Abstract
Purpose
To review what is known about the normal maturation of stereoacuity, the stereoacuity deficits associated with infantile and accommodative esotropia, the rationale for making improved stereoacuity a goal of treatment, and strategies for improving stereoacuity outcomes.
Methods
Studies of stereoacuity maturation during normal development, studies of stereoacuity outcomes following treatment for infantile and accommodative esotropia, and studies of primate models of esotropia are reviewed.
Results
Stereoacuity maturation normally proceeds rapidly during the first year of life. Infantile and accommodative esotropia are associated with profound and permanent disruption of stereopsis. While rehabilitation of stereoacuity following treatment of esotropia remains a challenge, even the achievement of subnormal stereoacuity may have real benefits to the child.
Conclusions
Some abnormalities in stereoacuity may exist before the onset of esotropia, but others may result directly from abnormal binocular experience. Several strategies for improving stereoacuity outcomes in esotropia are currently under active investigation. Improved stereoacuity outcomes are associated with better long term stability of alignment, reduced risk for and/or severity of amblyopia, improved achievement of sensorimotor developmental milestones, better reading ability, and improved long-term quality of life.
Keywords: stereoacuity, infantile esotropia, accommodative esotropia
Normal Maturation of Stereoacuity
Prior to 1980, there was scarcely any information about the normal maturation of stereopsis during infancy. As a result of the development of the forced-choice preferential looking (FPL) method for testing infant visual perception1, we were able for the first time to quantitatively assess stereoacuity maturation. The first studies of infant stereoacuity began at MIT and Vanderbilt University in 1979, with the first publications in 1980.2-4 Since that time, a variety of psychophysical, electrophysiological, and eye movement laboratory protocols for the assessment of local and global stereopsis during infancy and early childhood have been developed.3-16
Regardless of the methodology, most studies agree that stereopsis has an abrupt onset at 3 to 4 months of age and that, during months 4-12, the rate of stereoacuity maturation is rapid 8-11. For example, Figure 1 provides results from several of our own studies that show good agreement among FPL and VEP protocols to study the maturation of local and global stereoacuity during the first year of life. Slower improvement in stereoacuity continues beyond 18 months of age 7, 10, 11. The onset of stereopsis is not determined simply by the maturation of eye alignment nor the maturation of visual acuity17, 18, although there is some evidence that contrast sensitivity may be a limiting factor in rate of stereoacuity improvement with age12. Infants older than 4 months of age are not only able to discriminate binocular disparity but appear to experience the perception of depth. They preferentially look at and track horizontally disparate stimuli that give rise to the perception of depth in adults but not vertically disparate stimuli3, 9. In addition, they have appropriate reaching behavior in response to horizontally disparate targets.19
Figure 1.
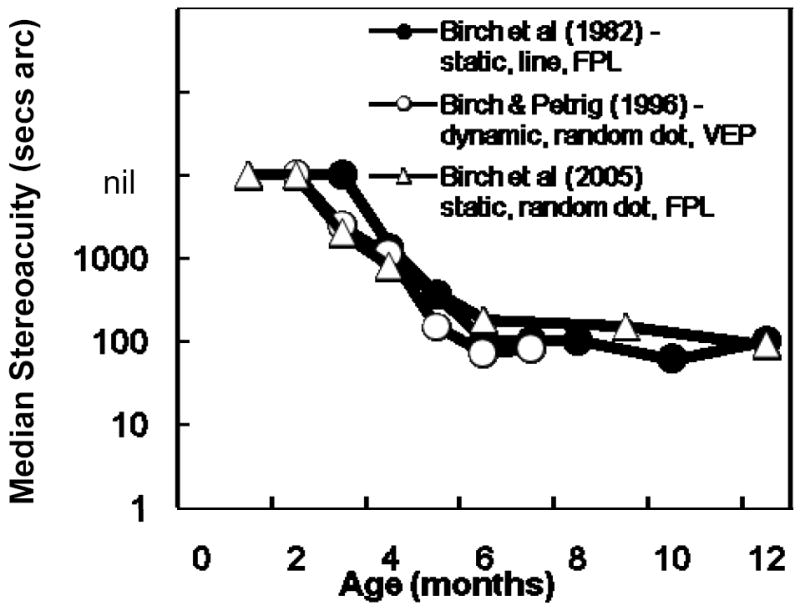
Maturation of local9 and global8, 10 stereoacuity during the first year of life evaluated by forced choice preferential looking (FPL)9, 10 and visual evoked potential (VEP)8 protocols.
Abnormal Stereopsis in Infantile and Accommodative Esotropia
The ability to assess stereopsis during infancy and early childhood has allowed us to examine the causes and rehabilitation of abnormal stereopsis in pediatric patients. Most research in this area has focused on esotropia, a convergent strabismus that typically has an onset during infancy (infantile esotropia) or early childhood (accommodative esotropia). Both infantile esotropia and accommodative esotropia are associated with abnormal binocular sensory function. Recently, clinical research on stereoacuity has been accelerated by the commercial availability of stereoacuity tests for infants and young children, such as the Infant Randot Stereoacuity Cards 10, 11 (which utilize the Teller Acuity Card format devised for rapid infant visual acuity assessment by Teller, Dobson, McDonald and colleagues20, 21), the Randot Preschool Stereoacuity Test22, 23, the Randot Stereo Smile Test 13, and the Distance Randot Stereoacuity Test 24-27. These new tests provide standardized, quick, and valid methods for assessing stereoacuity at near and at distance in infant and pediatric patients with binocular sensory dysfunction.
