Abstract
We report the case of a 56-year-old white man who presented at the Emergency Department for evaluation of dark-red urine. Rapid development of acute renal failure and haemolytic anaemia initially elicited the hypothesis of a haemolytic-uremic syndrome. A previous exposure to a gas mixture containing arsenic and copper was later recognized as the probable aetiology while other differential diagnoses were excluded. Chelating treatment was promptly initiated before laboratorial confirmation of arsenic and copper poisoning. Renal and haematological recovery was gradually observed and the patient survived with no sequelae.
Introduction
Haemolytic anaemia may be secondary to a wide range of causes either inherited or acquired. The former include disturbances affecting haemoglobin, the membrane-cytoskeleton complex and enzyme deficiency states. The latter include mechanical destruction, drugs, infections, auto-immune disorders or toxic agents.
Toxic agents are an uncommon cause of haemolysis, which may develop even in people without metabolic abnormalities. Many chemicals, due to their oxidative potential, can lead to haemolysis. Other agents act through non oxidative, largely unknown mechanisms, such as arsine, stibine, copper, and lead [1].
Acute poisoning with heavy metals is a rare cause for evaluation in the Emergency Department. Its identification may prove difficult particularly in unconscious patients with no obvious history. A low-level exposure may even be disregarded by the patient himself. This may challenge the initial approach for a problem which can result in a lethal outcome.
Case presentation
A 56-year-old white man was referred to our Emergency Department (ED) for evaluation of dark-red urine.
The patient complained of a general feeling of sickness, diffuse muscle pain, transient episodes of diaphoresis and chills, with no fever, associated with nausea and bilious vomiting for the past 24 hours. The appearance of dark-red coloured urine, resembling blood, motivated his search for medical care.
He denied abdominal pain or any recent traumatic event. His past medical history was positive for chronic gastritis. He had no past of nephrolithiasis or haematological disorders. He was taking a protein-pump inhibitor and denied use of any over-the-counter substances. He was an occasional pipe smoker and had no history of alcohol abuse or illicit drug consumption. His family history was unremarkable.
On physical examination, his blood pressure was 132/78 mmHg, with a heart rate of 78 beats per minute, a tympanic temperature of 36.8ºC, and a respiratory rate of 18 breaths per minute. There were no signs of dehydration. He had no costovertebral angle tenderness. He had no chronic liver disease stigmata. Further examination, including neurological evaluation, was unremarkable. Initial laboratory data included: Hb = 13.8 g/dL, MCV = 101.4 fL, MCHC = 32.3 g/dL, RDW = 79.8 fl, WBC = 18.01 × 109/L, PLT = 209 × 109/L; CRP = 8.5 mg/L, Cr = 1.55 mg/dL, Urea = 80 mg/dL. Urinalysis revealed proteinuria (3+), leukocyturia (80 cells/HPF), numerous renal tubular epithelial cells, no erythrocyturia, and absent nitrites or urobilinogen. Urinary tract ultrasound excluded signs of lithiasis or obstruction and revealed bilateral renal parenchyma hyperechogenicity and slight perirenal oedema at the right kidney; bladder wall visualization did not show suspicious lesions and the prostate was normal.
He was admitted to the Urology Department for a suspected urinary tract infection. A few hours later his condition deteriorated. He developed jaundice, fever (38.6°C), diarrhoea and mental confusion. His blood panel revealed a normocytic normochromic anaemia with anysocytosis (Hb = 8.0 g/dL, RDW = 83.2 fl), predominantly indirect hyperbilirrubinemia (TB = 5.82 mg/dL, DB = 0.51 mg/dL), high LDH (4415 U/L), elevation of inflammatory markers (WBC = 20.85 × 109/L, CRP = 108 mg/L) and worsening renal dysfunction (Cr = 3.59 mg/dL, Urea = 188 mg/dL) (Table 1).
Table 1.
