Key exclusion criteria were: (1) pre-existing illness with a life expectancy of <6 months; (2) pre-existing cognitive impairment, or communication/language barrier; (3) transfer into a study site ICU with pre-existing ALI of >24 hours duration; (4) >5 days of mechanical ventilation before ALI; (5) no fixed address; and (6) patient or family requests for no escalation of care, such as no vasopressors or no hemodialysis.
Measurement of baseline and ICU-related exposures
Patient demographics and the Acute Physiology and Chronic Health Evaluation (APACHE) II severity of illness score (12) were obtained upon admission to the study site ICU. The Sequential Organ Failure Assessment (SOFA) score (13), minimum blood glucose, and the dose, route and frequency of administration of selected medications (corticosteroids, narcotics, and benzodiazepines) were collected daily after ALI diagnosis while patients were in the ICU. Patients’ pre-hospitalization functional status (independence in activities of daily living [ADLs] (14)), years of formal education, and employment status were collected by interview with the patient (or proxy, when unable to obtain patient response). Mood symptoms prior to the onset of the illness that necessitated hospital admission were assessed by retrospective patient interview using the depression/anxiety dimension of the EQ-5D quality of life instrument (15). In the present analysis, a response of “moderate” or “extreme” depression/anxiety (versus “none”) was considered a positive response for abnormal baseline mood. All potential risk factors measured on a continuous scale were modeled as tertiles using clinically relevant thresholds closest to the 33rd and 67th percentile in order to characterize non-linear associations and prevent undue influence from outlying observations.
Measurement of Depressive Symptoms after Discharge
The outcome of interest was depressive symptoms at six months after ALI diagnosis, measured using the depression subscale of the Hospital Anxiety and Depression (HAD) instrument (16). The HAD instrument contains two seven-item subscales that separately measure symptoms of depression and anxiety. These subscales define separable symptom complexes based on factor analysis of this instrument (17). The depression subscale of the HAD instrument was specifically designed to screen for depression in medically ill patients, primarily measuring anhedonia rather than the “physical” symptoms of depression (e.g., loss of appetite) that frequently occur in conjunction with physical illness. We did not evaluate the HAD anxiety subscale because the symptoms it measures do not closely correspond to a specific psychiatric syndrome and have not been clearly linked to ICU-related factors in ALI survivors (6).
The HAD depression subscale score ranges from 0 to 21, with a higher score indicating greater depressive symptoms. A score of 8 – 10 indicates “possible depression” and ≥11 indicates “likely clinical depression” (16). In the primary analysis, we modeled the depression subscale score as a continuous variable. In a secondary analysis, we used a binary measure of depression, defined a priori using the standard cut-off threshold of ≥8. Although the HAD is a validated instrument, it does not permit a clinical diagnosis of depression, which requires a clinical psychiatric interview. Consequently, we refer to the outcome measure as “depressive symptoms,” rather than “depression.”
Statistical Analysis
We compared the distribution of all baseline and ICU-related exposure variables by depression screening status (HAD depression sub-scale <8 vs. ≥8) using Fisher’s exact test (categorical variables) and Student’s t-test (continuous variables) (Table 1). For each of these exposure variables, we calculated the univariable p-value for its association with the mean depression score using linear regression, and for its association with a positive depression screening test (HAD depression sub-scale score ≥8) using Fisher’s exact test or logistic regression, as appropriate. All exposure variables having a crude association with depressive symptoms (p ≤0.15) were analyzed in multivariable linear (mean depression score) or logistic (positive depression screening) models. All significance tests were two-sided, with statistical significance defined as p<0.05.
Table 1.