Infantile esotropia has a prevalence of 0.3 – 1% 28-33; onset of a constant, large angle nasalward misalignment of the visual axes occurs by 6 months of age. Infantile esotropia is associated with an increased risk of amblyopia and abnormal binocular sensory function. Outcomes with regard to amblyopia are generally good. For example, in a recent prospective study of 129 children diagnosed with infantile esotropia by 6 months of age and followed for a minimum of 5 years, over 90% of the children were treated for amblyopia one or more times during follow-up, yet fewer than 10% had persistent amblyopia at the final outcome visit and the majority of persistent cases of amblyopia were mild34. On the other hand, treatment for binocular sensory dysfunction is rarely successful. Even with optical correction and early surgery, less than 0.5% of this prospective cohort developed normal stereoacuity by 5 years of age and over 60% had nil stereoacuity (Figure 2).34
Figure 2.
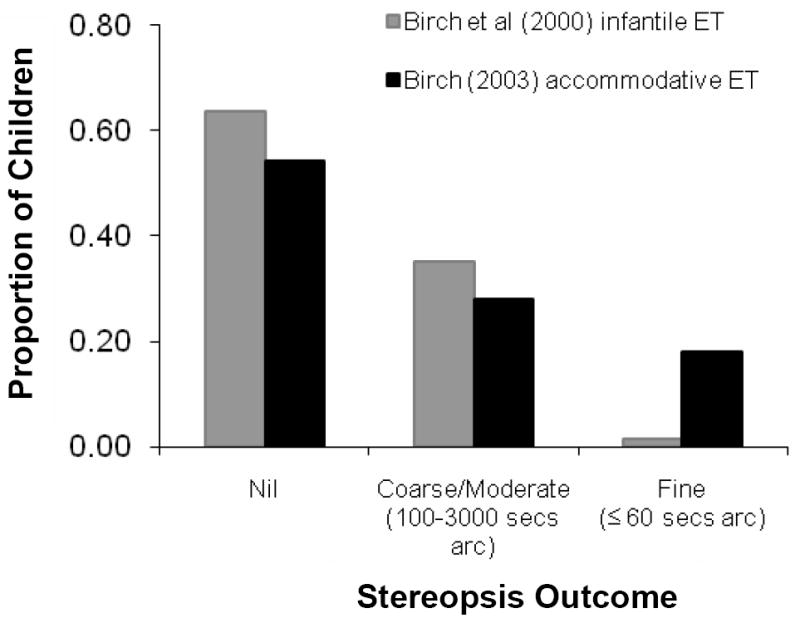
Stereoacuity outcomes at age ≥5 years following treatment for infantile or accommodative esotropia.34, 36
Accommodative esotropia has a prevalence of 1 – 2%28-30, 33, 35 onset of an initially intermittent nasalward misalignment of the visual axes usually begins at 18-48 months of age, associated with hypermetropia and/or an abnormal AC/A ratio. Despite the later age of onset, accommodative esotropia frequently is associated with abnormal binocular sensory function. In fact, even when children with accommodative esotropia were examined at the earliest stage of their disease, while the deviation was still intermittent, over 40% had abnormal stereoacuity 36. Even more children develop deficits in stereoacuity later in the course of the disease, so that only 18% had normal stereoacuity at the final visit 4-11 years later (Figure 2).36
Should Rehabilitation of Stereopsis Be a Goal for Treatment of Esotropia?
In patients with infantile esotropia, even the achievement of subnormal stereoacuity postoperatively may have real benefits to the child. Early surgical correction of infantile esotropia is associated with both improved stereoacuity outcomes34 and improved achievement of sensorimotor developmental milestones during infancy37. This is not surprising since it is appreciated that accurate prehension, particularly grasp-point selection, is known to rely on binocular disparity cues38-40. Stereovision continues to influence acquisition of sensorimotor skills into early childhood, including skills as simple as learning to catch a ball 41. Among kindergarteners and first graders with average intelligence, stereoacuity is correlated with standardized reading scores and teachers’ ratings of reading ability.42, 43
Children with stereopsis also have better long term stability of alignment, meaning that they are less likely to need additional surgery to restore horizontal alignment.44-46 Because there are fewer recurrent and consecutive deviations, stereopsis is also associated with improved long-term quality of life, including self-image, self-confidence, employment opportunities, satisfaction in interpersonal relationships, and success in school and sports.47-51 Stereopsis is also associated with reduced risk for and/or severity of amblyopia.52-54 If stereopsis prevents amblyopia, it may further impact quality of life, because visual acuity of the amblyopic eye has been shown to be an important determinant of quality of life.55
Why Are Stereoacuity Outcomes So Poor in Infantile and Accommodative Esotropia?
There is substantial evidence that the profound stereoacuity deficits associated with infantile esotropia do not result from a congenital absence of binocular sensory function, and do not reflect persistence of a neonatal pre-binocular state.56-60 Instead, the stereodeficit appears after an initial period of normal maturation, suggesting that the abnormality results from prolonged abnormal experience. Data from a primate model of infantile esotropia similarly show an initial period of low vulnerability to abnormal experience, followed by a period of high susceptibility to abnormal experience that peaks just after the onset of stereopsis 61, 62. Moreover, the benefit of early repair of esodeviation is more strongly associated with decreased duration of abnormal experience than with earlier age at repair in both human disease and primate models of infantile esotropia.34, 63 Taken together, the data suggest that very early surgical repair of esodeviation, near the time of onset of stereopsis, might yield excellent stereoacuity outcomes. It was unexpected, then, to find that early surgical repair of infantile esotropia increased the prevalence of stereopsis.34, 44, 64-66 but normal stereoacuity remained a very rare outcome34, 44, 64-66 (Table 1). Possible reasons for this will be addressed later, together with the question of whether or not normal stereoacuity can be achieved in infantile esotropia.