In-hospital laboratorial course
| Day 1 | Day 1* | Day 2 | Day 3 | Day 5 | Day 6 | Day 8 | Day 10 | Day 18 | |
|---|---|---|---|---|---|---|---|---|---|
| Hb, g/dL | 13.8 | 11.5 | 8.0 | 9.4 | 8.7 | 7.9 | 9.3 | 9.9 | 10 |
| WBC × 109/L | 18.01 | 18.44 | 20.85 | 10.56 | 8.71 | 8.64 | 12.39 | 11.82 | 12.74 |
| PLT × 109/L | 209 | 167 | 141 | 69 | 89 | 89 | 124 | 250 | 299 |
| CRP, mg/L | 8.5 | 25.4 | 108.7 | 52 | 40.9 | 28.8 | 85.5 | 104 | 87.9 |
| AST, U/L | 112 | 121 | 207 | 57 | 34 | 35 | |||
| ALT, U/L | 33 | 36 | 193 | 156 | 91 | 31 | |||
| γGT, U/L | 18 | 19 | 27 | ||||||
| ALP, U/L | 71 | 62 | 43 | ||||||
| TB, mg/dL | 2.06 | 5.82 | 6.16 | 0.94 | 1.02 | 0.66 | 0.63 | ||
| DB, mg/dL | 0.43 | 0.51 | 0.48 | 0.23 | 0.72 | 0.15 | 0.14 | ||
| LDH, U/L | 4115 | 1430 | 997 | 659 | 549 | 289 | |||
| Urea, mg/dL | 80 | 135 | 188 | 182 | 144 | 112 | 70 | 65 | 59 |
| Cr, mg/dL | 1.55 | 2.56 | 3.59 | 4.96 | 4.43 | 4.34 | 3.45 | 3.86 | 2.79 |
Exposure to a gas mixture containing arsenic and copper occurred nearly 32 hours before initial medical evaluation. One session of plasmapheresis was performed at in-hospital day 2. Penicillamine was administrated from day 4 to 7, followed by dimercaprol during 10 days. Transfusion with 1 pack of red blood cells was performed at day 2. Haemolytic anaemia, renal dysfunction and hepatotoxicity recovered gradually after chelation therapy.
* Laboratorial results at approximately 12 hours of admission.
He was transferred to the Intermediate Care Unit of the Internal Medicine Department. Additional exams included an ECG with normal sinus rhythm and normal QTc interval (416 ms), a normal chest X-ray, a blood smear with rare schizocytes, negative direct and indirect Coombs tests and a fractional excretion of sodium of 3.49%. These data pointed to a haemolytic cause for the rapidly worsening anaemia (Table 1) and acute renal failure in this context.
The association of acute renal failure and haemolytic anaemia elicited the hypothesis of a haemolytic-uremic syndrome. Considering other causes for a haemolytic physiopathology which could not be immediately excluded and the severity of the clinical condition of the patient, plasmapheresis with fresh frozen plasma was initiated and RBC transfused as needed.
Clinical history was revisited. The patient owned a factory of zippers where chemical compounds were frequently used in plating techniques. At this time the patient recalled accidental inhalation of a possible toxic gas nearly 32 hours before, while performing a new plating process wearing no protection. The gas resulted from the mixture of a so-called “tin’s oxidant” with a metal alloy. This exposure lasted no longer than 5 minutes and nearly 4 hours later he remembered feeling “sick”. Detailed information about this chemical substance was immediately requested.
At day 3, nearly 90 hours after the accidental exposure, the composition of the toxic substance was known. “Tin’s oxidant”, as the patient named it, included 40-45% of chlorhydric acid (HCl), 1.2-1.4% of copper sulphate (CuSO4), and 5-6% of arsenic trioxide (As2O3). Plasmapheresis was stopped at this time.
Blood and 24-hour urine samples were sent to the laboratory for measurement of arsenic and copper levels. Chelation treatment was initiated with Penicillamin (1800mg/day per os), before laboratorial confirmation, in order to address both copper and arsenic intoxication. Initial therapy with intramuscular Dimercaprol was postponed due to thrombocytopenia.
The patient completed 3 days of Penicillamin, followed by the administration of intramuscular Dimercaprol 200 mg at the following regime: 4-hourly in the first 6 days, then 6-hourly at day 7 and 8, and 12-hourly at day 9 and 10. Simultaneously, forced alkalinisation was performed, with fluid therapy as necessary.
