Abstract
Objectives To determine whether informed consent introduces selection bias in prospective observational studies using data from medical records, and consent rates for such studies.
Design Systematic review.
Data sources Embase, Medline, and the Cochrane Library up to March 2008, reference lists from pertinent articles, and searches of electronic citations.
Study selection Prospective observational studies reporting characteristics of participants and non-participants approached for informed consent to use their medical records. Studies were selected independently in duplicate; a third reviewer resolved disagreements.
Data extraction Age, sex, race, education, income, or health status of participants and non-participants, the participation rate in each study, and susceptibility of these calculations to threats of selection and reporting bias.
Results Of 1650 citations 17 unique studies met inclusion criteria and had analysable data. Across all outcomes there were differences between participants and non-participants; however, there was a lack of consistency in the direction and the magnitude of effect. Of 161?604 eligible patients, 66.9% consented to use of data from their medical records.
Conclusions Significant differences between participants and non-participants may threaten the validity of results from observational studies that require consent for use of data from medical records. To ensure that legislation on privacy does not unduly bias observational studies using medical records, thoughtful decision making by research ethics boards on the need for mandatory consent is necessary.
Introduction
Information from review of medical charts is often used to carry out audits, perform non-interventional observational studies, create disease registries, and do other types of health services research. Informed consent is not always necessary for these types of research, which involve abstraction of data from patients’ records. Many such studies do not influence practice or patients’ outcomes and therefore confer no risk and no benefit to participants. That notwithstanding, recent legislation to protect the privacy and confidentiality of patients’ information in medical research introduced in many jurisdictions (for example, the regulations to the Health Insurance Portability and Accountability Act in the United States) has resulted in increased requests from research ethics boards to obtain informed consent to use data from medical records for such observational studies.1 As early as 1977 concerns were voiced about the possible negative impact of privacy laws on epidemiological research.2 More recently, editorial reviews highlighted the negative impact of mandatory informed consent on observational research through conservative interpretation of privacy legislation.3 4 5
As with many other aspects of research, requirements for informed consent to use data from medical records vary across research ethics boards within and among countries. For example, in a multisite study involving a review of children’s charts who presented to emergency departments with bronchiolitis, 34 research ethics boards arrived at divergent requirements for consent at their institutions, ranging from none to mandatory written consent.6 Four of the invited 34 sites did not participate owing to the investigator perceived hurdles with research ethics boards pertaining to informed consent.
Of greater concern is the impact of informed consent on the validity of the research in observational studies, audits, or registries. Mandatory informed consent in such no risk or low risk studies can create challenges to implementation and biased results. For example, in the Canadian Stroke Registry, investigators identified important differences between participants and non-participants in prognostic characteristics.w1 The selection bias introduced by informed consent was sufficiently serious to jeopardise the overall validity of the study, and investigators effectively shut down the registry by discontinuing follow-up surveys and record linkage studies.w1 Furthermore, case studies documenting the challenges of implementing informed consent recently reported low consent rates and poor efficiency of recruitment.w1 w2
The primary objective of our systematic review was to determine whether informed consent for use of data from medical records introduces selection bias by examining differences in key personal characteristics between participants and non-participants in prospective observational studies requiring informed consent for access to medical records. Our secondary objective was to determine the rates of consent in these studies.
Methods
We sought all studies reporting characteristics of participants and non-participants approached for informed consent to use data from their medical records for prospective observational studies or registries. We included studies reporting at least one of the following characteristics: age, sex, race, education, income, or health status. We also included studies that requested consent for access to medical records in addition to self administered or interview administered surveys or biological samples. However, we excluded studies of interventions (for example, randomised controlled trials) and studies using self administered or interviewer administered surveys or biological samples (for example, biobanks) alone. Owing to limitations on resources, we included only English language studies.
After consultation with a librarian in health sciences, we searched Embase (1980 to week 13 2008), Medline (1966 to March week 3 2008), and the Cochrane Library (Issue 1, 2008) (see web extra appendix A for full search strategy). To identify further articles from each included study, we searched reference lists, used the PubMed “related articles” feature, carried out a search of cited references in Thompson Scientific (Web of Science), and used the Google Scholar “cited by” feature.
Independently and in duplicate (MEK, MD) we scanned citations first by title and then by abstract. We reviewed full reports of all potentially relevant abstracts and calculated inter-rater reliability for included studies using the ? statistic. We subsequently resolved all disagreements through consensus; an independent adjudicator (MCB) resolved outstanding disagreements. Study population and setting, disease status, and recruitment methods were extracted.
We calculated the participation rate in each study7 and assessed the susceptibility of calculations on participation rate against threats of selection and reporting bias.8 For each study we determined the number of eligible participants, number approached for consent, number who responded to the request for consent, number of active consents, and number of active declines.