Table 1.
Stereoacuity outcomes at ≥ 5 years of age from children who had surgery for infantile ET.34,44,64-66
| Age at Alignment | Nil | 3000-100 secs arc | ≤ 60 secs arc |
|---|---|---|---|
| ≤6 months | 24% | 75% | 1% |
| 7-12 months | 64% | 35% | 1% |
| 13-18 months | 61% | 38% | 1% |
For several reasons, stereoacuity outcomes following the treatment of accommodative esotropia might be anticipated to be excellent. First, this disease typically has an onset after 18 months of age, after the bulk of stereoacuity maturation is normally complete.67 Second, accommodative esotropia most often presents as an intermittent deviation so that the child enjoys some normal binocular experience even after the onset of disease.67 Third, in many cases, the esodeviation can be eliminated by providing optical correction alone.67 Indeed, there are numerous publications in the literature that utilize a cohort of children with accommodative esotropia as a comparison group for an infantile esotropia cohort, with the implicit assumption that they have normal binocular vision. Somewhat surprisingly, then, even at the earliest intermittent stage, 40% of children with accommodative esotropia have subnormal stereoacuity.22, 68 These stereodeficits may be primary. In some cases, the stereodeficit may be genetic; in other cases, it may be due to other factors that caused a stereodeficit prior to the onset of esotropia such as a perinatal event or anisometropia. There is evidence for a genetic basis for both accommodative esotropia69 and for stereodeficits (primary monofixation syndrome)70 as well as evidence for an association between accommodative esotropia and hyperopic anisometropia.71 Additional evidence for the primary nature of these stereodeficits is that hyperopic refractive error alone is not sufficient to cause accommodative esotropia; 63% of children with ≥+4.00 D and 79% of children with ≥+3.50 D never develop an esodeviation.72, 73 Thus, hyperopic refractive error may need to occur in conjunction with abnormal stereoacuity in order to precipitate the onset of accommodative esotropia, particularly in cases of moderate hyperopia. With additional follow-up, more children with accommodative esotropia develop stereodeficits so that, by age 6 years, less than 20% have normal stereoacuity.69 Periods of constant esotropia of ≥4 months duration, due to failure of spectacle treatment and/or noncompliance with spectacle wear, are associated with permanent stereodeficits even in patients who have normal stereoacuity at the onset of esotropia;74 thus, periods of abnormal binocular experience also may contribute to subnormal stereoacuity outcomes in accommodative esotropia.
Can Normal Stereoacuity Be Achieved in Infantile Esotropia?
On the basis of our 25 years of data on the maturation of stereopsis in infantile esotropia, its susceptibility to disruption by abnormal experience, and its potential for rehabilitation via early surgery34, 46, 56, 60, 64-66, 75, 76, it is clear that despite predictions, early surgery is not sufficient to support the development of normal stereoacuity. Here we consider other factors that may contribute to poor stereoacuity outcomes in infantile esotropia.
First, current surgical techniques for infantile esotropia may not be sufficiently accurate to restore alignment within Panum’s area and support stereopsis22, 77. Panum’s area includes corresponding points on the two retinas as well as small areas of surrounding noncorresponding retinal points that can still be fused with resultant stereopsis. Panum’s area is ±5-20 min of arc (0.1 – 0.6 prism diopter (pd)) in the fovea and alignment within this window may be necessary to support high grade stereoacuity 78. Other authors have suggested that alignment within 2 or 3 deg (4 to 6 pd) is necessary to achieve stereopsis. For example, Tychsen79 states that surgery must realign the eyes within 2.5 to 5 deg (5 to 10 pd) so that the ocular dominance columns (ODCs) mediating fusion in the visual cortex will be separated by no more than 1 to 2 horizontal neuron lengths. Leske & Holmes80 evaluated patients with various angles of strabismus and concluded that stereopsis may only be achievable when the horizontal alignment is within 4 pd. However, as is apparent in the Leske & Holmes80 data set, alignment within 4 pd, while it supports stereopsis, is not sufficient to guarantee high grade stereoacuity.
The hypothesis that subnormal stereoacuity outcomes result from inaccurate surgical alignment is supported by data from children with infantile esotropia who were treated with botulinum toxin rather than surgery. Very early (≤6 months of age) treatment with botulinum toxin results in better stereoacuity outcomes than very early surgical alignment 66, 81, 82(Figure 3). Better stereoacuity outcomes may arise from the temporary incomitance that results from the injection providing an opportunity for the infant to refine alignment to orthotropia by developing a face turn. Note that botulinum appears to be less beneficial for stereoacuity outcomes if the treatment is delayed 81 (Figure 3).
Figure 3.