Patient Characteristics, by HAD Depression Score at 6 Months After Acute Lung Injury
| Variable | Depression Screening Result, n (%)a |
Total n = 160 | P valueb | |
|---|---|---|---|---|
| Positive (HAD ≥8) n = 41 | Negative (HAD <8) n = 119 | |||
| Pre-ICU Characteristics | ||||
| Age, yearsc | 48 (12) | 49 (15) | 49 (14) | 0.82 |
| Male sex | 21 (51%) | 68 (57%) | 89 (56%) | 0.59 |
| Race | ||||
| Caucasian | 28 (68%) | 67 (56%) | 95 (59%) | 0.29 |
| African-American | 13 (32%) | 48 (40%) | 61 (38%) | |
| Other | 0 (0%) | 4 (3%) | 4 (3%) | |
| Education, years (n=152) | ||||
| <12 | 10 (25%) | 35 (31%) | 45 (30%) | 0.20 |
| 12 | 17 (43%) | 30 (27%) | 47 (31%) | |
| >12 | 13 (32%) | 47 (42%) | 60 (39%) | |
| Employmentd | ||||
| Employed | 15 (37%) | 52 (44%) | 67 (42%) | 0.72 |
| Disabled | 12 (29%) | 32 (27%) | 44 (28%) | |
| Other | 14 (34%) | 35 (29%) | 49 (31%) | |
| BMI (kg/m2) | ||||
| <25 | 12 (29%) | 46 (39%) | 58 (36%) | 0.13 |
| 25–30 | 10 (24%) | 33 (28%) | 43 (27%) | |
| 30–40 | 12 (29%) | 33 (28%) | 45 (28%) | |
| >40 | 7 (17%) | 6 (5%) | 13 (8%) | |
| Pre-admission dependence in ≥ 1 activity of daily livinge(n = 159) | 10 (24%) | 16 (14%) | 26 (16%) | 0.14 |
| Pre-admission depression/anxietyf (n = 133) | ||||
| None | 12 (36%) | 65 (65%) | 77 (58%) | 0.01 |
| Moderate | 17 (52%) | 29 (29%) | 46 (35%) | |
| Extreme | 4 (12%) | 6 (6%) | 10 (8%) | |
| ICU-Related Characteristics | ||||
| Type of ICU | ||||
| Medical | 28 (68%) | 86 (72%) | 114 (71%) | 0.22 |
| Surgical | 9 (22%) | 14 (12%) | 23 (14%) | |
| Trauma | 4 (10%) | 19 (16%) | 23 (14%) | |
| Length of ICU stay | ||||
| <10 days | 11 (27%) | 30 (25%) | 41 (26%) | 0.51 |
| 11–21 days | 15 (37%) | 55 (46%) | 70 (44%) | |
| >21 days | 15 (37%) | 34 (29%) | 49 (31%) | |
| Ventilation duration | ||||
| ≤7 days | 14 (34%) | 44 (37%) | 58 (36%) | 0.97 |
| 8–14 days | 12 (29%) | 35 (29%) | 47 (29%) | |
| >14 days | 15 (37%) | 40 (34%) | 55 (34%) | |
| APACHE II scoreg | ||||
| ≤20 | 13 (32%) | 51 (43%) | 64 (40%) | 0.36 |
| 21–25 | 9 (22%) | 26 (22%) | 35 (22%) | |
| >25 | 19 (46%) | 42 (35%) | 61 (38%) | |
| Worst SOFA scoreh | ||||
| <8 | 9 (22%) | 39 (33%) | 48 (30%) | 0.18 |
| 8–10 | 14 (34%) | 46 (39%) | 60 (37%) | |
| >10 | 18 (44%) | 34 (29%) | 52 (33%) | |
| Lowest PaO2/FiO2 ratio | ||||
| <100 | 28 (68%) | 76 (64%) | 104 (65%) | 0.81 |
| 100–199 | 13 (32%) | 40 (34%) | 53 (33%) | |
| 200–299 | 0 (0%) | 3 (3%) | 3 (2%) | |
| Steroids (prednisone-equivalent) | ||||
| None | 18 (44%) | 53 (45%) | 71 (44%) | 0.95 |
| ≤25 mg/day | 11 (27%) | 29 (24%) | 40 (25%) | |
| >25 mg/day | 12 (29%) | 37 (31%) | 49 (31%) | |
| Narcotics (morphine-equivalent) | ||||
| <50 mg/day | 15 (37%) | 48 (40%) | 63 (39%) | 0.74 |
| 50–150 mg/day | 13 (32%) | 41 (34%) | 54 (34%) | |
| >150 mg/day | 13 (32%) | 30 (25%) | 43 (27%) | |
| Benzodiazepines (midazolam-equivalent) | ||||
| <25 mg/day | 15 (37%) | 50 (42%) | 65 (41%) | 0.07 |
| 25–75 mg/day | 8 (20%) | 39 (33%) | 47 (29%) | |
| >75 mg/day | 18 (44%) | 30 (25%) | 48 (30%) | |
| Any hypoglycemia (<60 mg/dL) | 17 (41%) | 41 (34%) | 58 (36%) | 0.45 |
| Tracheotomy | 12 (29%) | 28 (24%) | 40 (25%) | 0.53 |
| Liberated from ventilator pre-discharge | 112 (94%) | 37 (90%) | 149 (93%) | 0.48 |
HAD, Hospital Anxiety and Depression scale; BMI, body mass index; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment.
