Abstract
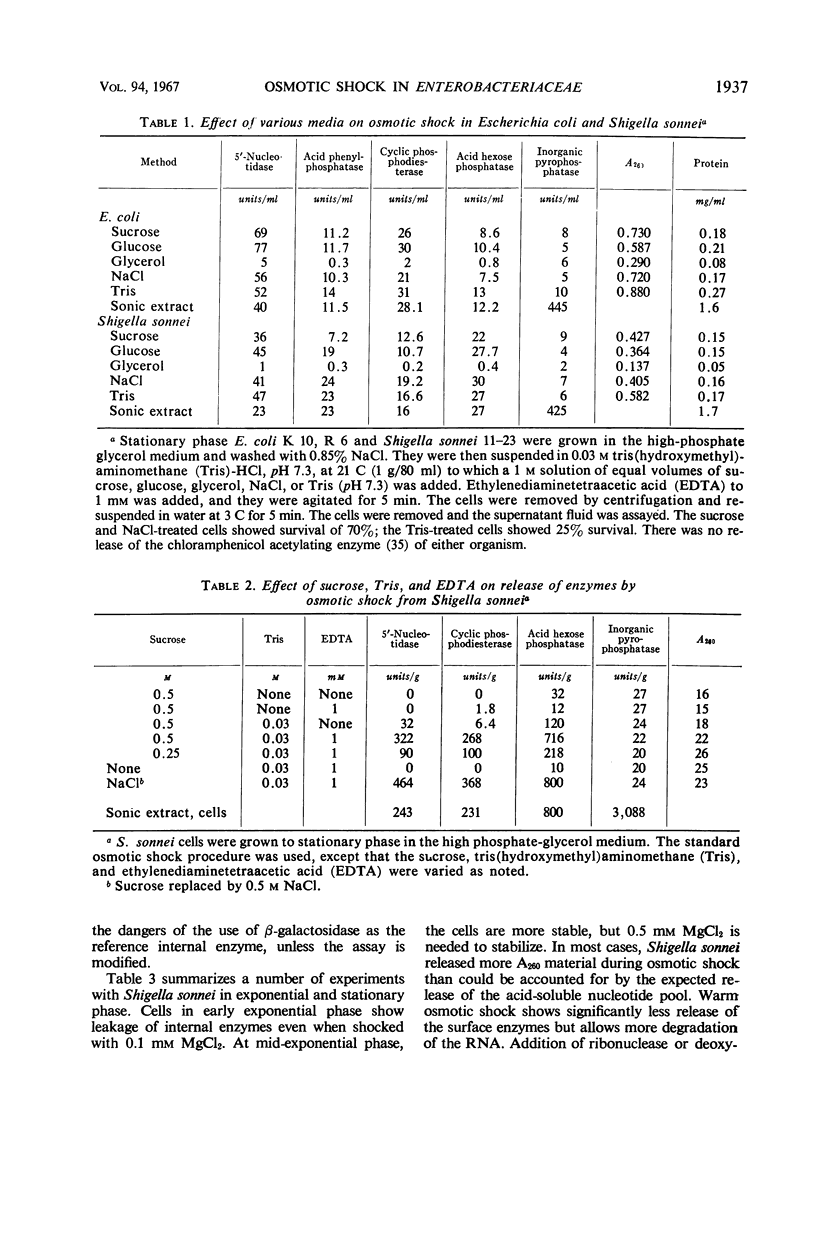
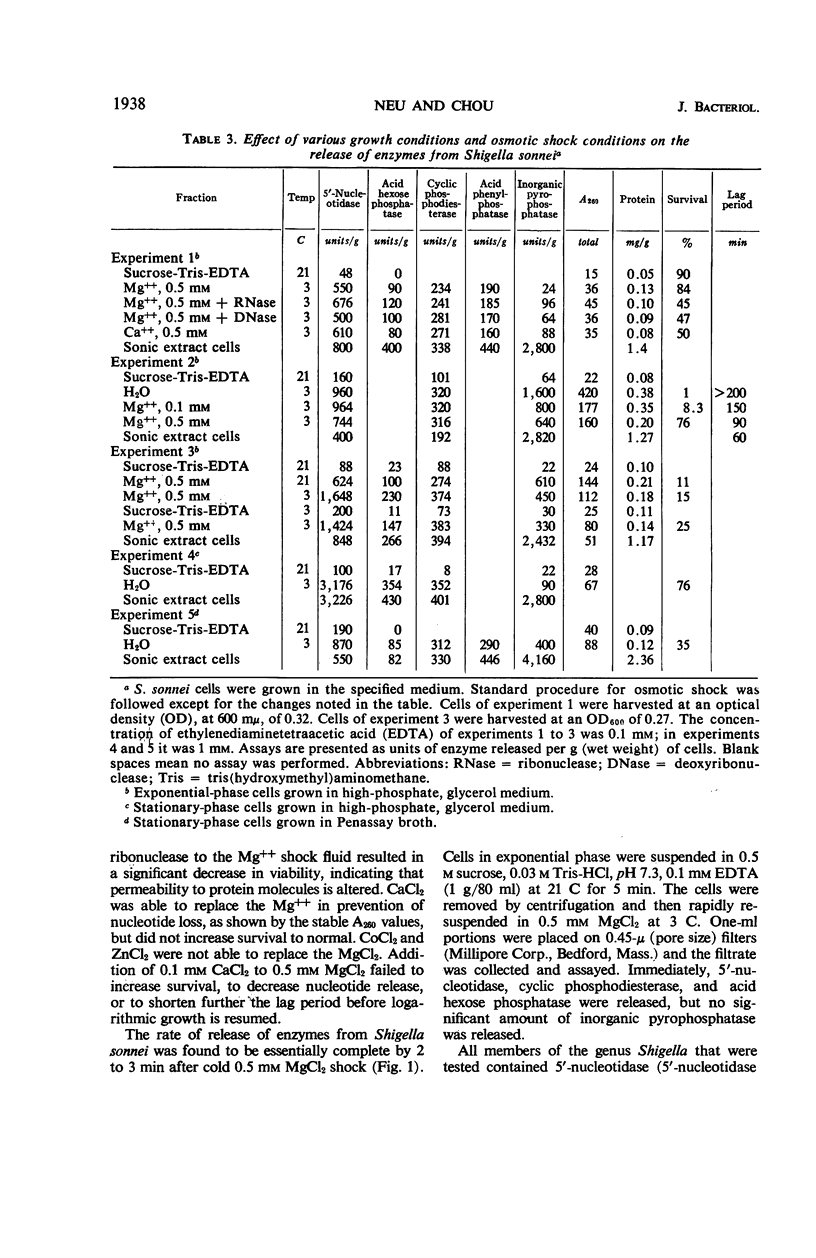
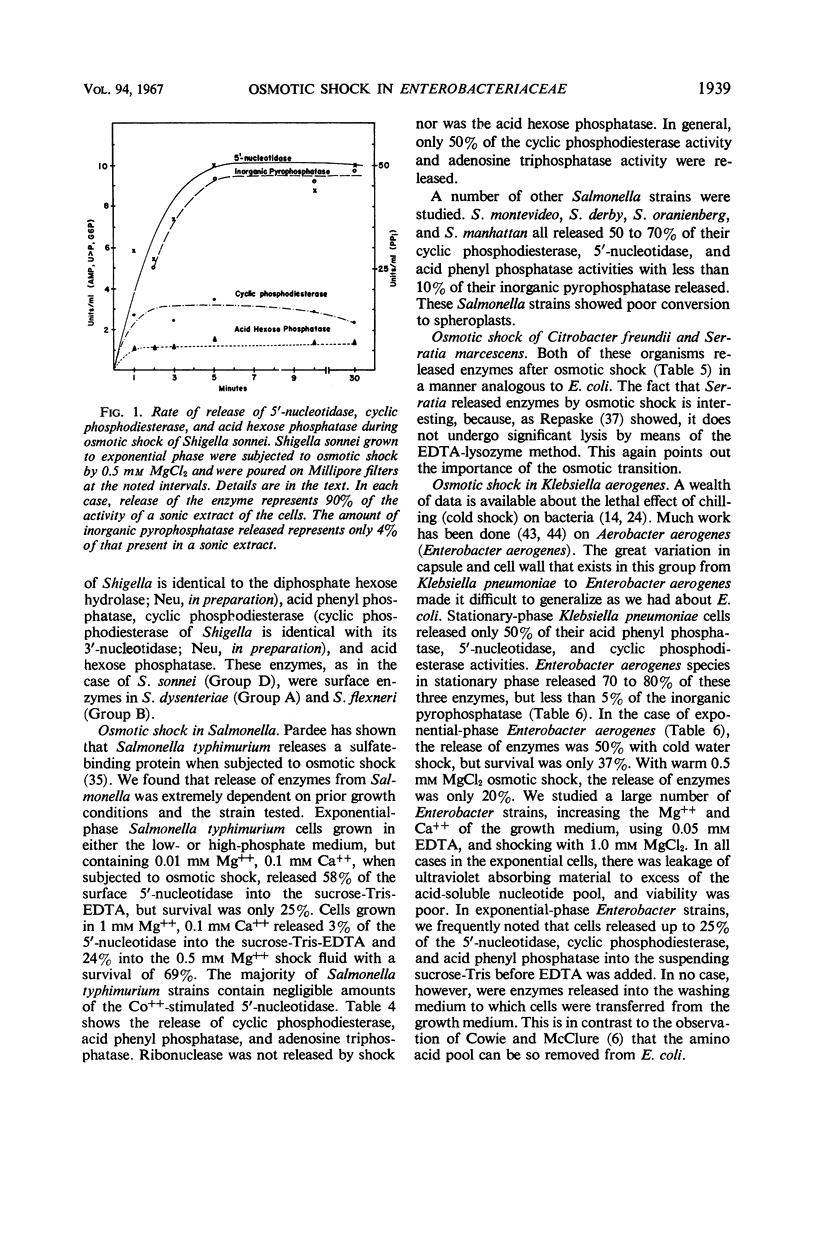
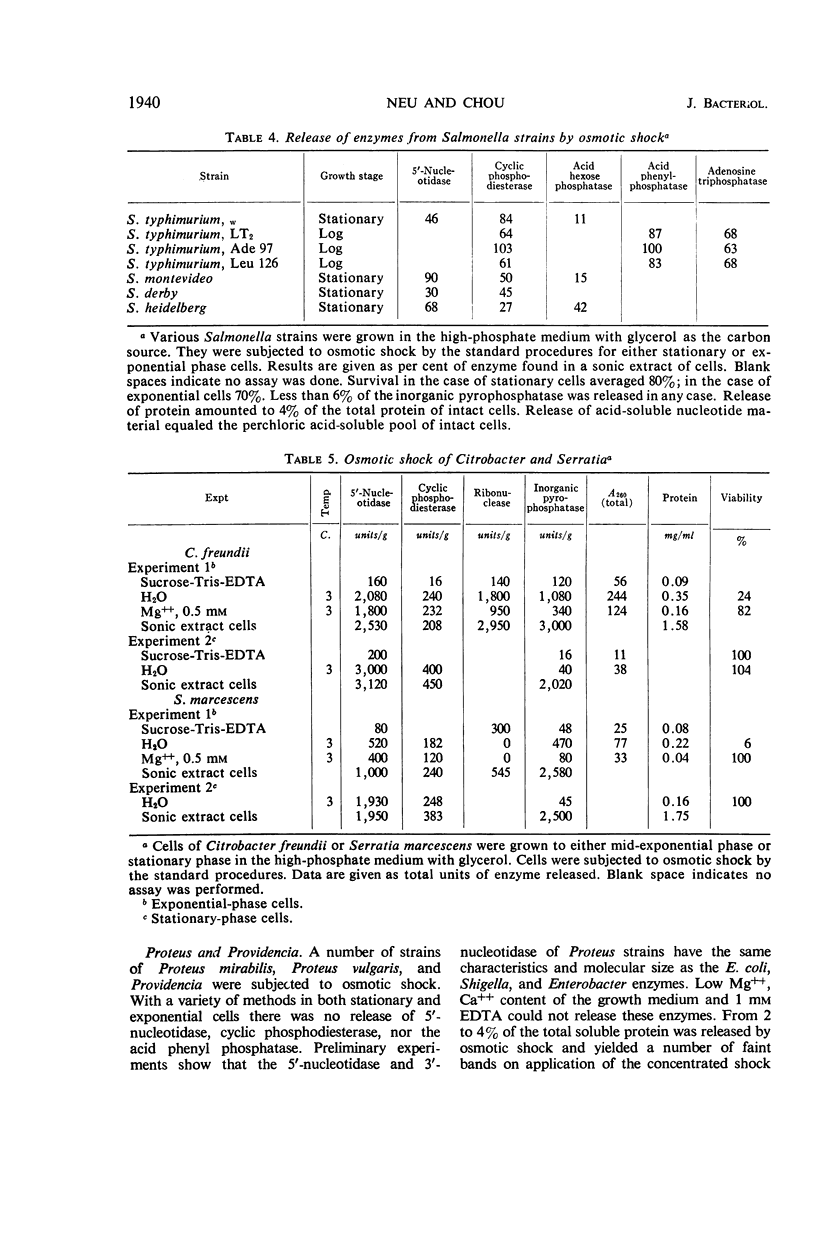
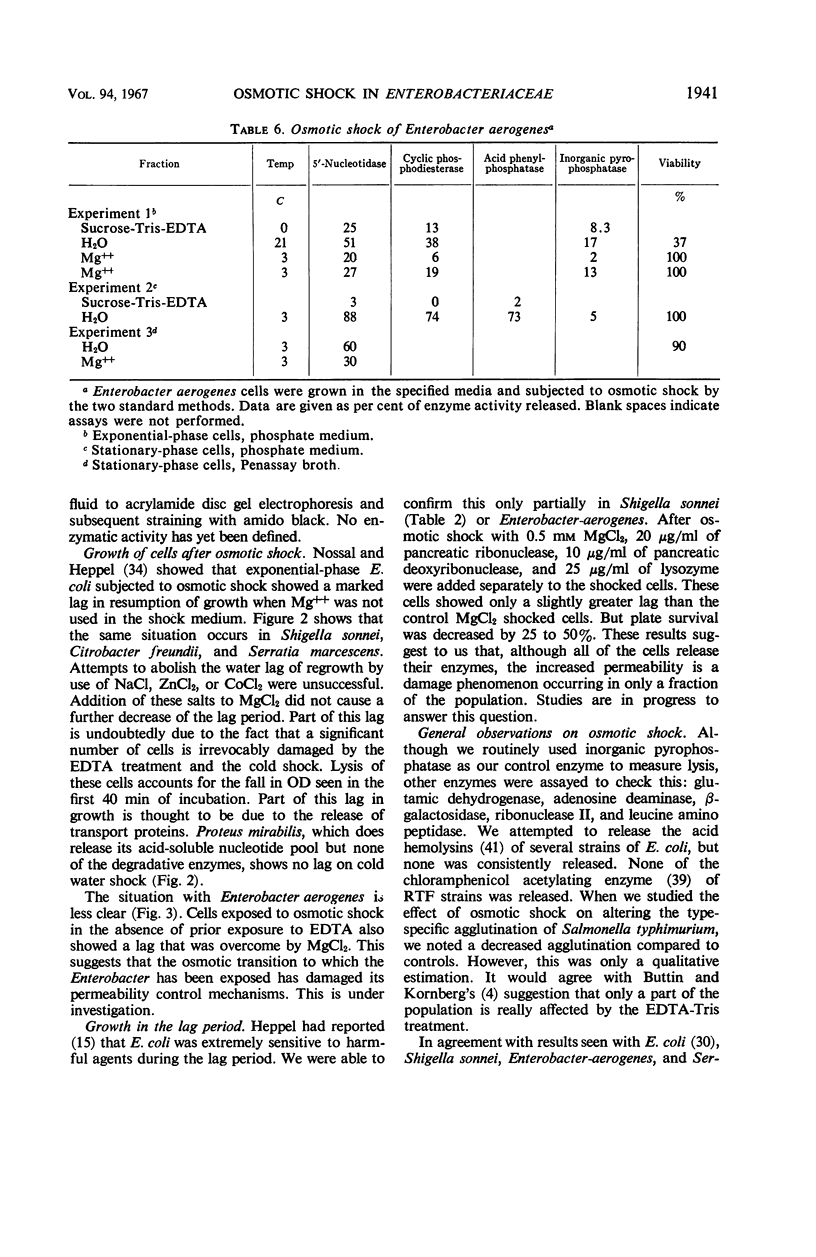
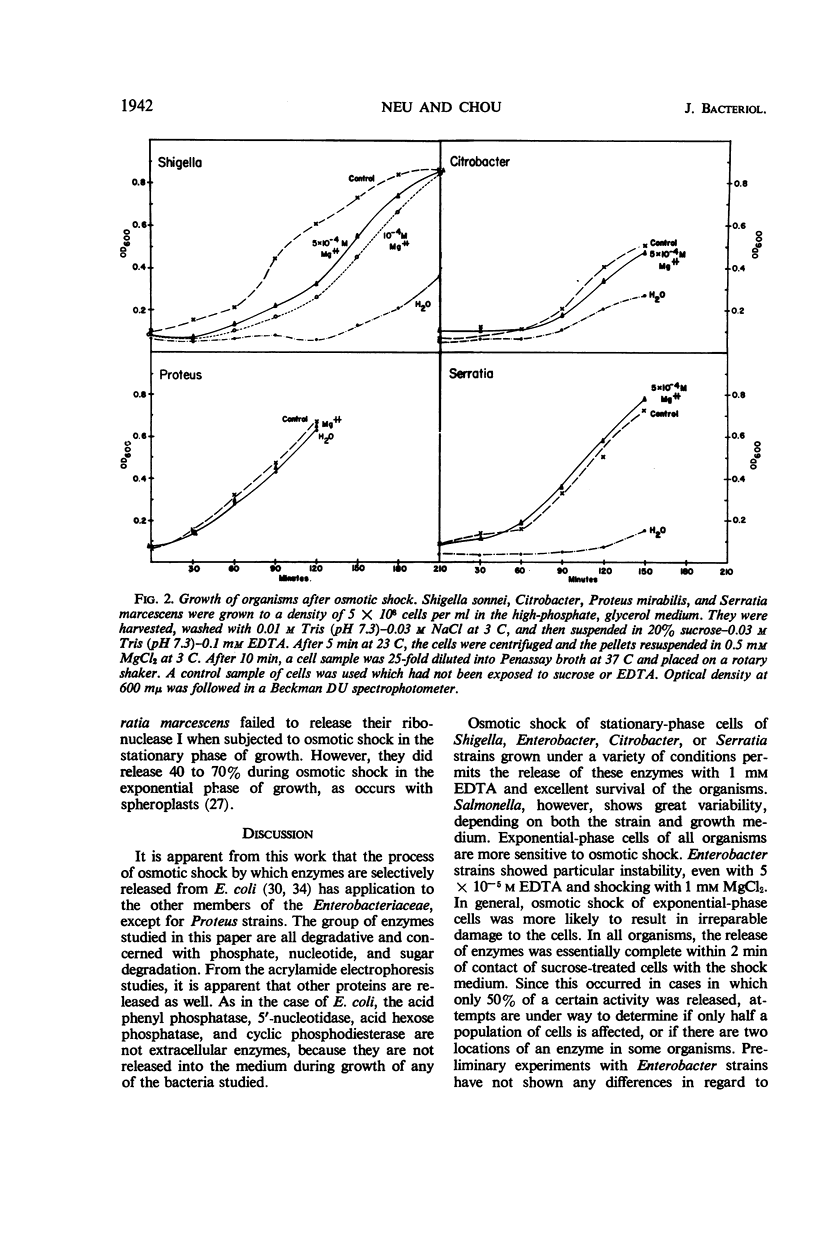
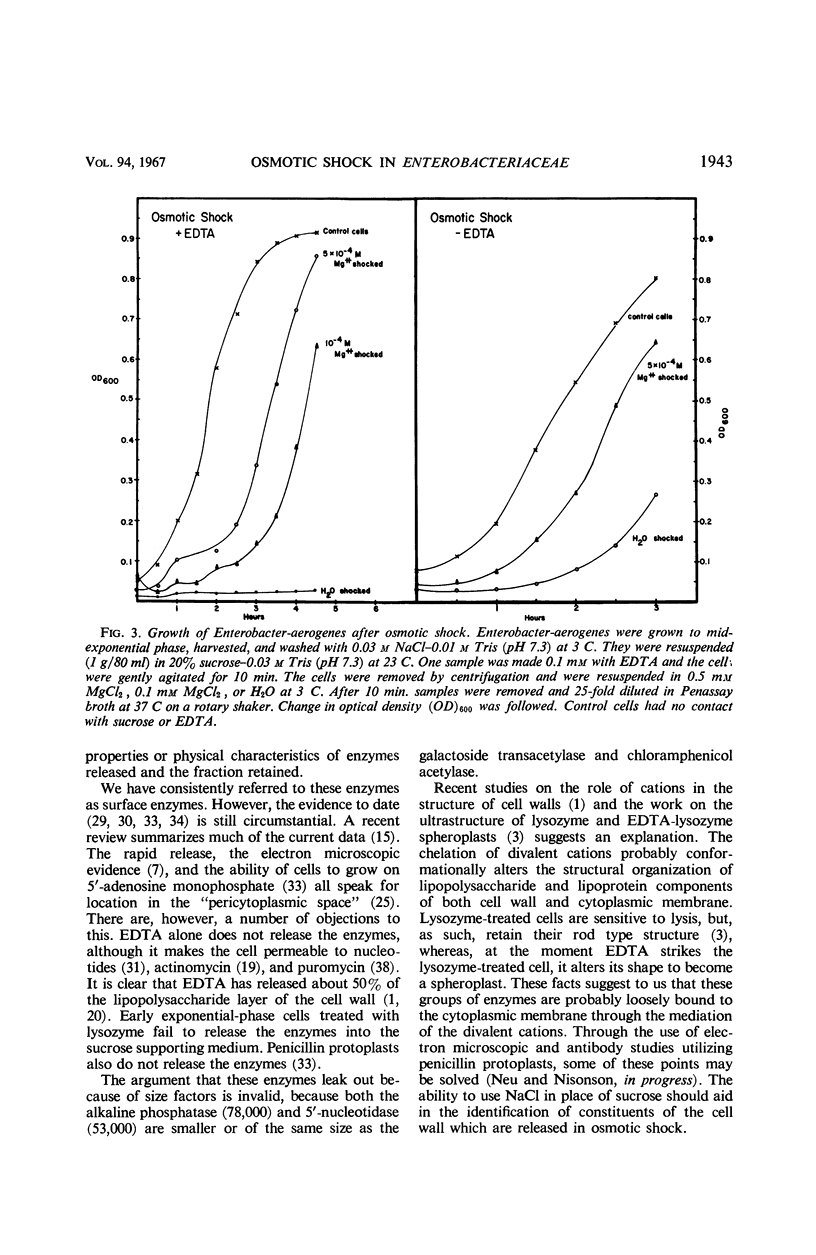
The process of osmotic shock, which has been used to release degradative enzymes from Escherichia coli, can be applied successfully to other members of the Enterobacteriaceae. Cyclic phosphodiesterase (3′-nucleotidase), 5′-nucleotidase (diphosphate sugar hydrolase), acid hexose phosphatase, and acid phenyl phosphatase are released from Shigella, Enterobacter, Citrobacter, and Serratia strains. Some