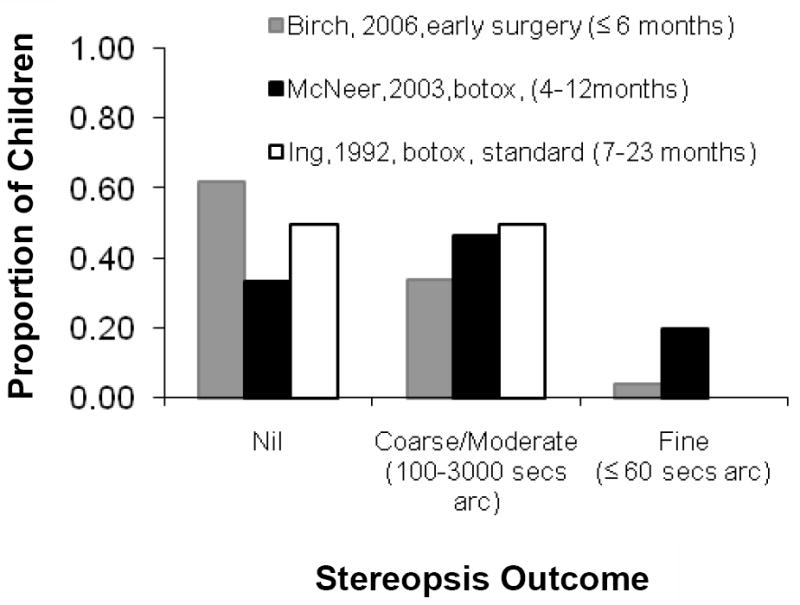
Stereoacuity outcomes at ≥5 years following treatment for infantile esotropia with botulinum toxin at <12 months of age,82 surgery at ≤6 months of age,66 or botulinum toxin at 7-23 months of age.81
Second, early surgery for infantile esotropia (at ≤6 months of age) may still not be early enough. Highly developed stereoacuity is dependent on a normal complement of binocular neurons in the visual cortex. Researchers agree that the presence of highly sensitive disparity encoding mechanisms in the early stages of cortical processing in V1 is a prerequisite for stereopsis in normal subjects83. However, relative disparity appears to be calculated in V2 and stereopsis has been attributed to V284. Using a macaque model of infantile esotropia, Kumagami et al61 have shown that V1 susceptibility to abnormal experience peaks just after the onset of stereopsis. Maruko et al 85 show a similar critical period for V2 with considerable maturation of disparity sensitivity between 2 and 8 weeks of age, that parallels monkey psychophysical stereoacuity development86 and (converting weeks to months) human stereoacuity development. If as these studies suggest, the repair of esotropia must occur before the onset of stereopsis to avoid abnormal binocular suppression in the visual cortex and to achieve the optimal disparity sensitivity, then surgical alignment in humans would need to be completed by 2-3 months of age (corresponding to 2-3 weeks in the macaque). Recently, the Pediatric Eye Disease Investigator Group has defined a clinical profile for which persistence of esotropia was sufficiently likely (>98%) that early surgery could be considered, namely <+3.00D and ≥40 pd esotropia on at least 2 visits at ≥2.5 months of age 87. This profile could be used to identify patients that may be excellent candidates for very early surgery.
Third, even a brief delay (≤3 months) in surgery from the time of onset of esotropia may be too long. Evidence from a macaque monkey model of infantile esotropia shows that V1 can be severely affected by very brief periods of abnormal visual experience. Mori et al62 found that a brief 2-week misalignment after the emergence of stereopsis is sufficient to drastically reduce the functional binocular connections in V1, and longer periods of strabismus result in little additional loss in disparity sensitivity. Zhang et al.88 found that 3 days of optically imposed strabismus (decorrelation) at the peak of the critical period strikingly altered the V1 cortical circuits that support binocular vision. Thus, V1 is extremely sensitive to very brief periods of interocular decorrelation of input signals just after the onset of stereopsis. In human infants, surgery for infantile esotropia is associated with better stereopsis outcomes when the surgery is completed early enough to limit the duration of misalignment to less than 3 months 34. Similarly, Tychsen79, working with a macaque monkey model of infantile esotropia, found that the visual cortex could repair horizontal connections as long as the image decorrelation caused by esotropia was repaired by 3 weeks of age (equivalent to 3 months in humans); a full complement of V1 connections with normal metabolic activity (no suppression) was recovered. Both data from animal models and human clinical data suggest that delay between the onset of infantile esotropia and the age at surgical alignment is a critical factor in determining binocular sensory outcomes. Based on these data, Tychsen79 proposed that alignment within 60 days of onset of esotropia is necessary to achieve high grade stereoacuity.
Fourth, while there is substantial evidence that a congenital absence of binocular sensory function is not found in infantile esotropia 56-60, the possibility remains that binocular function is abnormal. There may be genetic, gestational, or perinatal factors that subtly affect brain development. Evidence from identical twins shows precision of stereoscopic depth perception may be heritable89. The fact that the prevalence of primary monofixation syndrome in parents of children with congenital esotropia90 is higher than in the general population also supports the hypothesis that a hereditary abnormality in disparity sensitivity may be associated with infantile esotropia.
In summary, stereoacuity maturation normally proceeds rapidly during the first year of life. Infantile and accommodative esotropia are associated with profound and permanent disruption of stereopsis. Some abnormalities in stereoacuity may exist before the onset of esotropia, but others may result directly from abnormal binocular experience. Maintaining or rehabilitating normal stereoacuity remains a challenge, especially with our limited understanding of genetic factors that are associated with esotropia. However, several strategies for improving stereoacuity outcomes in esotropia are currently under active investigation. New surgical approaches and devices that may improve alignment accuracy are currently under development. In addition, new approaches to early accurate diagnosis are being developed so that very early surgery will be an option. Improved stereoacuity outcomes are associated with better long term stability of alignment, reduced risk for and/or severity of amblyopia, improved achievement of sensorimotor developmental milestones, better reading ability, and improved long-term quality of life.