Heterogeneity among the studies in study design, recruitment methods, requests for consent, populations enrolled, and research settings precluded quantitative synthesis of the data.9 10 We used RevMan v 4.2.8 (Cochrane Collaboration) to calculate odds ratios (binary data), weighted mean differences (continuous data), and 95% confidence intervals. We used the ?2 statistic for comparisons of nominal data (>2 categories)11 using SPSS version 16.0.
Results
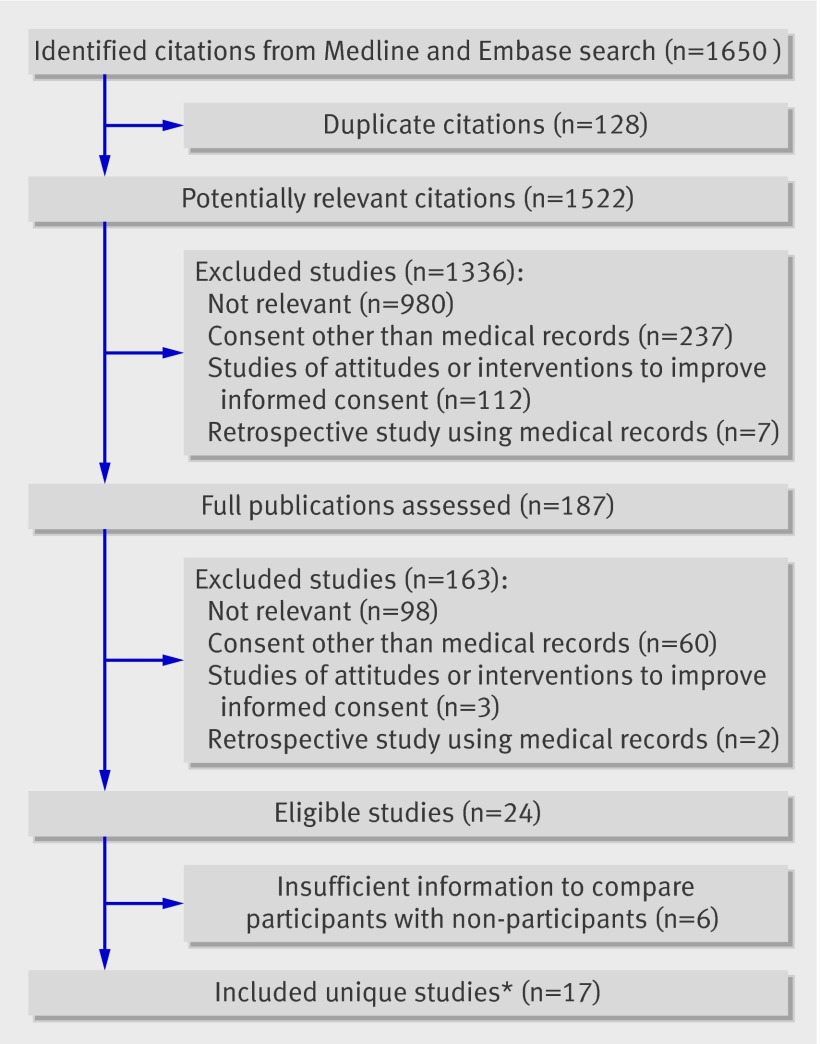
The electronic search identified 1650 citations, of which 128 were duplicates and 1335 were excluded after review of the title or abstract. Of 187 publications reviewed in full, 24 representing 23 unique studies met the eligibility criteria.w1-w24 The inter-rater reliability for included studies was 0.84 (95% confidence interval 0.83 to 0.86). Of the 23 eligible studies, 17 reported sufficient information for analyses of participants and non-participants and form the basis of this review.w1 w2 w4 w5 w7 w8 w10-w13 w15 w16 w18-w21 w23 w24 The figure outlines the flow of included studies12 and table 1 summarises the characteristics of the studies.
Flow diagram of included studies. *Two separate publications reported different outcomes from same studyw18 w19
Table 1.