strains of Salmonella also release these enzymes. Members of Proteus and Providencia groups fail to release enzymes when subjected to osmotic shock and do not show a lag in regrowth, although they do release their acid-soluble nucleotide pools. In contrast to E. coli, release of enzymes from other members of the Enterobacteriaceae studied is affected by growth conditions and strain of organism. None of the organisms was as stable to osmotic shock in exponential phase of growth as was E. coli. Exponential-phase cells of Shigella, Enterobacter, and Citrobacter could be shocked only with 0.5 mm MgCl2 to prevent irreparable damage to the cells. These observations suggest that this group of degradative enzymes is probably loosely bound to the cytoplasmic membrane through the mediation of divalent cations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXI. Utilization of deoxyribonucleoside triphosphates by Escherichia coli cells. J Biol Chem. 1966 Nov 25;241(22):5419–5427. [PubMed] [Google Scholar]
- COWIE D. B., McCLURE F. T. Metabolic pools and the synthesis of macromolecules. Biochim Biophys Acta. 1959 Jan;31(1):236–245. doi: 10.1016/0006-3002(59)90460-3. [DOI] [PubMed] [Google Scholar]
- Cordonnier C., Bernardi G. Localization of E. coli endonuclease I. Biochem Biophys Res Commun. 1965 Sep 8;20(5):555–559. doi: 10.1016/0006-291x(65)90434-1. [DOI] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G. The relationship between poly A polymerase and the ribosomes. Biochemistry. 1966 Nov;5(11):3676–3684. doi: 10.1021/bi00875a042. [DOI] [PubMed] [Google Scholar]
- Hegarty C. P., Weeks O. B. Sensitivity of Escherichia coli to Cold-Shock during the Logarithmic Growth Phase. J Bacteriol. 1940 May;39(5):475–484. doi: 10.1128/jb.39.5.475-484.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Kundig W., Kundig F. D., Anderson B., Roseman S. Restoration of active transport of glycosides in Escherichia coli by a component of a phosphotransferase system. J Biol Chem. 1966 Jul 10;241(13):3243–3246. [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. The effect of sudden chilling on Escherichia coli. J Gen Microbiol. 