Acknowledgments
This research was supported in part by NIH EY05236.
Footnotes
The authors have no commercial associations that constitute a conflict of interest with any aspect of the submitted manuscript.
References
- 1.Teller DY, Boothe R. Development of vision in infant primates. Trans Ophthalmol Soc U K. 1979;99:333–7. [PubMed] [Google Scholar]
- 2.Held R, Birch EE, Gwiazda J. Stereoacuity of human infants. Proc Natl Acad Sci U S A. 1980;77:5572–4. doi: 10.1073/pnas.77.9.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox R, Aslin RN, Shea SL, Dumais ST. Stereopsis in human infants. Science. 1980;207:323–4. doi: 10.1126/science.7350666. [DOI] [PubMed] [Google Scholar]
- 4.Shea SL, Fox R, Aslin RN, Dumais ST. Assessment of stereopsis in human infants. Invest Ophthalmol Vis Sci. 1980;19:1400–4. [PubMed] [Google Scholar]
- 5.Archer SM, Helveston EM, Miller KK, Ellis FD. Stereopsis in normal infants and infants with congenital esotropia. Am J Ophthalmol. 1986;101:591–6. doi: 10.1016/0002-9394(86)90950-5. [DOI] [PubMed] [Google Scholar]
- 6.Birch EE, Fawcett SL, Morale SE, Jeffrey BG, O’Connor AR. Measurement of stereoacuity outcomes during infancy: infant random dot stereocards. Invest Ophthalmol Vis Sci. 2002;43 E-Abstract 2937. [Google Scholar]
- 7.Birch EE, Hale L. Operant assessment of stereoacuity. Clin Vis Sci. 1989;4:295–300. [Google Scholar]
- 8.Birch EE, Petrig B. FPL and VEP measures of fusion, stereopsis and stereoacuity in normal infants. Vision Res. 1996;36:1321–7. doi: 10.1016/0042-6989(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 9.Birch EE, Gwiazda J, Held R. Stereoacuity development for crossed and uncrossed disparities in human infants. Vision Res. 1982;22:507–13. doi: 10.1016/0042-6989(82)90108-0. [DOI] [PubMed] [Google Scholar]
- 10.Birch EE, Morale SE, Jeffrey BG, O’Connor AR, Fawcett SL. Measurement of stereoacuity outcomes at ages 1 to 24 months: Randot Stereocards. J AAPOS. 2005;9:31–6. doi: 10.1016/j.jaapos.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Birch EE, Salomao S. Infant random dot stereoacuity cards. J Pediatr Ophthalmol Strabismus. 1998;35:86–90. doi: 10.3928/0191-3913-19980301-06. [DOI] [PubMed] [Google Scholar]
- 12.Brown AM, Lindsey DT, Satgunam P, Miracle JA. Critical immaturities limiting infant binocular stereopsis. Invest Ophthalmol Vis Sci. 2007;48:1424–34. doi: 10.1167/iovs.06-0718. [DOI] [PubMed] [Google Scholar]
- 13.Ciner EB, Schanel-Klitsch E, Herzberg C. Stereoacuity development: 6 months to 5 years. A new tool for testing and screening. Optom Vis Sci. 1996;73:43–8. doi: 10.1097/00006324-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Leguire L, Rogers G, Fellows R. Toward a clinical test for stereopsis in human infants. Invest Ophthalmol Vis Sci. 1983;24 [Google Scholar]
- 15.Petrig B, Julesz B, Kropfl W, Baumgartner G, Anliker M. Development of stereopsis and cortical binocularity in human infants: electrophysiological evidence. Science. 1981;213:1402–5. doi: 10.1126/science.7268443. [DOI] [PubMed] [Google Scholar]
- 16.Wattam-Bell J. Stereoscopic and motion Dmax in adults and infants. Invest Ophthalmol Vis Sci. 1995;36:S910. [Google Scholar]
- 17.Birch EE. Stereopsis in infants and its developmental relationship to visual acuity. In: Simons K, editor. Early Visual Development: Normal and Abnormal. New York: Oxford University Press; 1993. pp. 224–36. [Google Scholar]
- 18.Birch EE, Gwiazda J, Held R. The development of vergence does not account for the onset of stereopsis. Perception. 1983;12:331–6. doi: 10.1068/p120331. [DOI] [PubMed] [Google Scholar]
- 19.Granrud CE. Binocular vision and spatial perception in 4-and 5-month-old infants. J Exp Psychol Hum Percept Perform. 1986;12:36–49. doi: 10.1037//0096-1523.12.1.36. [DOI] [PubMed] [Google Scholar]
- 20.Dobson V, McDonald MA, Kohl P, Stern N, Samek M, Preston K. Visual acuity screening of infants and young children with the acuity card procedure. J Am Optom Assoc. 1986;57:284–9. [PubMed] [Google Scholar]
- 21.Teller DY, McDonald MA, Preston K, Sebris SL, Dobson V. Assessment of visual acuity in infants and children: the acuity card procedure. Dev Med Child Neurol. 1986;28:779–89. doi: 10.1111/j.1469-8749.1986.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 22.Birch EE, Williams C, Drover J, et al. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS. 2008;12:23–6. doi: 10.1016/j.jaapos.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birch EE, Williams C, Hunter J, Lapa MC. Random dot stereoacuity of preschool children. ALSPAC “Children in Focus” Study Team. J Pediatr Ophthalmol Strabismus. 1997;34:217–22. doi: 10.3928/0191-3913-19970701-08. quiz 247-8. [DOI] [PubMed] [Google Scholar]