Characteristics of included studies
| Study | Setting | Eligible participants | Recruitment methods | Authorisation request | Reported outcomes |
|---|---|---|---|---|---|
| Bryant 2006w4 | Cohort study, Canada | English speaking adults aged between 35 and 69 with no known history of cancer, residing in Alberta | Random digit dialling and interview by research team, recruitment period October 2000 to June 2002 | Request for periodic data linkages with Alberta cancer registry and data on utilisation of health services | Age, sex |
| Buckley 2007w5 | Cohort study, Ireland; multicentre (n=35) | Adults with established ischaemic heart disease | Postal survey from researchers, recruitment period not stated (follow-up of CoHeart Study) | Request for access to medical records, survey | Age, sex, income, health status |
| Dunn 2004w7 | Cohort study, UK; multicentre (1-5 centres, depending on study) | Adults meeting eligibility criteria for six different studies | Postal survey (senders unspecified), recruitment period August 1996 to June 2002 | Separate requests for access to medical records and future contact | Age, sex |
| Edlund 1985w8 | Cohort study, USA; single centre | Adult inpatients with tardive dyskinesia | Personal contact in hospital (recruiters unspecified), recruitment period not reported | Request for access to medical records | Age |
| Harris 2004w10 | Cohort study, UK; two centres | Adults aged more than 65 from two London practices | Postal survey (senders unspecified), recruitment period not stated | Separate requests for access to medical records, survey or questionnaire | Age, sex, race, income, health status |
| Huang 2007w11 | Cohort study, Taiwan | Adults aged 20 or more from 2001 Taiwanese National Health Interview Survey with valid identification number | Personal contact by researchers, recruitment period not reported | Request for access to national health insurance records | Age, sex, race, income, education, health status |
| Jacobsen 1999w12 | Registry, USA; single centre | Adult outpatients receiving medical care at Mayo Clinic, Rochester, MN | Postal survey from researchers, recruitment period January to April 1997 | Request for access to medical records | Age, sex, health status (Charlson comorbidity) |
| Klassen 2005; NICU and healthy childrenw13 | Cohort study, Canada; two centres | Children born between 1996 and 1997 in two British Columbia hospitals and three NICUs | Postal survey from researchers, recruitment period not stated | NICU and healthy children; separate requests for access to medical records (mother and baby), survey | Maternal age, sex, income |
| Matsui 2005; non-genetic and geneticw15 | Cohort study, Japan; Takashima study | Adults from geographical catchments in four Japanese areas attending annual health check up | Personal contact by researchers, physicians and nurses, recruitment period 2002-3 | Non-genetic: two catchments, separate requests for access to medical records, survey, blood sample; genetic: two catchments, same as for non-genetic plus genetic analysis, DNA analysis | Sex |
| McKinney 2005w16 | Registry, England; multicentre (n=5) | Children admitted to PICU | Personal contact by PICU staff, recruitment period May to July 2003 | Request for access to medical records | Age, sex, race, income, health status |
| Schwartz 2005w19; Phipps 2004w18 | Registry, USA; three centres | Adults with stroke or traumatic brain injury from 1 of 3 rehabilitation service systems in south eastern Pennsylvania | Personal contact at clinic by “site recruiters”, recruitment period not specified | Separate requests for medical records and future contact | Age, sex, race |
| Tate 2006w20 | Cohort study, UK; Millennium cohort | Children born between September 2000 and January 2002 | Personal contact, recruitment period not stated | Request for access to medical records (mother and baby) | Maternal age, race, income, education |
| Tu 2004; phases I and IIw1 | Registry, Canada; multicentre (n=20), Canadian Stroke Registry Network | Adults with acute stroke, transient ischaemic attacks, or both | Personal contact in hospital by research nurses, two recruitment phases: June 2001 to February 2002, June 2002 to December 2002 | Separate requests for access to medical records, future contact, interview, release of aggregate data to commercial organisation | Age, sex, race |
| Woolf 2000w21 | Registry, USA; single centre | Adults in primary care for practice based research network | Personal contact by clinic administrative staff, recruitment May to November 1999 | Combined request for access to medical records and future contact | Age, sex, race, income, education, health status |
| Yawn 1998w23 | Registry, USA; single centre | Adult and paediatric attendees at medical centre | Personal contact and postal survey (emergency department patients) by administrative clinic staff, recruitment period January to February 1997 | Request for access to medical records | Age, sex |
| Young 2001w24 | Cohort study, Australia; Australian Longitudinal Study on Women’s Health | Women participating in Australian Longitudinal Study on Women’s Health | Postal survey from researchers, recruitment period March 1997 | Request for access to medical records | Age, education |
NICU=neonatal intensive care unit; PICU=paediatric intensive care unit.
Participation rates and susceptibility to bias
All 17 studies described eligibility criteria. Three disclosed no information on how investigators identified eligible participants for informed consent.w15 w19 w23 Four approached all eligible participants and two randomly selected eligible participants.w12 w21 In five studies, investigators were prevented from approaching all potentially eligible participants owing to physician approval,w1 w2 w5 patient availability,w1 and study specific barriers.w13 All but one study presented sufficient information to reconstruct the outcomes of participation (table 1).w15
Of 161?604 eligible patients in the 17 studies, 108?033 (66.9%, 95% confidence interval 66.6% to 67.1%) provided active consent for use of data from their medical records. Consent rates for eligible participants varied across the studies (from 36.6%w21 to 92.9%w20) and approximated a normal distribution (not shown). Table 2 outlines the methodological information related to obtaining consent and table 3 outlines the rates of participation in each study.
Table 2.