Percentages may not add to 100% due to rounding.
Fisher’s exact test or Student’s t-test.
Reported as mean (standard deviation).
“Disabled” refers to patients who were unemployed due to health problems prior to ALI. “Other” includes students, homemakers, retired persons, and patients who were unemployed for non-health-related reasons.
Six items describing pre-ICU functional status: bathing, dressing, toileting, transferring, continence, and feeding (14). Proxy response was used when patients could not respond.
Per EQ-5D quality of life survey (15) administered retrospectively to patients after ICU discharge.
Severity of illness score ranging from 0 (best) to 71 (worst) based on physiologic variables within 24 hours of ICU admission, age and chronic comorbidities (12).
Organ dysfunction score, recorded daily in the ICU, ranging from 0 (best) to 24 (worst) based on six major organ systems (13).
For the binary outcome measure (depression sub-scale score ≥8), we calculated relative risks using log-binomial regression models. When the log-binomial model failed to converge, a Poisson regression model with robust variance calculation was used (18). Missing data (which was limited to only education level, pre-ICU functional status, and pre-ICU depression/anxiety) were imputed using mean imputation based on a best-subset regression model utilizing all other variables to model the missing data (19). Influence was evaluated using added-variable plots. The effect of outlier data values on regression coefficients was estimated using Dfbeta statistics, and collinearity was assessed using variance inflation factors (19,20). Goodness-of-fit was evaluated using deviance residuals (21). All analyses were performed using STATA version 9.2 (Stata Corp., College Station, TX, USA).
This study was approved by the Institutional Review Boards of all participating institutions.
Results
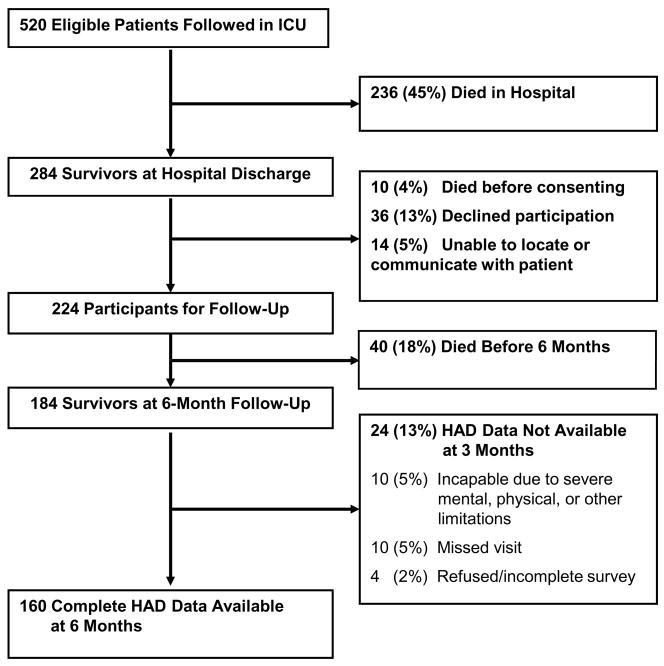
Of 520 patients enrolled in the cohort study, 284 (55%) survived to hospital discharge. Of these survivors, 50 (18%) died before consent or 6 month follow-up, 36 (13%) declined participation in long-term follow-up, and 14 (5%) were lost to follow-up, leaving 184 survivors at 6 month follow-up (Figure 1). A total of 174 (95%) 6-month survivors were available for follow-up assessment, of whom 160 (92%) were able to complete the entire HAD questionnaire. The median (interquartile range, IQR) HAD depression score was 5 (2–8), and the mean (standard deviation) was 5.2 (4.2). The prevalence of a positive screening test for depression (score ≥8) was 26% (41/160) (Table 1). Using a more stringent threshold (HAD depression score ≥11), the prevalence of a positive depression screen was 11% (18/160).
Figure 1. Study Flow Diagram.
ICU, intensive care unit; HAD, Hospital Anxiety and Depression scale.