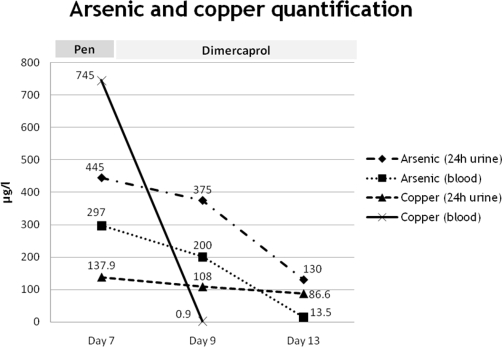
Quantitative measurements were available at in-hospital day 7 and confirmed high levels of arsenic and copper in urine and blood (Table 2). Further measurements demonstrated a progressive decline under the expected chelation reaction (Figure 1). In repeated blood gas analysis there was no methaemoglobin, a possible consequence of copper intoxication.
Table 2.
Arsenic and copper blood and urine concentration 90 hours after exposure (available at in-hospital day 7)
| Results (µg/L) | Normal values | |
|---|---|---|
| Arsenic | ||
| 24h urine | 445 | <100 µg/L |
| Blood | 297 | <70 µg/L |
| Copper | ||
| 24h urine | 137.9 | 15-60 µg/L |
| Blood | 745 | 0.7-1.5 µg/L |
Figure 1.
Plotted concentrations of arsenic and copper in blood and urine samples under chelation therapy. Chelating therapy was preceded, three days before, by one session of plasmapheresis. Penicillamine (Pen) was administrated between days 4 and 7, followed by dimercaprol from days 8 to 18. Normal values: arsenic (24 h urine) <100 µg/L; arsenic (blood) <70 µg/L; copper (24 h urine) = 15-60 µg/L; copper (blood) = 0.7-1.5 µg/L.
During in-hospital stay, patient’s symptoms gradually vanished and recovery of haematological, renal and liver disturbances was observed. He did not present any neurological or respiratory abnormalities. Syalorrhea and inflammatory signs at intramuscular injection sites developed as a consequence of dimercaprol treatment. After 13 days of chelation he was discharged and referred for follow-up appointments at the Internal Medicine and Nephrology Outpatient Clinic.
One month later he remained asymptomatic. Physical examination was unremarkable. Six months later his haemoglobin level and renal function were normal (Hb = 14.8 g/dL; MCV = 89.1 fL; MCHC = 34.1 g/dL; Cr = 1.02 mg/dL, Urea = 46 mg/dL). The patient was discharged.
Discussion
Arsenic is one the four most hazardous toxicants, along with cadmium, lead, and mercury. Metal poisoning can result from exposure to inhaled dusts, fumes or vapours. Another possible route comes from ingestion of contaminated food, drinks or by hand-to-mouth exposure [2-4].
This patient presented with many of the classical clinical findings attributed to arsenic acute intoxication. Initial unspecific features of acute gastrointestinal findings may be underappreciated, particularly if a recent exposure is not known. Acute toxicity with arsenic manifests within hours as nausea, vomiting, diarrhea, abdominal pain. Severe poisoning may result in multi-organic failure, delirium, seizures, coma, and ultimately death. If the patient survives, bone marrow suppression, peripheral neuropathy and skin lesions may develop [2-4].
Haemolytic anemia secondary to acute intoxication with arsenic is a well-recognized effect [2-4]. Arsenic-related haemolysis may also be a consequence of chronic intoxication [5].
The gaseous state is one the most toxic forms of arsenic [6]. Cases of intoxication with arsine gas have been published mostly as a result of accidental work-related exposures [6-9]. Because arsine is nonirritating and produces no immediate symptoms, persons exposed to hazardous levels may be unaware of its presence. In contrast with reported cases following arsenic poisoning [8,10-17], our patient did not develop respiratory failure.
Additionally, direct heart toxicity may result in disturbances of the electrical conduction system [2-4,15]. One report described an early manifestation of torsade de pointes after acute arsenic poisoning [18].
We can only retrospectively speculate about the initial exposure dosage, which possibly was not high enough to implicate direct respiratory or cardiac damage.
Treatment should be promptly initiated under high suspicion of a recent intoxication. Primary treatment in acute arsenic poisoning involves rapid decontamination and early initiation of chelation therapy with agents such as dimercaprol (British Anti-Lewsite, BAL), edentate (EDTA), succimer (DMSA, dimercaptosuccinic acid) and penicillamine. No specific antidote is available and there are no controlled trials regarding different therapeutic strategies [2-4].