- 24.Adams WE, Leske DA, Hatt SR, Mohney BG, Birch EE, Weakley DR, Jr, Holmes JM. Improvement in distance stereoacuity following surgery for intermittent exotropia. J AAPOS. 2008;12:141–4. doi: 10.1016/j.jaapos.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu VL, Birch EE, Holmes JM. Assessment of a new Distance Randot stereoacuity test. J AAPOS. 2006;10:419–23. doi: 10.1016/j.jaapos.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Holmes JM, Birch EE, Leske DA, Fu VL, Mohney BG. New tests of distance stereoacuity and their role in evaluating intermittent exotropia. Ophthalmology. 2007;114:1215–20. doi: 10.1016/j.ophtha.2006.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leske DA, Birch EE, Holmes JM. Real depth vs randot stereotests. Am J Ophthalmol. 2006;142:699–701. doi: 10.1016/j.ajo.2006.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman Z, Neumann E, Hyams SW, Peleg B. Ophthalmic screening of 38,000 children, age 1 to 2 1/2 years, in child welfare clinics. J Pediatr Ophthalmol Strabismus. 1980;17:261–7. doi: 10.3928/0191-3913-19800701-16. [DOI] [PubMed] [Google Scholar]
- 29.Graham PA. Epidemiology of strabismus. Br J Ophthalmol. 1974;58:224–31. doi: 10.1136/bjo.58.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg AE, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood esotropia: a population-based study. Ophthalmology. 2007;114:170–4. doi: 10.1016/j.ophtha.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 31.Kornder LD, Nursey JN, Pratt-Johnson JA, Beattie A. Detection of manifest strabismus in young children. I. A prospective study. Am J Ophthalmol. 1974;77:207–10. doi: 10.1016/0002-9394(74)90674-6. [DOI] [PubMed] [Google Scholar]
- 32.Stayte M, Johnson A, Wortham C. Ocular and visual defects in a geographically defined population of 2-year-old children. Br J Ophthalmol. 1990;74:465–8. doi: 10.1136/bjo.74.8.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U. S. Department of Health Education and Welfare. Natl Vital and Health Statistics, Series 11. Rockville, MD: U.S National Center for Health Statistics; 1972. Eye Examination Findings among Children,United States. [PubMed] [Google Scholar]
- 34.Birch EE, Fawcett S, Stager DR. Why does early surgical alignment improve stereoacuity outcomes in infantile esotropia? J AAPOS. 2000;4:10–4. doi: 10.1016/s1091-8531(00)90005-3. [DOI] [PubMed] [Google Scholar]
- 35.Chew E, Remaley NA, Tamboli A, Zhao J, Podgor MJ, Klebanoff M. Risk factors for esotropia and exotropia. Arch Ophthalmol. 1994;112:1349–55. doi: 10.1001/archopht.1994.01090220099030. [DOI] [PubMed] [Google Scholar]
- 36.Birch EE. Binocular sensory function in accommodative esotropia. J AAPOS. 2003;7:369–73. doi: 10.1016/j.jaapos.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Drover JR, Stager DR, Sr, Morale SE, Leffler JN, Birch EE. Improvement in motor development following surgery for infantile esotropia. J AAPOS. 2008;12:136–40. doi: 10.1016/j.jaapos.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradshaw MF, Elliott KM, Watt SJ, Hibbard PB, Davies IR, Simpson PJ. Binocular cues and the control of prehension. Spat Vis. 2004;17:95–110. doi: 10.1163/156856804322778288. [DOI] [PubMed] [Google Scholar]
- 39.Melmoth DR, Storoni M, Todd G, Finlay AL, Grant S. Dissociation between vergence and binocular disparity cues in the control of prehension. Exp Brain Res. 2007;183:283–98. doi: 10.1007/s00221-007-1041-x. [DOI] [PubMed] [Google Scholar]
- 40.Watt SJ, Bradshaw MF. The visual control of reaching and grasping: binocular disparity and motion parallax. J Exp Psychol Hum Percept Perform. 2003;29:404–15. doi: 10.1037/0096-1523.29.2.404. [DOI] [PubMed] [Google Scholar]
- 41.Mazyn LI, Lenoir M, Montagne G, Delaey C, Savelsbergh GJ. Stereo vision enhances the learning of a catching skill. Exp Brain Res. 2007;179:723–6. doi: 10.1007/s00221-007-0957-5. [DOI] [PubMed] [Google Scholar]
- 42.Kulp MT, Schmidt PP. Visual predictors of reading performance in kindergarten and first grade children. Optom Vis Sci. 1996;73:255–62. doi: 10.1097/00006324-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Kulp MT, Schmidt PP. A pilot study. Depth perception and near stereoacuity: is it related to academic performance in young children? Binocul Vis Strabismus Q. 2002;17:129–34. [PubMed] [Google Scholar]
- 44.Birch E, Stager D, Wright K, Beck R. The natural history of infantile esotropia during the first six months of life. Pediatric Eye Disease Investigator Group. J AAPOS. 1998;2:325–8. doi: 10.1016/s1091-8531(98)90026-x. [DOI] [PubMed] [Google Scholar]
- 45.Birch EE, Fawcett SL, Stager DR., Sr Risk factors for the development of accommodative esotropia following treatment for infantile esotropia. J AAPOS. 2002;6:174–81. doi: 10.1067/mpa.2002.122962. [DOI] [PubMed] [Google Scholar]