Three ICU-related factors were significantly associated with depressive symptoms, whether measured as a continuous score (Table 2) or dichotomized as a positive screening test for depression (Table 3). Patients admitted to a surgical (versus medical or trauma) ICU had an increase in mean HAD score of 2.7 points (95% confidence interval [CI]: 0.8, 4.5), and a relative risk for a positive depression screening test of 2.2 (95% CI: 1.1, 4.2). Similarly, patients receiving a mean daily benzodiazepine dose of ≥75mg of midazolam-equivalent had a mean HAD increase of 2.0 points (95% CI: 0.6, 3.5), and those with a maximum organ dysfunction (SOFA) score of >10 had an increase of 1.6 points (95% CI: 0.2, 3.0). Both of these latter factors were associated with a relative risk for a positive depression screening test of 2.1 (95% CI: 1.2, 3.5). Patients admitted to surgical ICUs (versus medical or trauma ICUs) had a lower median [IQR] daily benzodiazepine dose (midazolam-equivalent dose: 3.4 [0.9–32.0] vs. 38.5 [15.8–94.2] mg, p<0.001) and a similar median [IQR] value for maximum SOFA score (9 [8–11] vs. 9 [7–11], p>0.9).
Table 2.
Factors Associated with Depressive Symptoms at 6 Months After Acute Lung Injury
| Factor | Increase (95% CI) in Mean HAD Depression Scorea |
|||
|---|---|---|---|---|
| Univariable | P value | Multivariable | P valueb | |
| Pre-ICU Characteristics | ||||
| Education ≤ 12 years | 1.7 (0.2, 3.1) | 0.02 | 1.7 (0.3, 3.0) | 0.01 |
| Pre-admission depression/anxietyc | 2.5 (1.0, 4.0) | 0.001 | 1.6 (0.1, 3.1) | 0.03 |
| Caucasian race | 1.2 (−0.2, 2.6) | 0.08 | 1.1 (−0.3, 2.4) | 0.10 |
| BMI >40 kg/m2 | 2.6 (0.1, 5.0) | 0.04 | 1.6 (−0.7, 4.0) | 0.16 |
| Unemployed at baseline | 1.5 (0.2, 2.9) | 0.02 | 0.7 (−0.7, 2.0) | 0.30 |
| Dependent in ≥ 1 ADL at baselined | 1.7 (−0.2, 3.5) | 0.07 | 0.8 (−1.0, 2.6) | 0.36 |
| ICU-Related Characteristics | ||||
| Admitted to surgical ICU | 2.4 (0.5, 4.3) | 0.01 | 2.7 (0.8, 4.5) | 0.004 |
| Benzodiazepine dose >75mg midazolam per day | 1.1 (−0.4, 2.6) | 0.12 | 1.9 (0.5, 3.4) | 0.008 |
| Maximum SOFA score >10e | 1.2 (−0.3, 2.6) | 0.10 | 1.5 (0.1, 2.9) | 0.03 |
| Steroid dose >25mg prednisone- equivalent per day | −1.1(−2.6, 0.4) | 0.13 | −1.3 (−2.7, 0.1) | 0.07 |
| Liberated from ventilator after ICU discharge | 2.5 (−0.2, 5.1) | 0.06 | 1.4 (−1.1, 4.0) | 0.25 |
| Any hypoglycemia <60 mg/dL | 1.1 (−0.3, 2.5) | 0.11 | 0.4 (−1.0, 1.7) | 0.56 |
| ICU length of stay ≥3 weeks | 1.6 (0.1, 3.1) | 0.03 | 0.2 (−1.3, 1.6) | 0.83 |
CI, confidence interval; HAD, Hospital Anxiety and Depression scale; ICU, intensive care unit; BMI, body mass index; ADL, activity of daily living; SOFA, Sequential Organ Failure Assessment.
Measured by the 7-item Hospital Anxiety and Depression (HAD) instrument’s depression sub-scale, a continuous measure ranging from 0 to 21 with pre-defined thresholds for depression screening of ≥8 and ≥11 (16). Results are presented as the increase in mean HAD depression score, comparing patients with the factor of interest to those without that factor, using a linear regression model.
P-values calculated using linear least-squares regression. Where two contiguous tertiles did not differ in their mean HAD depression score (p>0.15), they were combined to create a binary variable. All factors with a univariable p-value <0.15 were included in this Table.
Per EQ-5D quality of life survey (15) administered retrospectively to patients after ICU discharge.
Six items describing pre-ICU functional status: bathing, dressing, toileting, transferring, continence, and feeding (14). Proxy response was used when patients could not respond.
