Abstract
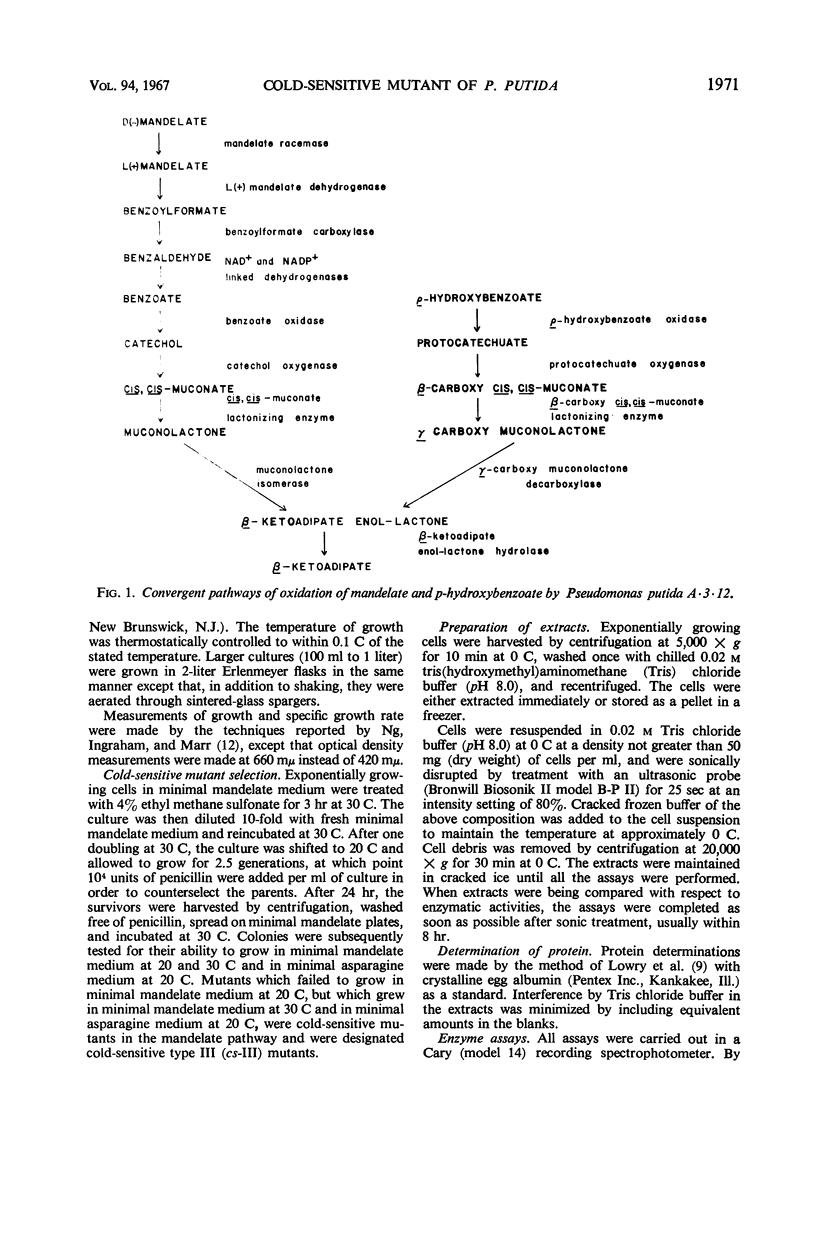
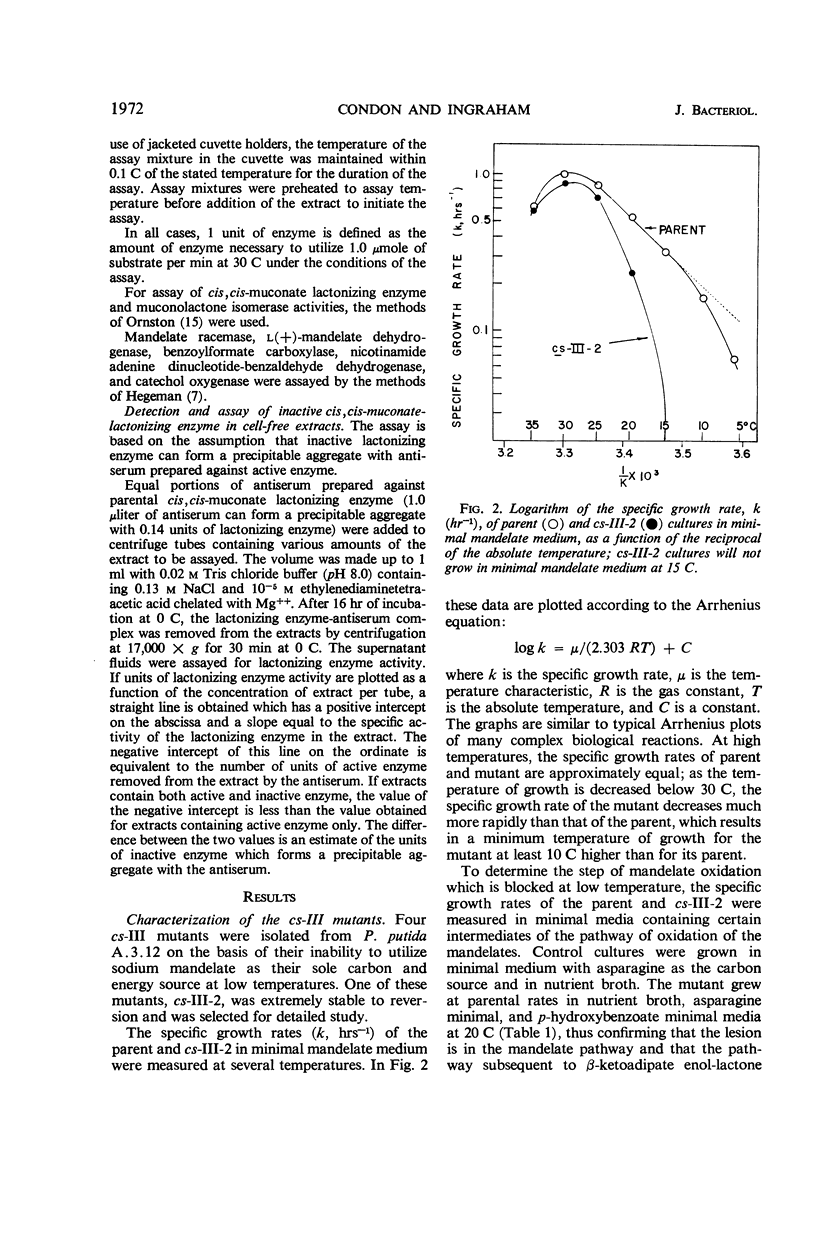
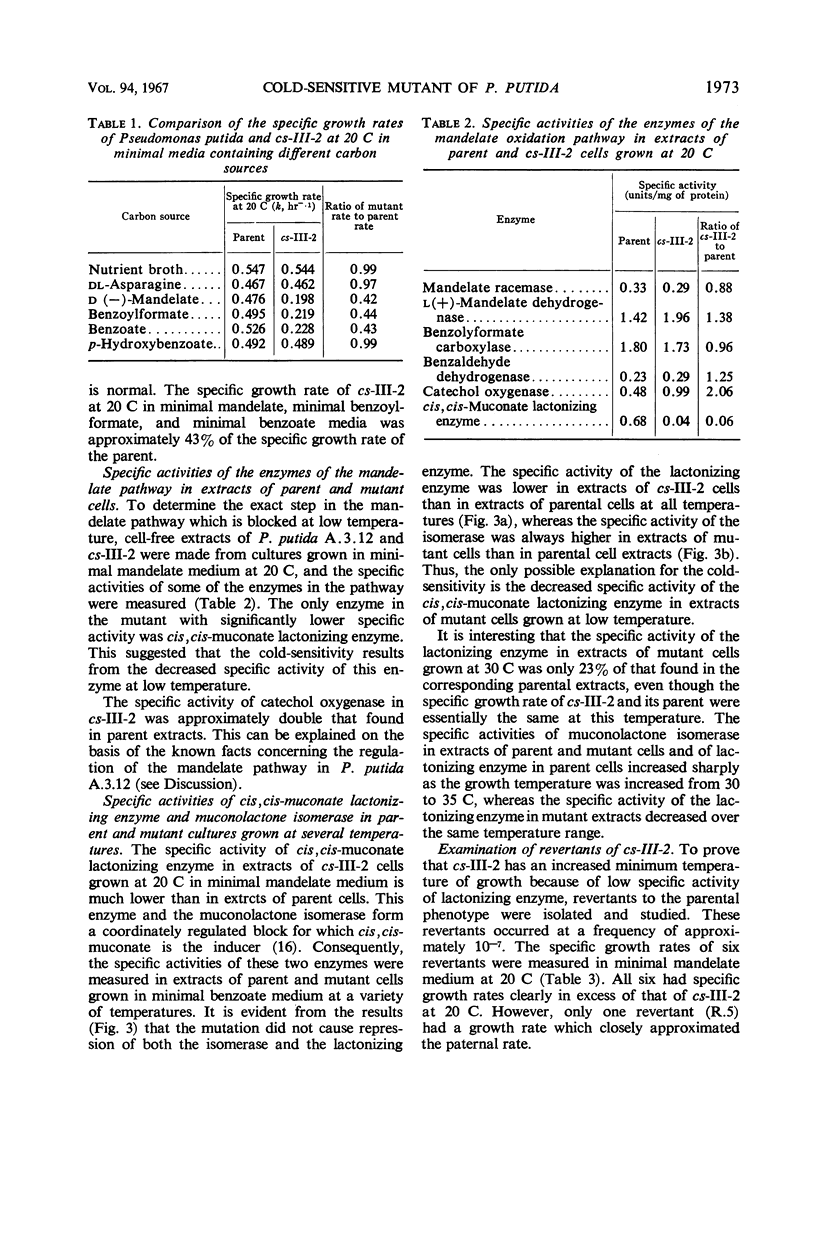
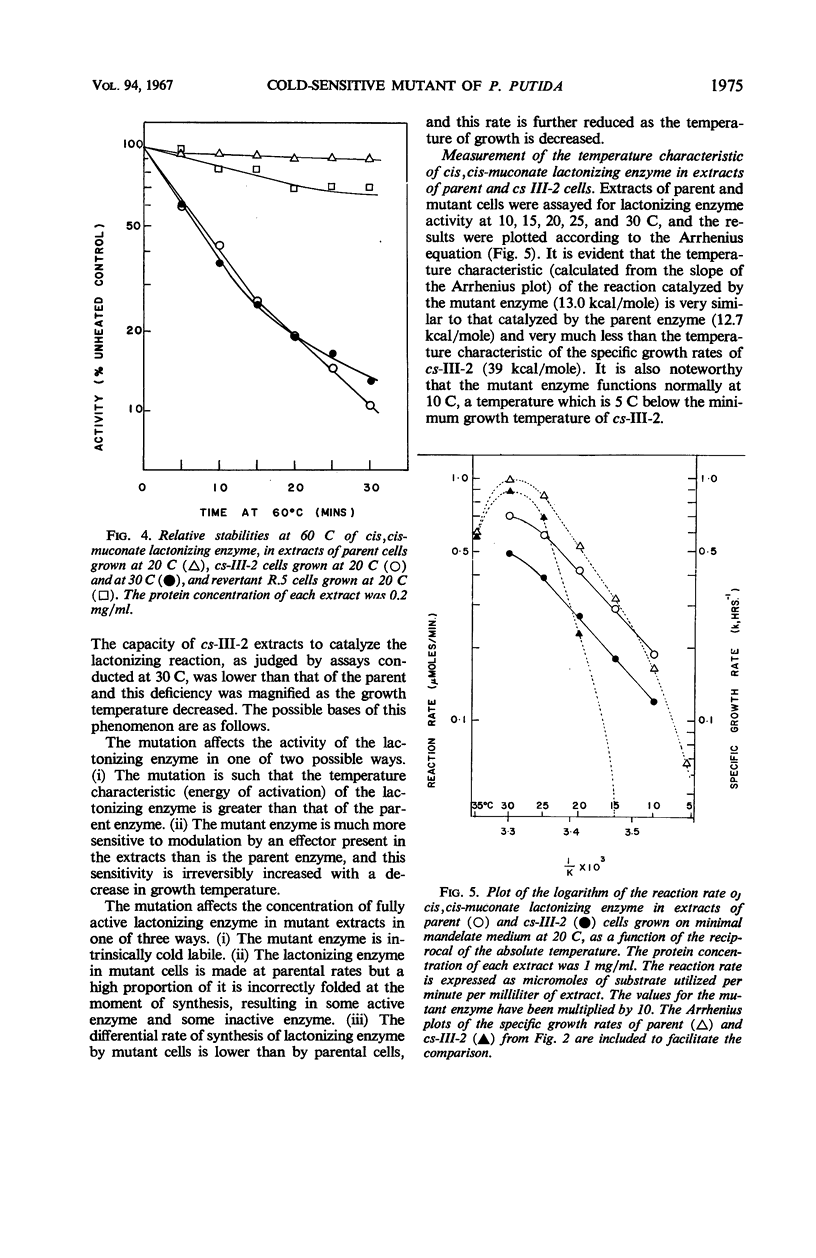
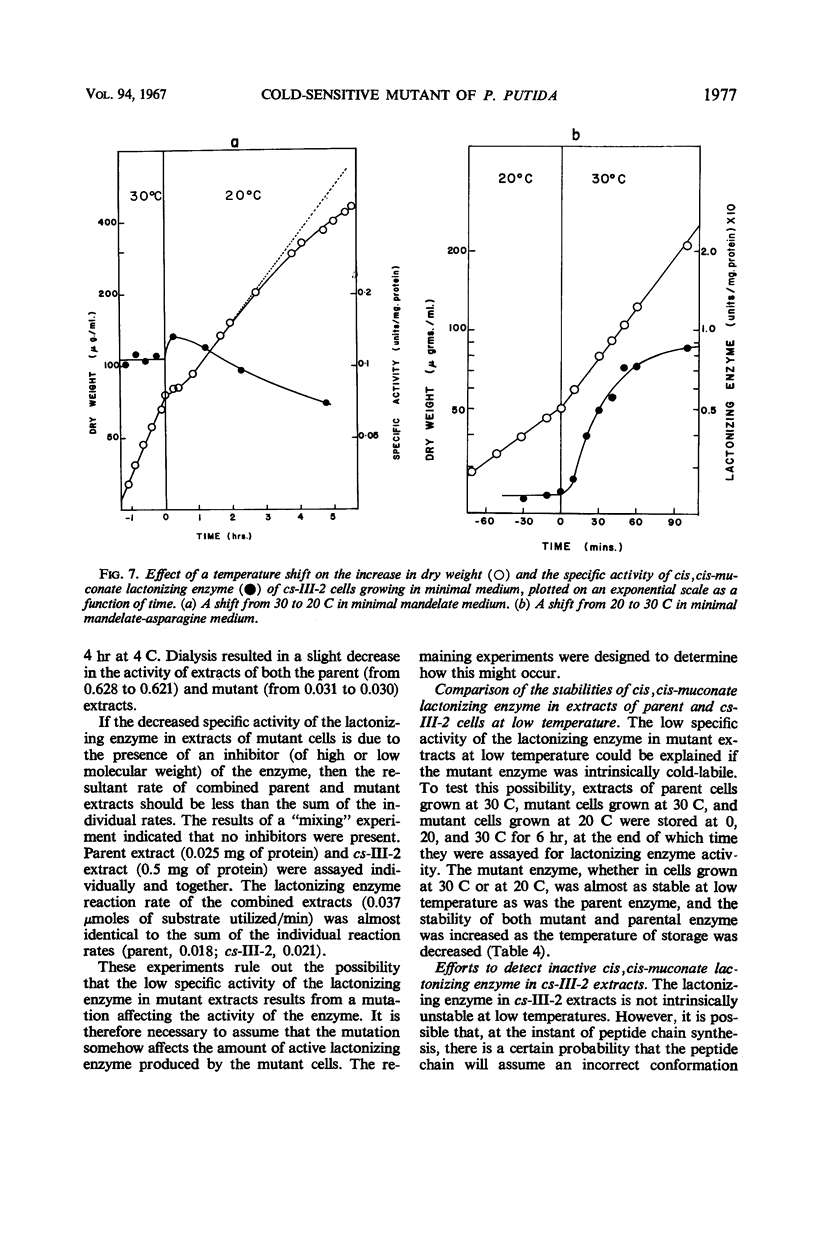
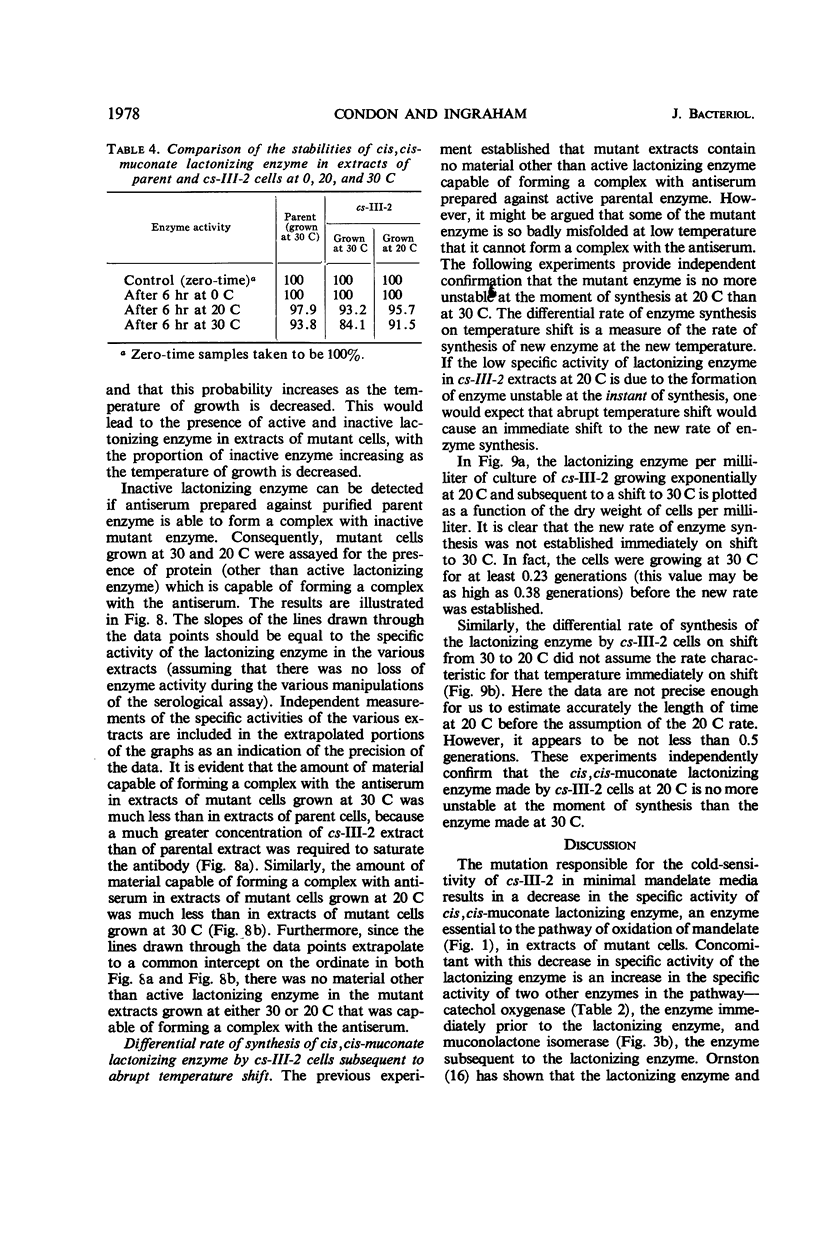
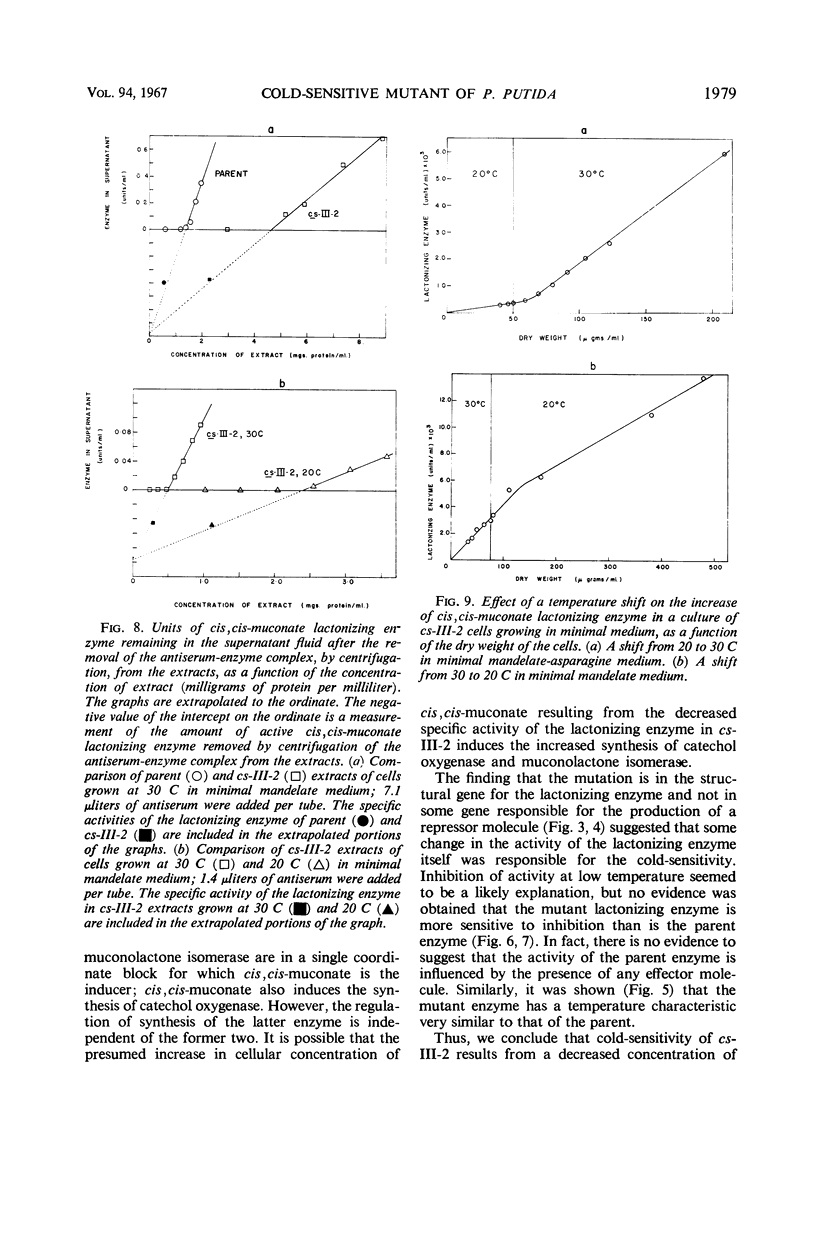
A cold-sensitive mutant of Pseudomonas putida has been isolated which grows normally at 30 C but is unable to grow on mandelate as a source of carbon at 15 C. The mutation results in the inability of the strain to carry out the