- 46.Birch EE, Stager DR, Sr, Berry P, Leffler J. Stereopsis and long-term stability of alignment in esotropia. J AAPOS. 2004;8:146–50. doi: 10.1016/j.jaapos.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Coats DK, Paysse EA, Towler AJ, Dipboye RL. Impact of large angle horizontal strabismus on ability to obtain employment. Ophthalmology. 2000;107:402–5. doi: 10.1016/s0161-6420(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 48.Menon V, Saha J, Tandon R, Mehta M, Khokhar S. Study of the psychosocial aspects of strabismus. J Pediatr Ophthalmol Strabismus. 2002;39:203–8. doi: 10.3928/0191-3913-20020701-07. [DOI] [PubMed] [Google Scholar]
- 49.Olitsky SE, Sudesh S, Graziano A, Hamblen J, Brooks SE, Shaha SH. The negative psychosocial impact of strabismus in adults. J AAPOS. 1999;3:209–11. doi: 10.1016/s1091-8531(99)70004-2. [DOI] [PubMed] [Google Scholar]
- 50.Satterfield D, Keltner JL, Morrison TL. Psychosocial aspects of strabismus study. Arch Ophthalmol. 1993;111:1100–5. doi: 10.1001/archopht.1993.01090080096024. [DOI] [PubMed] [Google Scholar]
- 51.Packwood EA, Cruz OA, Rychwalski PJ, Keech RV. The psychosocial effects of amblyopia study. J AAPOS. 1999;3:15–7. doi: 10.1016/s1091-8531(99)70089-3. [DOI] [PubMed] [Google Scholar]
- 52.Bosworth R, Birch E. Binocular function and optotype-grating acuity discrepancies in amblyopic children. Invest Ophthalmol Vis Sci. 2003;43 E-Abstract 3183. [Google Scholar]
- 53.Bosworth R, Borowy C, Lapa MC, Salomao SR, Birch EE. Relationship between stereo acuity and severity of amblyopia in children with strabismus and anisometropia. Paper presented at the AAPOS meeting; Washington, DC. March 2004. [Google Scholar]
- 54.McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- 55.van de Graaf ES, van der Sterre GW, van Kempen-du Saar H, Simonsz B, Looman CW, Simonsz HJ. Amblyopia and Strabismus Questionnaire (A&SQ): clinical validation in a historic cohort. Graefes Arch Clin Exp Ophthalmol. 2007;245:1589–95. doi: 10.1007/s00417-007-0594-5. [DOI] [PubMed] [Google Scholar]
- 56.Birch EE, Stager DR. Monocular acuity and stereopsis in infantile esotropia. Invest Ophthalmol Vis Sci. 1985;26:1624–30. [PubMed] [Google Scholar]
- 57.Birch EE, Fawcett S, Stager D. Co-development of VEP motion response and binocular vision in normal infants and infantile esotropes. Invest Ophthalmol Vis Sci. 2000;41:1719–23. [PubMed] [Google Scholar]
- 58.Bosworth RG, Birch EE. Motion detection in normal infants and young patients with infantile esotropia. Vision Res. 2005;45:1557–67. doi: 10.1016/j.visres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Bosworth RG, Birch EE. Direction-of-motion detection and motion VEP asymmetries in normal children and children with infantile esotropia. Invest Ophthalmol Vis Sci. 2007;48:5523–31. doi: 10.1167/iovs.07-0666. [DOI] [PubMed] [Google Scholar]
- 60.Stager DR, Birch EE. Preferential-looking acuity and stereopsis in infantile esotropia. J Pediatr Ophthalmol Strabismus. 1986;23:160–5. doi: 10.3928/0191-3913-19860701-03. [DOI] [PubMed] [Google Scholar]
- 61.Kumagami T, Zhang B, Smith EL, 3rd, Chino YM. Effect of onset age of strabismus on the binocular responses of neurons in the monkey visual cortex. Invest Ophthalmol Vis Sci. 2000;41:948–54. [PubMed] [Google Scholar]
- 62.Mori T, Matsuura K, Zhang B, Smith EL, 3rd, Chino YM. Effects of the duration of early strabismus on the binocular responses of neurons in the monkey visual cortex (V1) Invest Ophthalmol Vis Sci. 2002;43:1262–9. [PubMed] [Google Scholar]
- 63.Tychsen L. Causing and curing infantile esotropia in primates: the role of decorrelated binocular input (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:564–93. [PMC free article] [PubMed] [Google Scholar]
- 64.Birch EE, Stager DR, Berry P, Everett ME. Prospective assessment of acuity and stereopsis in amblyopic infantile esotropes following early surgery. Invest Ophthalmol Vis Sci. 1990;31:758–65. [PubMed] [Google Scholar]
- 65.Birch EE, Stager DR, Everett ME. Random dot stereoacuity following surgical correction of infantile esotropia. J Pediatr Ophthalmol Strabismus. 1995;32:231–5. doi: 10.3928/0191-3913-19950701-07. [DOI] [PubMed] [Google Scholar]
- 66.Birch EE, Stager DR., Sr Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS. 2006;10:409–13. doi: 10.1016/j.jaapos.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 67.American Academy of Ophthalmology. Basic Science and Clinical Course. San Francisco, CA: American Academy of Ophthalmology; 2005. Pediatric Ophthalmology and Strabismus. [Google Scholar]