1958 Oct;19(2):380–389. doi: 10.1099/00221287-19-2-380. [DOI] [PubMed] [Google Scholar]
- Melo A., Glaser L. Nucleotide diphosphate hexose pyrophosphatases. Biochem Biophys Res Commun. 1966 Mar 8;22(5):524–531. doi: 10.1016/0006-291x(66)90306-8. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. SOME OBSERVATIONS ON THE "LATENT" RIBONUCLEASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Jun;51:1267–1274. doi: 10.1073/pnas.51.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Neu H. C., Ashman D. F., Price T. D. Effect of ethylenediaminetetraacetic acid-Tris(hydroxymethyl)aminomethane on release of the acid-soluble nucleotide pool and on breakdown of ribosomal ribonucleic acid in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1360–1368. doi: 10.1128/jb.93.4.1360-1368.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Effect of chilling on Aerobacter aerogenes in aqueous suspension. J Gen Microbiol. 1962 Dec;29:719–730. doi: 10.1099/00221287-29-4-719. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., POSTGATE J. R. PENETRATION OF SUBSTANCES INTO COLD-SHOCKED BACTERIA. J Gen Microbiol. 1964 Sep;36:393–403. doi: 10.1099/00221287-36-3-393. [DOI] [PubMed] [Google Scholar]
- Sellin H. G., Srinivasan P. R., Borek E. Studies of a phage-induced DNA methylase. J Mol Biol. 1966 Aug;19(1):219–222. doi: 10.1016/s0022-2836(66)80065-7. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Simmonds S., Toye N. O. Peptidases in spheroplasts of Escherichia coli K-12. J Biol Chem. 1966 Aug 25;241(16):3852–3860. [PubMed] [Google Scholar]
- Snyder I. S., Koch N. A. Production and characteristics of hemolysins of Escherichia coli. J Bacteriol. 1966 Feb;91(2):763–767. doi: 10.1128/jb.91.2.763-767.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C., Bickel W. D., Haskins W. T., Milner K. C., Ribi E. Chemical, biological, and structural properties of stable Proteus L forms and their parent bacteria. J Bacteriol. 1967 Mar;93(3):1143–1159. doi: 10.1128/jb.93.3.1143-1159.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