A recent case report has suggested plasma exchange as an effective early treatment intervention for acute arsenic poisoning [19]. In our case, plasmapheresis was performed regarding the hypothesis of a haemolytic-uremic syndrome, before any toxicodrome was suspected. However, it may have contributed for the benign clinical course since chelating treatment was only initiated nearly 100 hours after exposure.
In this case, it is not possible to separate the haemolytic effect of copper from the arsenic one, since copper intoxication may also cause haemolysis [20]. Methaemoglobinemia is a frequent complication of copper poisoning which may require additional therapeutic strategies [20]. Its absence in the presented case points against a major contribution of this metal in patient’s clinical course.
Conclusion
We report a rare case of haemolytic anaemia following arsenic and copper acute intoxication. The unusual nature of this aetiology and the delayed awareness of a possible toxic exposure challenged the diagnosis.
Common consequences of arsenic poisoning are haemolysis and renal failure.
Because of the severity of the clinical course, including multi-organic failure and a high risk of death, therapeutic strategies should presumptively be initiated without formal laboratory confirmation. Evidence-based treatment options are limited. Use of chelating agents, such as dimercarprol, is recommended. Whether plasma exchange should be a primary, an alternative or additional treatment option remains unclear, although its early use may be an effective treatment.
Acknowledgments
The authors are grateful to Prof. Dra. Maria de Lourdes Bastos, Head of the Laboratory of Toxicology of the Faculty of Pharmacy of the University of Porto, for the collaboration in toxicological analyses.
Abbreviations
- ALP
alkaline phosphatase
- Cr
creatinine
- CRP
C-reactive protein
- DB
direct bilirubin
- FENa
fractional excretion of sodium
- γGT
gamma glutamyl transpeptidase
- Hb
haemoglobin
- HPF
high power field
- LDH
lactate dehydrogenase
- MCHC
mean corpuscular haemoglobin concentration
- MCV
medium corpuscular volume
- PLT
platelet
- RDW
red cell distribution width
- TB
total bilirubin
- WBC
white blood cell
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for the review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NC gathered patient information, interpreted data, did literature research, and wrote the first draft of the manuscript. CC contributed majorly to preparation of the manuscript and participated in management of plasmapheresis. JPA, JA and FF were responsible for the patient during admission to Intermediate Care Unit, including differential diagnosis, confirmation of final diagnosis and treatment decisions. AA was responsible for the management of the patient after discharge from the Intermediate care Unit and follow up at the outpatient clinic. All authors were involved in the clinical management of the patient, and read and approved the final manuscript.
This article is available from: http://casesjournal.com/casesjournal/article/view/7768
Contributor Information
Nuno Correia, Email: nunovox@gmail.com.
Catarina Carvalho, Email: catarinagcarvalho@sapo.pt.
Fernando Friões, Email: fbfrioes@gmail.com.
José P Araújo, Email: pauloaraujo@iol.pt.
Jorge Almeida, Email: jorge.salmeida@hsjoao.min-saude.pt.
Ana Azevedo, Email: anazev@med.up.pt.