Methodological elements to describe informed consent
| Study | Element | |||
|---|---|---|---|---|
| Did investigators describe how eligible people were identified? | Were eligible people equally likely to be approached to participate? | If eligible people were not equally likely to be approached to participate, how were people chosen for participation? | Did investigators report consent related outcomes for all eligible people? | |
| Al-Shahi 2005w2 | Yes | No | Approval by general practitioner or hospital consultant | Yes |
| Bryant 2006w4 | Yes | Yes | NA | Yes |
| Buckleyw5 | Yes | No | Contact by general practitioner | Yes |
| Dunn 2004w7 | Yes | Yes | NA | Yes |
| Edlund 1985w8 | Yes | Yes | NA | Yes |
| Harris 2004w10 | Yes | No | Approval by general practitioner or district nurses, no dementia, alive and living in practice area; on the electoral register and had contact with the practice within the last 5 years* | Yes |
| Huang 2007w11 | Yes | Yes | NA | Yes |
| Jacobsen 1999w12 | Yes | Yes | Random selection | Yes |
| Klassen 2005; healthy childrenw13 | Yes | No | Ability to contact, English language, vital status of mother or baby | Yes |
| Klassen 2005; NICUw13 | Yes | No | Ability to contact, English language, vital status of mother or baby | Yes |
| Matsui; genetic 2005w15 | Yes | No | Not stated | No |
| Matsui; non-genetic 2005w15 | Yes | No | Not stated | No |
| McKinney 2005w16 | Yes | No | Not stated | Yes |
| Schwartz 2005w19 | Yes | No | Not stated | No |
| Tate 2006w20 | Yes | Yes | NA | Yes |
| Tu 2004; phase Iw1 | Yes | No | Doctor approval, patient availability | Yes |
| Tu 2004; phase IIw1 | Yes | No | Doctor approval, patient availability | Yes |
| Woolf 2000w21 | Yes | Yes | Random selection | Yes |
| Yawn 1998w23 | Yes | No | Not stated | Yes |
| Young 2001w24 | Yes | Yes | NA | Yes |
NICU=neonatal intensive care unit; NA=not available. Criteria informed by Guyatt and Rennie.8
*Reasons for exclusion reported in Harris et al.13
Table 3.
Participation rates by study and associated participation rates
| Study | Eligible | Approached | Responded | Active consent | Active decline | No response | Not approached | Participation rate (%) |
|---|---|---|---|---|---|---|---|---|
| Al-Shahi 2005w2 | 187 | 131 | 111 | 111 | 0 | 20 | 56 | 59.4 |
| Bryant 2006w4 | 11 865* | 11 865 | 11 865 | 11 525 | 338 | 0 | 0 | 97.1 |
| Buckley 2007w5 | 1383† | 1269 | 876 | 574 | 302 | 393 | 114 | 41.5 |
| Dunn 2004w7 | 33 101 | 33 101 | 22 644 | 18 172 | 4472 | 11 239 | 0 | 53.6 |
| Edlund 1985w8 | 93 | 93 | 93 | 40 | 53 | 0 | 0 | 43.0 |
| Harris 2004w10 | 2843‡ | 2276 | 1704 | 1565 | 139 | 572 | 567§ | 55.0 |
| Huang 2007w11 | 15 413¶ | 15 413 | 15 413 | 13 504** | 1909 | 0 | 0 | 87.6 |
| Jacobsen 1999w12 | 2463 | 2463 | 2023 | 1941†† | 82 | 440†† | 0 | 78.8 |
| Klassen 2005; healthy childrenw13 | 691 | 592 | 393 | 274 | 119 | 199 | 126 | 38.2 |
| Klassen 2005; NICUw13 | 2098 | 1692 | 1140 | 832 | 308 | 552 | 529 | 37.5 |
| Matsui; genetic 2005w15 | 2195 | NR | 2195 | 1855 | 340‡‡ | 84.5 | ||
| Matsui; non-genetic 2005w15 | 3166 | NR | 3166 | 2900 | 266‡‡ | 91.6 | ||
| McKinney 2005w16 | 422 | 183 | 183 | 182 | 1 | 0 | 239 | 43.1 |
| Schwartz 2005w19 | 2422 | 2164 | 1817 | 1256 | 563 | 346 | 258 | 51.9 |
| Tate 2006w20 | 18 505 | 18 505 | 17 221 | 17 195 | 26 | 1284 | 0 | 92.9 |
| Tu 2004; phase Iw1 | 4825 | 2078 | 2078 | 1684 | 394 | 0 | 2207§§ | 39.3 |
| Tu 2004; phase IIw1 | 2823 | 1761 | 1761 | 1428 | 333 | 0 | 1062§§ | 50.6 |
| Woolf 2000w21 | 1229 | 1106 | 1021 | 743 | 278 | 85 | 123 | 36.3 |
| Yawn 1998w23 | 15 997 | 15 789 | 15 069 | 14 493 | 576 | 720 | 208 | 90.6 |
| Young 2001w24 | 39 883 | 39 883 | 20 864 | 19 700 | 1164 | 19 019 | 0 | 49.3 |
NR-not reported.
*Includes two transgendered people for whom consent information was not available.
†Authors reported 1609 eligible; we adjusted this number to 1383 after excluding 226 from the original cohort who were dead at the time of the follow-up study and not eligible for inclusion.