- 68.Birch EE, Fawcett SL, Morale SE, Weakley DR, Jr, Wheaton DH. Risk factors for accommodative esotropia among hypermetropic children. Invest Ophthalmol Vis Sci. 2005;46:526–9. doi: 10.1167/iovs.04-0618. [DOI] [PubMed] [Google Scholar]
- 69.Birch EE. Marshall Parks lecture. Binocular sensory outcomes in accommodative ET. J AAPOS. 2003;7:369–73. doi: 10.1016/j.jaapos.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Scripture K, Helveston EM. Stereo vision in parents of children with congenital esotropia. AAPOS. 1992 [Google Scholar]
- 71.Weakley DR, Jr, Birch E, Kip K. The role of anisometropia in the development of accommodative esotropia. J AAPOS. 2001;5:153–7. doi: 10.1067/mpa.2001.114662. [DOI] [PubMed] [Google Scholar]
- 72.Atkinson J. Infant vision screening: prediction and prevention of strabismus and amblyopia from refractive screening in the Cambridge Photorefractive Program. In: Simons K, editor. Early Visual Development: Normal and Abnormal. New York: Oxford University Press; 1993. pp. 335–48. [Google Scholar]
- 73.Dobson V, Sebris SL. Longitudinal study of acuity and stereopsis in infants with or at-risk for esotropia. Invest Ophthalmol Vis Sci. 1989;30:1146–58. [PubMed] [Google Scholar]
- 74.Fawcett S, Leffler J, Birch EE. Factors influencing stereoacuity in accommodative esotropia. J AAPOS. 2000;4:15–20. doi: 10.1016/s1091-8531(00)90006-5. [DOI] [PubMed] [Google Scholar]
- 75.Birch EE, Felius J, Stager DR, Sr, Weakley DR, Jr, Bosworth RG. Pre-operative stability of infantile esotropia and post-operative outcome. Am J Ophthalmol. 2004;138:1003–9. doi: 10.1016/j.ajo.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 76.Birch EE, Shimojo S, Held R. Preferential-looking assessment of fusion and stereopsis in infants aged 1-6 months. Invest Ophthalmol Vis Sci. 1985;26:366–70. [PubMed] [Google Scholar]
- 77.Tran HM, Mims JL, 3rd, Wood RC. A new dose-response curve for bilateral medial rectus recessions for infantile esotropia. J AAPOS. 2002;6:112–9. doi: 10.1067/mpa.2002.121617. [DOI] [PubMed] [Google Scholar]
- 78.Evans B, Doshi S. Binocular Vision and Orthoptics: Investigation and Management. Boston: Butterworth-Heinemann; 2001. [Google Scholar]
- 79.Tychsen L. Can ophthalmologists repair the brain in infantile esotropia? Early surgery, stereopsis, monofixation syndrome, and the legacy of Marshall Parks. J AAPOS. 2005;9:510–21. doi: 10.1016/j.jaapos.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Leske DA, Holmes JM. Maximum angle of horizontal strabismus consistent with true stereopsis. J AAPOS. 2004;8:28–34. doi: 10.1016/j.jaapos.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Ing MR. Botulinum alignment for congenital esotropia. Trans Am Ophthalmol Soc. 1992;90:361–7. discussion 367-71. [PMC free article] [PubMed] [Google Scholar]
- 82.McNeer KW, Tucker MG, Guerry CH, Spencer RF. Incidence of stereopsis after treatment of infantile esotropia with botulinum toxin A. J Pediatr Ophthalmol Strabismus. 2003;40:288–92. doi: 10.3928/0191-3913-20030901-10. [DOI] [PubMed] [Google Scholar]
- 83.Chino YM, Smith EL, 3rd, Hatta S, Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosci. 1997;17:296–307. doi: 10.1523/JNEUROSCI.17-01-00296.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen G, Lu HD, Roe AW. A map for horizontal disparity in monkey V2. Neuron. 2008;58:442–50. doi: 10.1016/j.neuron.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maruko I, Zhang B, Tao X, Tong J, Smith EL, 3rd, Chino YM. Postnatal development of disparity sensitivity in visual area 2 (v2) of macaque monkeys. J Neurophysiol. 2008;100:2486–95. doi: 10.1152/jn.90397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Dell C, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vision Res. 1997;37:2675–84. doi: 10.1016/s0042-6989(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 87.The Pediatric Eye Disease Investigator Group (PEDIG) Spontaneous resolution of early-onset esotropia: experience of the Congenital Esotropia Observational Study. Am J Ophthalmol. 2002;133:109–18. doi: 10.1016/s0002-9394(01)01316-2. [DOI] [PubMed] [Google Scholar]
- 88.Zhang B, Bi H, Sakai E, et al. Rapid plasticity of binocular connections in developing monkey visual cortex (V1) Proc Natl Acad Sci U S A. 2005;102:9026–31. doi: 10.1073/pnas.0500280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilmer J, Backus B. Precision of depth judgement from binocular disparity is heritable. [February 16, 2009];J Vision. 2007 7:831. abstract-831a. Available at: http://www.journalofvision.org/7/9/831/
- 90.Scott MH, Noble AG, Raymond WRt, Parks MM. Prevalence of primary monofixation syndrome in parents of children with congenital esotropia. J Pediatr Ophthalmol Strabismus. 1994;31:298–301. doi: 10.3928/0191-3913-19940901-06. [DOI] [PubMed] [Google Scholar]