This organ dysfunction score is recorded daily in the ICU and ranges from 0 (best) to 24 (worst) based on six major organ systems (13).
Table 3.
Factors Associated with Positive Depression Screening Test at 6 Months After Acute Lung Injury
| Factor | Relative Risk (95% CI) for Positive Depression Screening Test |
|||
|---|---|---|---|---|
| Univariable | P value | Multivariable | P valuea | |
| Pre-ICU Characteristics | ||||
| Pre-admission depression/anxietyb | 2.4 (1.2, 4.5) | 0.005 | 2.0 (1.1, 3.6) | 0.02 |
| BMI >40 kg/m2 | 2.3 (1.3, 4.2) | 0.02 | 1.9 (1.0, 3.4) | 0.05 |
| Dependent in ≥ 1 ADL at baselinec | 1.7 (0.9, 3.0) | 0.11 | 1.4 (0.7, 2.4) | 0.34 |
| ICU-Related Characteristics | ||||
| Admitted to surgical ICU | 1.7 (0.9, 3.1) | 0.11 | 2.2 (1.1, 4.2) | 0.02 |
| Benzodiazepine dose >75mg midazolam per day | 1.8 (1.0, 3.1) | 0.03 | 2.1 (1.2, 3.5) | 0.007 |
| Maximum SOFA score >10d | 1.6 (0.9, 2.8) | 0.07 | 2.1 (1.2, 3.5) | 0.008 |
CI, confidence interval; SOFA, Sequential Organ Failure Assessment; BMI, body mass index; ADL, activity of daily living.
P-values for univariable analyses using Fisher’s exact test (categorical exposures) or logistic regression (continuous exposures). Where two contiguous tertiles did not differ in their risk of a positive depression screening test (p>0.15), they were combined to create a binary variable. All factors with a univariable p-value <0.15 were included in this Table.
Per EQ-5D quality of life survey (15) administered retrospectively to patients after ICU discharge.
Six items describing pre-ICU functional status: bathing, dressing, toileting, transferring, continence, and feeding (14). Proxy response was used when patients could not respond.
Organ dysfunction score, recorded daily in the ICU, ranging from 0 (best) to 24 (worst) based on six major organ systems (13).
None of the baseline patient characteristics evaluated in this analysis were significantly associated with both mean depression score and positive screening results. However, patients with education ≤12 years had significantly higher mean HAD scores than patients with more education (1.7 points; 95% CI: 0.3, 3.0), and pre-admission depression/anxiety was significantly associated with a positive screening test (relative risk 2.0; 95% CI: 1.1, 3.6).
Two ICU-related factors were associated with mean HAD depression score (p<0.15) in univariable analysis but excluded from further analyses due to collinearity with ICU length of stay: duration of mechanical ventilation >7 days (univariable increase of 1.1 points; 95% CI −2.5, 0.3; p = 0.12) and tracheotomy (increase of 1.2 points; 95% CI −0.4, 2.7; p = 0.13). Separately including these variables in the multivariable model revealed that neither variable was significantly associated with the mean HAD depression score (p>0.2 for each), and neither variable materially changed the other multivariable results. The maximum variance inflation factor in the final multivariable model (Table 2) was 1.28, suggesting little collinearity.
Discussion
This prospective analysis of 160 acute lung injury survivors demonstrates that depressive symptoms at 6 months after ALI are both common and potentially associated with features of critical illness and intensive care. In this research, representing the largest published study of long-term psychiatric morbidity in ALI survivors to date, the factors most consistently associated with depressive symptoms included admission to a surgical (versus medical or trauma) ICU, maximum organ dysfunction score, and higher mean daily benzodiazepine dose in the ICU.
The 26% prevalence of a positive depression screening test at 6 months after ALI in this study is similar to the 28% prevalence observed earlier in our cohort at 3 month follow-up (22) and the 25% – 32% rates reported in other populations of mechanically ventilated patients at 6 to 12 months after discharge (23–25). This prevalence is substantially higher than the 8% prevalence of a positive depression screening test (using HAD depression sub-scale score ≥8) in a general adult population (26) and further confirms the high burden of psychiatric morbidity in ALI survivors.