reaction catalyzed by cis,cis-muconate lactonizing enzyme at low temperature and must lie in the structural gene for that enzyme, because the mutant enzyme produced at 30 C shows altered thermal stability. The mutant enzyme is not intrinsically cold-labile, nor is it cold-labile at the moment of synthesis. The activity of the mutant enzyme is not inhibited at low temperature. Evidence is presented to establish that this mutation in the structural gene coding for cis,cis-muconate lactonizing enzyme results in the lack of expression of that gene at low temperature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., SPIEGELMAN S., ROBERTS R. B., DUERKSEN J. D. Ribosome-bound beta-galactosidase. Proc Natl Acad Sci U S A. 1961 Jan 15;47:114–122. doi: 10.1073/pnas.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Co-linearity of beta-galactosidase with its gene by immunological detection of incomplete polypeptide chains. Science. 1966 Nov 25;154(3752):1027–1029. doi: 10.1126/science.154.3752.1027. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN A., GOLDSTEIN D. B., LOWNEY L. I. PROTEIN SYNTHESIS OF 0 DEGREES C IN ESCHERICHIA COLI. J Mol Biol. 1964 Jul;9:213–235. doi: 10.1016/s0022-2836(64)80102-9. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARR A. G., INGRAHAM J. L., SQUIRES C. L. EFFECT OF THE TEMPERATURE OF GROWTH OF ESCHERICHIA COLI ON THE FORMATION OF BETA-GALACTOSIDASE. J Bacteriol. 1964 Feb;87:356–362. doi: 10.1128/jb.87.2.356-362.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG H., INGRAHAM J. L., MARR A. G. Damage and derepression in Escherichia coli resulting from growth at low temperatures. J Bacteriol. 1962 Aug;84:331–339. doi: 10.1128/jb.84.2.331-339.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Ingraham J. L. Cold-sensitive mutants of Escherichia coli resulting from increased feedback inhibition. Proc Natl Acad Sci U S A. 1965 Aug;54(2):451–457. doi: 10.1073/pnas.54.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'donovan G. A., Kearney C. L., Ingraham J. L. Mutants of Escherichia coli with High Minimal Temperatures of Growth. J Bacteriol. 1965 Sep;90(3):611–616. doi: 10.1128/jb.90.3.611-616.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- SHUKUYA R., SCHWERT G. W. Glutamic acid decarboxylase. III. The inactivation of the enzyme at low temperatures. J Biol Chem. 1960 Jun;235:1658–1661. [PubMed] [Google Scholar]
- SHUSTER C. W., DOUDOROFF M. A cold-sensitive D(-) beta-hydroxybutyric acid dehydrogenase from Rhodospirillum rubrum. J Biol Chem. 1962 Feb;237:603–607. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]