‡Number eligible reported in Harris et al.13
§Reasons for not being approached reported in Harris et al.13
¶802 of 15 413 eligible people did not complete all parts of demographic survey and were not included in demographic analyses.
**Includes 593 people who consented to access of data from medical records but did not complete all parts of demographic survey.
††Per authors, non-respondents were considered as positive consent per Minnesota law. Table includes those who actively consented.
‡‡Aggregate data reported.
§§Not approached because of language barrier, surrogate decision maker unavailable and other, and patients died or left hospital.
Differences between participants and non-participants
Authors represented the characteristics of participants and non-participants in four different ways: continuous data,w1 w2 w8 w11 w21 proportions,w1 w2 w4 w5 w7 w10 w11 w19 w21 w23 w24 regression analyses,w13 and the weighted proportion of patients declining consent after adjustment for study design.w12 w20 Studies reported comparisons between participants and non-participants with different denominators: four studies reported consent of those eligible,w1 w2 w16 w23 eight of those approached,w4 w7 w8 w12 w19-w21 w24 and four of those who responded.w5 w10 w13 w15 One study reported the denominator for consent on the basis of the availability of personal characteristics.w11 We describe comparisons between participants and non-participants according to age, sex, race, income, education, and health status. Table 4 summarises differences by these outcomes.
Table 4.
Summary of differences between participants and non-participants by study
| Study | Personal characteristics | |||||
|---|---|---|---|---|---|---|
| Age | Sex | Race | Income | Education | Health status | |
| Al-Shahi 2005w2 | NS | NS | NR | NS | NR | Less disability (Rankin score); P<0.001 |
| Bryant 2006w4 | Varied by age strata; P<0.001 | More females: odds ratio 2.21 (95% CI 1.77 to 2.75) | NR | NR | NR | NR |
| Buckley 2007w5 | NS | Fewer females: odds ratio 0.73 (95% CI 0.54 to 0.97) | NR | NS | NR | NR |
| Dunn 2004w7 | Varied by age strata; P<0.001 | More females: odds ratio 1.07 (95% CI 1.03 to 1.12) | NR | NR | NR | NR |
| Edlund 1985w8 | NS | NR | NR | NR | NR | NR |
| Harris 2004w10 | NS | NS | NS | NS | NR | More disability (disability score); P<0.001 |
| Huang 2007w11 | Varied by age strata; P<0.001 | Fewer females: odds ratio 0.90 (95% CI 0.81 to 1.0) | Varied by race strata; P<0.001 | Varied by income strata; P<0.001 | Varied by education strata; P<0.001 | Varied by SF-36 subscale |
| Jacobsen 1999w12 | Varied by age strata; P<0.001 | NS | NR | NR | NR | More comorbidity (Charlson index =2); P=0.008 |
| Klassen 2005; healthyw13 | NS | NS | NR | Varied by income strata | NS | NR |
| Klassen 2005; NICUw13 | NS | NS | NR | NS | NS | NR |
| Matsui; genetic 2005w15 | NR | NS | NR | NR | NR | NR |
| Matsui; non-genetic 2005w15 | NR | Fewer females: odds ratio 0.62 (95% CI 0.46 to 0.82) | NR | NR | NR | NR |
| McKinney 2005w16 | NS | NS | NS | NS | NR | Varied by paediatric risk of mortality score; P=0.024 |
| Schwartz 2005w19 and Phipps 2004w18 (race) | Younger: 58.7 (20.2) v 67.7 (18.6) | Fewer females: odds ratio 0.67 (95% CI 0.49 to 0.91) | NS | NR | Varied by education strata; P=0.019 | NR |
| Tate 2006w20 | Varied by age strata | NR | Varied by race strata | Varied by income strata | Varied by education strata | NR |
| Tu 2004; phase Iw1 | Younger; 69 v 72, P<0.001 | NS | Varied by race strata; P<0.001 | NR | NR | NR |
| Tu 2004; phase IIw1 | NS | Fewer females: odds ratio 0.79 (95% CI 0.68 to 0.91) | Varied by race strata; P<0.001 | NR | NR | NR |
| Woolf 2000w21 | NS | Fewer females: odds ratio 0.59 (95% CI 0.42 to 0.81) | Fewer black patients; P=0.013 | NS | NS | More physical disability; weighted mean difference -2.5 (95% CI -3.98 to -1.02) |
| Yawn 1998w23 | Varied by age strata; P=0.018 | NS | NR | NR | NR | NR |
| Young 2001w24 | Varied by age strata; P<0.001 | NR | NR | NR | More education beyond school level; P<0.001 | NR |
NR=not reported; NS=no statistically significant difference between participants and non-participants. See web extra appendix B for detailed information on each characteristic.