Among the 12 characteristics we examined in the ICU, three (admission to surgical ICU, maximum organ dysfunction, and benzodiazepine dose) were significantly associated with depressive symptoms, when measured as both a continuous score and as a binary outcome (positive screening test). However, given the relatively small body of literature in this field, we caution that these associations are exploratory in nature. Regarding ICU type, specifically, we caution that only 14% of our cohort was admitted to a surgical ICU, thus limiting our ability to compare surgical versus medical ICUs. Furthermore, in order to avoid overlooking associations of potential clinical importance, we did not adjust for multiple comparisons; hence, statistical chance may play a role in our findings.
Using a subset of the present cohort (n = 104, 65% of the present sample), we previously investigated the association of ICU hypoglycemia with depressive symptoms at 3 months post-ALI. This variable had a significant association, as did two patient baseline factors (pre-admission anxiety/depression and morbid obesity) and, to a lesser extent, one other ICU-related factor (higher daily benzodiazepine dose) (22). These associations with depressive symptoms at 3 months were all re-confirmed using the full cohort analyzed in this study (data not shown). Of these four factors, only benzodiazepine dose remained significantly associated with depressive symptoms at 6 months post-ALI. In addition, two new ICU-related factors – admission to a surgical ICU and maximum ICU organ dysfunction score – were also significantly associated with depressive symptoms at 6 months. These differences in findings at 3 versus 6 months suggest that post-ALI depressive symptoms may follow a dynamic course; notably, only 61% of patients with a positive depression screening test at 6 months had a positive screen at 3 months. These differences may also reflect statistical chance; alternatively, surgical issues or severity of illness during intensive care may result in lasting physical morbidity that influences psychopathology more in the medium term than shortly after hospital discharge.
Prior studies have suggested that post-ICU depression is associated with pre-ICU functional status (8) and duration of ICU stay or sedative administration (27). In univariable analyses, we found similar associations of all three variables with mean HAD score. However, the effects of these variables were markedly attenuated in our multivariable models, which adjusted for other covariates not included in the original studies (adjusted p = 0.59 for duration of benzodiazepines; other variables in Table 2). Though we did find a significant association between depressive symptoms and mean benzodiazepine dose, this relationship may not be causal; rather, it may reflect that patient distress in the ICU (prompting more sedation) identifies individuals who are more likely to be distressed in general (28). Our study also differs in methodology from prior studies. Weinert et al (8) evaluated patients with acute respiratory failure rather than ALI and combined results from 2- and 6-month follow-up into a single analysis. Nelson et al (27) used a retrospective questionnaire format administered to 24 patients at 6 – 41 months after ALI and evaluated the duration of any sedative use, evaluating both benzodiazepines and narcotics together. These differences in study design may provide additional explanation for the differences noted when comparing our results with these prior studies.
This research has a number of potential limitations. First, we measured depressive symptoms at follow-up with the seven-item HAD depression subscale. Although this screening instrument has been validated in medically ill patients and has been used in >700 studies (17), including numerous studies of ICU survivors (29–31), a positive screening test does not represent a clinical diagnosis of depression. In addition, the HAD instrument does not include measures of physical symptoms, guilt, worthlessness or suicidality, and while its sensitivity for clinical depression is high (17), it may miss depressed patients in whom anhedonia is not prominent. Future studies using clinical interviews of ICU survivors would be useful in this regard. Second, we limited our study to depressive symptoms and cannot comment on associations with other potentially important psychiatric outcomes, such as post-traumatic stress (6,32,33). Third, we did not collect detailed data on prior psychiatric history or post-ALI psychiatric treatment (e.g., psychotherapy and antidepressants), which might reduce the burden of depressive symptoms in patients who would otherwise meet clinical criteria for depression (8). Fourth, 18% of hospital survivors died within six months of ALI, and an additional 13% declined participation in this longitudinal study. Although losses to follow-up in this study were less than in prior longitudinal cohorts of ARDS survivors (3,23), our results may not generalize to all ALI survivors.
Conclusions
This prospective analysis of 160 acute lung injury survivors demonstrates that depressive symptoms are common at 6 months after ALI and appear to be associated with aspects of critical illness and intensive care management. Future studies should continue to investigate intensive care-related factors, as well as the effect of premorbid psychopathology and post-ICU factors arising during patients’ recovery, in order to better understand the mechanisms by which patients develop long-term psychiatric morbidity after critical illness.
Acknowledgments
We thank all patients who participated in the study and the dedicated research staff who assisted with the study including Ms. Rachel Bell, Ms. Kim Boucher, Dr. Sanjay Desai, Ms. Carinda Feild, Ms. Thelma Harrington, Dr. Praveen Kondreddi, Ms. Frances Magliacane, Ms. Stacey Murray, Dr. Kim Nguyen, Dr. Susanne Prassl, Dr. Abdulla Damluji, Ms. Arabela Sampaio, Ms. Kristin Sepulveda, Dr. Shabana Shahid, and Ms. Michelle Silas.