References
- Luzzato L. Hemolytic Anemias and Anemia Due to Acute Bood Loss. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s Principle Internal Medicine. 17. New-York: McGraw-Hill; 2008. pp. 652–662. [Google Scholar]
- Arsenic exposure and poisoning. [http://www.uptodate.com. ]
- Ratnaike RH. Acute and chronic arsenic toxicity. Postgrad Medl J. 2003;79:391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. Arsenic. In: Goldfrank LR, Flomenbaum NE, Lewin N, Howland MA, Hoffman R, Nelson L, editors. Goldfrank's toxicologic emergencies. 7. New York: McGraw-Hill; 2002. pp. 1183–1195. [Google Scholar]
- Lee JJ, Kim YK, Cho SH, Park KS, Chung IJ, Cho D, Ryang DW, Kim HJ. Hemolytic anemia as a sequel of arsenic intoxication following long-term ingestion of traditional Chinese medicine. J Korean Med Sci. 2004;19:127–129. doi: 10.3346/jkms.2004.19.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakulska D, Czerczak S. Hazardous effects of arsine: a short review. Int J Occup Med Environ Health. 2006;19:36–44. doi: 10.2478/v10001-006-0003-z. [DOI] [PubMed] [Google Scholar]
- Romeo L, Apostoli P, Kovacic M, Martini S, Brugnone F. Acute arsine intoxication as a consequence of metal burnishing operations. Am J Ind Med. 1997;32:211–216. doi: 10.1002/(sici)1097-0274(199709)32:3<211::aid-ajim5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Barbee JY, Jr, Prince TS. Acute respiratory distress syndrome in a welder exposed to metal fumes. South Med J. 1999;92:510–512. doi: 10.1097/00007611-199905000-00012. [DOI] [PubMed] [Google Scholar]
- Sanz Guajardo A, Montero Garcia A, Moreno Heredia E, Sánchez Sicilia L. Hemolysis and acute renal failure in collective arsine poisoning (study of 5 cases) Rev Clin Esp. 1970;119:525–532. [PubMed] [Google Scholar]
- Dueñas-Laita A, Pérez-Miranda M, González-López MA, Martín-Escudero JC, Ruiz-Mambrilla M, Blanco-Varela J. Acute arsenic poisoning - case report. Lancet. 2005;365(9475):1982. doi: 10.1016/S0140-6736(05)66670-6. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Dawson AH. Arsenic trioxide poisoning: a description of two acute overdoses. Hum Exp Toxicol. 2004;23:359–364. doi: 10.1191/0960327104ht459cr. [DOI] [PubMed] [Google Scholar]
- Lai MW, Boyer EW, Kleinman ME, Rodig NM, Ewald MB. Acute arsenic poisoning in two siblings. Pediatrics. 2005;116:249–257. doi: 10.1542/peds.2004-1957. [DOI] [PubMed] [Google Scholar]
- Wang EE, Mahajan N, Wills B, Leikin J. Successful treatment of potentially fatal heavy metal poisonings. J Emerg Med. 2007;32:289–294. doi: 10.1016/j.jemermed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Vantroyen B, Heillier JF, Meulemans A, Michels A, Buchet JP, Vanderschueren S, Haufroid V, Sabbe M. Survival after a lethal dose of arsenic trioxide. J Toxicol Clin Toxicol. 2004;42:889–895. doi: 10.1081/CLT-200035344. [DOI] [PubMed] [Google Scholar]
- Civantos DP, López Rodríguez A, Aguado-Borruey JM, Narvaez JA. Fulminant malignant arrhythmia and multiorgan failure in acute arsenic poisoning. Chest. 1995;108:1774–1775. doi: 10.1378/chest.108.6.1774-a. [DOI] [PubMed] [Google Scholar]
- Greenberg C, Davies S, McGowan T, Schorer A, Drage C. Acute respiratory failure following severe arsenic poisoning. Chest. 1979;76:596–598. doi: 10.1378/chest.76.5.596. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, van Zijl P, Louw JA. Multiple organ failure with the adult respiratory distress syndrome in homicidal arsenic poisoning. Respiration. 1992;59:57–61. doi: 10.1159/000196026. [DOI] [PubMed] [Google Scholar]
- Ortega Carnicer J, Ruiz Lorenzo F, Mañas García D, Ceres Alabau F. Torsades de Pointes precoces y elevación sérica de la troponina I debidas a intoxicación aguda por arsénico. Med Intensiva. 2006;30:77–80. doi: 10.1016/S0210-5691(06)74473-4. [DOI] [PubMed] [Google Scholar]
- Song Y, Wang D, Li H, Hao F, Ma J, Xia Y. Severe acute arsine poisoning treated by plasma exchange. Clin Toxicol (Phila) 2007;45:721–727. doi: 10.1080/15563650701502675. [DOI] [PubMed] [Google Scholar]
- Franchitto N, Gandia-Mailly P, Georges B, Galinier A, Telmon N, Ducassé JL, Rougé D. Acute copper sulphate poisoning: a case report and literature review. Resuscitation. 2008;78(1):92–96. doi: 10.1016/j.resuscitation.2008.02.017. [DOI] [PubMed] [Google Scholar]