Age—Sixteen studies reported characteristics of the participants and non-participants by age (see web extra appendix B1).w1 w2 w4 w5 w7 w8 w10-w13 w16 w19-w21 w23 w24 Seven studies found no age related differences,w2 w5 w8 w10 w13 w16 w21 one found that participants were younger than non-participants,w19 and seven identified significant differences across age strata; however, no clear pattern emerged.w4 w7 w11 w12 w20 w23 w24 In the Canadian Stroke Registry Network, participants in phase I of the study were younger than non-participants, whereas in phase II there were no differencesw1 after a change in recruitment strategy.
Sex—Fourteen studies that recruited both males and females reported the characteristics of participants and non-participants by sex (see web extra appendix B2).w1 w2 w4 w5 w7 w10-w13 w15 w16 w19 w21 w23 Six studies reported no differences in the odds of females participating compared with males.w2 w10 w12 w13 w16 w23 In the six studies where there were differences, two determined that females were more likely to consent than males,w4 w7 whereas four determined that females were less likely to consent than males.w5 w11 w19 w21 In the remaining two studies the participation of females differed between subgroups. In the Takashima cohort study there was no difference in the likelihood of participation between females and males enrolled in a group requesting access to their medical records in addition to surveys and a blood sample. However, females were less likely to participate than males with the addition of genetic testing to the request for access to medical records, surveys, and a blood sample.w15 The Canadian Stroke Registry Network initially had no differences in participation rates between the sexes; after a change in recruitment strategy fewer females than males participated.w1
Race—Six studies reported the characteristics of participants and non-participants by race (see web extra appendix B3).w1 w10 w11 w18 w20 w21 Two found no difference in the odds of obtaining consent by race.w10 w18 Three studies determined higher participation rates in white or Caucasian patients than others,w1 w20 w21 and a Taiwanese study of national health records identified differences across four strata of race.w11
Income—Seven publications reported participants and non-participants by income (see web extra appendix B4).w5 w10 w11 w13 w16 w20 w21 Four studies found no differences by income.w5 w10 w16 w21 Across five strata of income, Huang et alw11 identified varying rates of participation for access to Taiwanese National Health Insurance records. Another study reported no differences in income in parents of babies in neonatal intensive care units, whereas there were differences across income categories in parents of healthy babies.w13 Women who never worked or who were unemployed long term were less likely to participate in the UK millennium cohort study; however, after adjusting for education, socioeconomic status did not independently predict participation.w20
Education—Six studies reported participants and non-participants by education (see web extra appendix B5).w11 w13 w19-w21 w24 Two studies found no differences related to educationw13 w21; however, in the Australian Longitudinal Study on Women’s Health, those women who had continued their education beyond school were more likely to participate. Three studies that described participants and non-participants by strata identified significant differences, although no clear patterns emerged.w11 w19 w20
Health status—All six studies that reported health status found differences between participants and non-participants (see web extra appendix B6).w2 w10-w12 w16 w21 In two studies participants had more disability or comorbidity than non-participants as measured by the Charlson comorbidity indexw12 and the physical components summary.w21 Two studies reported that participants had less disability than non-participants as measured by the modified Rankin scorew2 and disability score.w10 One study reported higher SF-36 subscale scores in physical function, role physical, vitality, and general health in participants and no differences in role emotional, social functioning, bodily pain, and mental health.w11 In a study that enrolled patients from the paediatric intensive care unit, participation varied by strata for risk of death.w16
Discussion
Bias results in systematic deviation from the underlying truth.8 Jacobsen et al used the term authorisation bias to describe statistically significant differences between participants and non-participants in research that used medical records.w12 In this systematic review we identified 17 unique studies comparing participants and non-participants in observational studies that requested access to medical records. Across all outcomes there were differences between participants and non-participants, although there was a lack of consistency in the direction and the magnitude of effect. Thus although results of this systematic review suggest that requirements for informed consent introduced a variety of biases into prospective observational studies using data from medical records, no systematic deviations occurred and the cause of the differences by age, sex, race, income, education, or health status that did emerge is unclear. Most studies did not explore reasons for refusal, non-response, or inability to contact patients. This is an important gap, as failure to ask for consent may indicate deficiencies in organisational planning that call for a different policy response than does refusal to participate. At this stage the state of the research is such that our ability to predict these differences with confidence and to guide researchers to avoid authorisation bias is limited.
In terms of our secondary objective, participation rates varied substantially. Studies with high participation rates showed selection biases, the proportion of eligible participants approached for enrolment differed across studies and we identified opportunities to improve the reporting of outcomes for consent. Whereas all studies reported how investigators identified eligible people, four did not report how those eligible were chosen for participation.w15 w16 w19 w23 Knowing such information helps us to better interpret how susceptible these four studies were to selection bias before the introduction of informed consent.