This research is supported by the National Institutes of Health (Acute Lung Injury SCCOR Grant # P050 HL 73994). Dr. Dowdy is supported by the National Institutes of Health, Medical Scientist Training Program Award (T32 GMO7309). Drs. Bienvenu, and Sevransky are supported by Mentored Patient-Oriented Research Career Development Awards from the National Institutes of Health (K23 MH64543, and K23 GM071399, respectively). Dr. Needham is supported by a Clinician-Scientist Award from the Canadian Institutes of Health Research. The funding bodies had no role in the study design, manuscript writing or decision to submit the manuscript for publication.
Footnotes
The authors do not have any potential conflicts of interest to disclose.
Reference List
- 1.Dowdy DW, Eid MP, Sedrakyan A, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31:611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 2.Dowdy DW, Eid MP, Dennison CR, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 4.Bercker S, Weber-Carstens S, Deja M, et al. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:711–715. doi: 10.1097/01.ccm.0000157969.46388.a2. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 6.Davydow DS, Desai SV, Needham DM, et al. Psychiatric Morbidity in Survivors of the Acute Respiratory Distress Syndrome: A Systematic Review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapfhammer HP, Rothenhausler HB, Krauseneck T, et al. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161:45–52. doi: 10.1176/appi.ajp.161.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47:399–407. doi: 10.1176/appi.psy.47.5.399. [DOI] [PubMed] [Google Scholar]
- 9.Weinert CR, Gross CR, Kangas JR, et al. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156:1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 10.Weinert C. Epidemiology and treatment of psychiatric conditions that develop after critical illness. Curr Opin Crit Care. 2005;11:376–380. doi: 10.1097/01.ccx.0000168529.23078.64. [DOI] [PubMed] [Google Scholar]
- 11.Needham DM, Dennison CR, Dowdy DW, et al. Study protocol: The Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care. 2006;10:R9. doi: 10.1186/cc3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 15.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 18.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 19.STATA Corp. STATA reference manual: release 8. College Station, TX: STATA Corp.; 2003. [Google Scholar]
- 20.Belsley D, Kuh E, Welsch R. Regression Diagnostics. New York: John Wiley & Sons; 1980. [Google Scholar]
- 21.McCullagh P, Nelder JA. Generalized linear models. 2. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- 22.Dowdy DW, Dinglas V, Mendez-Tellez PA, et al. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit Care Med. 2008 doi: 10.1097/CCM.0b013e31818781f5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopkins RO, Weaver LK, Chan KJ, et al. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004;10:1005–1017. doi: 10.1017/s135561770410711x. [DOI] [PubMed] [Google Scholar]
- 24.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31:1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 25.Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med. 2004;32:61–69. doi: 10.1097/01.CCM.0000098029.65347.F9. [DOI] [PubMed] [Google Scholar]
- 26.Crawford JR, Henry JD, Crombie C, et al. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40:429–434. doi: 10.1348/014466501163904. [DOI] [PubMed] [Google Scholar]
- 27.Nelson BJ, Weinert CR, Bury CL, et al. Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Crit Care Med. 2000;28:3626–3630. doi: 10.1097/00003246-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Jones C, Backman C, Capuzzo M, et al. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33:978–985. doi: 10.1007/s00134-007-0600-8. [DOI] [PubMed] [Google Scholar]
- 29.Scragg P, Jones A, Fauvel N. Psychological problems following ICU treatment. Anaesthesia. 2001;56:9–14. doi: 10.1046/j.1365-2044.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 30.Rattray JE, Johnston M, Wildsmith JA. Predictors of emotional outcomes of intensive care. Anaesthesia. 2005;60:1085–1092. doi: 10.1111/j.1365-2044.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- 31.Sukantarat K, Greer S, Brett S, et al. Physical and psychological sequelae of critical illness. Br J Health Psychol. 2007;12:65–74. doi: 10.1348/135910706X94096. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths J, Fortune G, Barber V, et al. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33:1506–1518. doi: 10.1007/s00134-007-0730-z. [DOI] [PubMed] [Google Scholar]
- 33.Jones C, Griffiths RD, Humphris G, et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29:573–580. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]