Our review indicates that consent rates for studies using medical records vary considerably, affecting recruitment efforts and potentially influencing study results. Accordingly, consideration of these factors in the study design, planning, and budgeting is essential. Willison et al offered practical advice for studies based on consent at local and systems levels after their experiences of involving multisites in the Canadian Stroke Registry Network, such as testing the consent process by using a pilot, close communication with research ethics boards and healthcare institutions, consideration of random sampling strategies, and ongoing monitoring and feedback on accrual.14 Recent recommendations reinforce the explicit reporting of personal comparisons between participants and non-participants as an important feature of publications on observational studies.15 Future research needs to systematically study why otherwise eligible patients are not approached for consent and the characteristics of patients associated with refusal to participate in studies using medical records.
Pragmatically what should researchers planning a prospective observational study that involves medical records do? The United Kingdom National Health Service Act 2006 (Section 251),16 the common rule of the Health Insurance Portability and Accountability Act,17 and the Canadian Institutes for Health Research18 offer guidance to researchers on informed consent for research involving medical records. Although consent is required for the collection of personal information from participants for medical procedures, medical examinations, and clinical trials, exemption from requiring consent may be appropriate for studies using medical records owing to impracticability of informed consent and the possibility of introducing biased study results.18
We suggest requesting a waiver of consent from the research ethics board for research using medical records because these studies confer no or minimal risk, do not directly benefit the patient, and because of the potential biases introduced through loss of data in ways that are not completely at random. We suggest explicitly outlining the procedures the research team will take to protect the privacy and confidentiality of each patient. For example, to minimise the risks of a breach of confidentiality, researchers could collect the minimum personal information necessary for identification from each record and incorporate strict access policies to the data at patient level.
However, if a waiver of consent is not possible, as in some European Union jurisdictions,19 we suggest collecting a minimum dataset of key prognostic variables on all eligible people for the study identified through screening. These data can be used to carry out a preliminary analysis comparing participants with non-participants on the key prognostic variables at predetermined times during study accrual, taking into account statistical adjustments for multiple significance testing. Such an approach may lead to revised recruitment strategies to address these concerns—for example, tailored recruitment, targeting participation of populations less likely to grant consent.
On the basis of findings from this review, the validity of results from observational studies that require consent for access to medical records may be threatened as a result of significant differences between participants and non-participants. Across the continuum of research we suggest three strategies to minimise the impact of authorisation bias at the inception, reporting, and interpretation of research. At inception we suggest widespread education aimed at clinicians, researchers, and research ethics boards on the conditions under which studies can proceed without individual consent. To help us better interpret differences between participants and non-participants we suggest standardised reporting of methods used to seek informed consent. We believe the elements we report in table 2 provide the minimum dataset for these purposes and could serve as the foundation for expectations on quality reporting. Similarly, we advocate standardised key metrics on informed consent such as participation rates, including eligible, approached, responded, active consent, and active declines (see table 3). Finally, in interpreting observational studies that exhibit significant differences between participants and non-participants, clinicians and researchers should be aware of differences in important prognostic variables and their possible impact on study results. The box summarises our recommended strategies to minimise the impact of bias from informed consent.
Five suggested strategies to minimise the impact of bias from informed consent
Request a waiver of consent from research ethics boards and explicitly outline procedures to protect the privacy and confidentiality of each patient
-
If a waiver is not possible then:
Collect a minimum dataset of key prognostic variables on all eligible people identified through screening
Complete a preliminary analysis comparing participants and non-participants on key prognostic variables at predetermined times
Revise the strategy for recruitment as necessary
Aim education at clinicians, researchers, and research ethics boards on conditions under which studies can proceed without individual consent
Standardise reporting of methods used to seek informed consent
Increase awareness by clinicians and researchers of the potential impact of selection bias introduced by informed consent and implications for interpretation of the study
Strengths and limitations of the study
Our review has several strengths. A priori we developed comprehensive search strategies with librarians in health sciences who were familiar with the indexing methods of electronic databases on health. We included both Medline and Embase, which are complementary bibliographic databases of the biomedical literature20; we supplemented included articles with searches of cited references and related articles. We used broad search strategies of published literature and reviewed all citations and included studies in duplicate that resulted in good agreement and transparent reporting.10 12
Our review also has limitations. Our search included only studies in English available in the peer review literature. Because of the variability in results we do not expect exclusion of non-English studies to impair the generalisability of our findings; however, this hypothesis needs to be confirmed in future research. Our review was limited by the published reports, including lack of clarity about the sample size and reporting standards for screening and consent procedures. Not all studies reported data on our outcomes of interest; authors may not have collected data on these outcomes or chose to report only significant differences between participants and non-participants.w7 w20 w24 For example, of the two studies reporting all six of our outcomes of interest, one identified statistically significant differences by sex, race, and health status and no differences by age, income, or education,w21 whereas the other study identified significant differences by age, race, income, education, and health status and no differences by sex.w11 Because these observational studies were not specifically designed to study differences in consent between participants and non-participants, we may have observed statistically significant differences across our outcomes of interest simply by chance.8
Conclusion
In conclusion, we observed authorisation bias in studies requiring informed consent for use of data from medical records. To assess better the impact of informed consent on prospective observational studies, consistent reporting of core personal factors of known prognostic significance between the characteristics of participants and non-participants is necessary. To ensure that legislation on privacy does not unduly threaten the validity of observational studies using data from medical records, education of bodies responsible for overseeing research and further investigations are urgently needed on the determinants and consequences of consent and non-consent for these studies.
What is already known on this topic
Privacy legislation has resulted in some research ethics boards requiring informed consent to use medical records
Whether mandatory informed consent creates selection bias in these observational studies is unknown
What this study adds
Of 1650 citations, 17 unique studies met inclusion criteria and had analysable data on the following six outcomes: age, sex, race, education, income, or health status
Across all outcomes, differences between participants and non-participants occurred, although there was a lack of consistency in the direction and the magnitude of effect
To ensure that legislation does not unduly bias observational studies using medical records, thoughtful decision making by research ethics boards on the need for mandatory consent is necessary
Contributors: MEK conceived and designed the study and drafted the article. She had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and is guarantor. MEK and MD acquired the data; MEK, MD, and MCB analysed the data; and MEK, MD, DJC, DJW, and MCB interpreted the data. MD, DJC, DJW, and MCB critically revised the manuscript for important intellectual content. All authors approved the final version to be published.
MEK is funded by a fellowship from the Canadian Institutes of Health Research (Clinical Research Initiative). DJC is a research chair of the Canadian Institutes of Health Research. The Canadian Institutes of Health Research had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation review, or approval of the manuscript.
Competing interests: None declared.
Ethical approval: Not required.
Cite this as: BMJ 2009;338:b866
References
- 1.O’Herrin JK, Fost N, Kudsk KA. Health Insurance Portability Accountability Act (HIPAA) regulations: effect on medical record research. Ann Surg 2004;239:772-6; discussion 776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordis L, Gold E, Seltser R. Privacy protection in epidemiologic and medical research: a challenge and a responsibility. Am J Epidemiol 1977;105:163-8. [DOI] [PubMed] [Google Scholar]
- 3.Gershon AS, Tu JV. The effect of privacy legislation on observational research. CMAJ 2008;178:871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junghans C, Jones M. Consent bias in research: how to avoid it. Heart 2007;93:1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra D, Gertz R, Singleton P, Inskip HM. Confidentiality of personal health information used for research. BMJ 2006;333:196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansbach M, Acholonu U, Clark S, Camargo CA. Variation in institutional review board responses to a standard, observational, pediatric research protocol. Acad Emerg Med 2007;14:377-80. [DOI] [PubMed] [Google Scholar]
- 7.Stang A, Ahrens W, Jockel K. Control response proportions in population-based case-control studies in Germany. Epidemiology 1999;10:181-3. [PubMed] [Google Scholar]
- 8.Guyatt GH, Rennie D, eds. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press, 2002.
- 9.Hatala R, Keitz S, Wyer P, Guyatt G, for the Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ 2005;172:661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Jadad AR, Klassen TP. Guides for reading and interpreting systematic reviews. III. How did the authors synthesize the data and make their conclusions? Arch Pediatr Adolesc Med 1988;152:915-20. [DOI] [PubMed] [Google Scholar]
- 11.Norman GR, Streiner DL. Biostatistics: the bare essentials. 2nd edn. Hamilton: BC Decker, 2000.
- 12.Cook DJ, Sackett DL, Spitzer WO. Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam Consultation on Meta-Analysis. J Clin Epidemiol 1995;48:167-71. [DOI] [PubMed] [Google Scholar]
- 13.Harris T, Cook DG, Victor C, Rink E, Mann AH, Shah S, et al. Predictors of depressive symptoms in older people—a survey of two general practice populations. Age Ageing 2003;32:501-8. [DOI] [PubMed] [Google Scholar]
- 14.Willison DJ, Kapral MK, Peladeau P, Richards JA, Fang J, Silver FL. Variation in recruitment across sites in a consent-based clinical data registry: lessons from the Canadian Stroke Network. BMC Med Ethics 2006;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol 2005;161:280-8. [DOI] [PubMed] [Google Scholar]
- 16.National Health Service Act 2006. 2008. www.opsi.gov.uk/Acts/acts2006/ukpga_20060041_en_1.
- 17.HIPAA Privacy Rule: information for researchers, 2006. http://privacyruleandresearch.nih.gov/pr_08.asp.
- 18.Canadian Institutes for Health Research. Canadian Institutes for health research best practices for protecting privacy in health research. Ottawa: CIHR, 2005.
- 19.Directive 2002/58/EC of the European Parliament and of the Council of 12 Jul 2002. 2008. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:201:0037:0047:EN:PDF.
- 20.Suarez-Almazor ME, Belseck E, Homik J, Dorgan M, Ramos-Remus C. Identifying clinical trials in the medical literature with electronic databases: MEDLINE alone is not enough. Control Clin Trials 2000;21:476-87. [DOI] [PubMed] [Google Scholar]