Abstract
Sialyl Tn (STn) and sialyl lactoside derivatives containing O-acetylated sialic acid residues have been synthesized using a one-pot three-enzyme system and conjugated to biotinylated human serum albumin (HSA) using an adipic acid para-nitrophenyl ester coupling reagent. This approach has been proven to be efficient for preparing carbohydrate-protein conjugates containing base-sensitive O-acetyl groups.
Introduction
Carbohydrates presented on the surface of mammalian cells and microorganisms are believed to play important roles in various molecular recognition processes.1 Many cell surface carbohydrates have terminal sialic acid residues2 which are directly involved in a variety of biological and pathological processes, including bacterial and viral infection, regulation of immue system,1a,3 and cancer metastasis.4
Some sialic acid-containing carbohydrates are considered to be tumor-associated carbohydrate antigens (TACAs) which are overexpressed by certain types of cancers.5 Sialyl-Tn antigen (STn, O-linked disaccharide Neu5Acα2,6GalNAc) is one of these TACAs. STn is expressed by 30% breast carcinomas6 and has been considered as an independent indicator for poor prognosis of cancer.7 Synthetic STn conjugated to a protein carrier: Keyhole Limpet Haemocyanin (KLH) has been developed by Biomira, Inc. (Edmonton, Alberta, Canada) as a cancer vaccine (Theratope®) for breast cancer patients.8 STn has also been used as one of seven epithelial cancer antigens and one of five prostate tumor-associated antigens in preparing unimolecular multivalent vaccines.9 A variety of chemical methods have been developed for the synthesis of STn and other sialylated oligosaccharides.10 More recently, enzymatic synthesis of STn oligosaccharides and glycopeptides has been achieved by using sialyltransferases, such as recombinant mouse ST6GalNAc-I,11 chicken ST6GalNAc-I,12 and α2,6-sialyltransferase from Photobacterium damsela (Pd2,6ST).13 The only type of sialic acid residues in these compounds, however, is N-acetylneuraminic acid (Neu5Ac), the predominant form of naturally occurring sialic acid. In nature, more than 50 structurally different sialic acid forms have been found, including Neu5Ac, N-glycolylneuraminic acid (Neu5Gc), keto-deoxynonulosonic acid (KDN), and their methylated, sulfated, lactylated, and single/multiple acetylated derivatives.2 Our groups have been interested in understanding the biological significance of naturally occurring sialic acid modifications. One of the approaches to elucidate the function of sialic acid modifications is to synthesize sialosides with naturally occurring modifications and couple them individually to an inert protein carrier for 96-well plate based high-throughput screening of sialic acid binding proteins. A general coupling method would be necessary to produce sialoside-protein conjugates, including those containing base-sensitive groups (e.g. acetyl groups).
Among coupling reagents that have been used for producing carbohydrate-protein conjugates, succinimide esters is very convenient and effective for amide bond formation.14 Its instability during chromatographic purification, however, limits its application. Maleimide-promoted conjugation, on the other hand, often results in disappointingly low yields as the result of a nonproductive disulfide dimerization of glycosides.15 Homobifunctional squaric acid diesters (e.g. diethyl squarate or didecyl squarate) have been used commonly as efficient cross-linking reagents for preparing carbohydrate-protein conjugations.16 The basic condition used for coupling (usually in aqueous solution of pH 9), however, limits its application on compounds containing base-sensitive groups such as O-acetyl groups. Bis(p-nitrophenyl) esters are known cross-linking agents for compounds with primary amines.17 Recently, one of these linkers, adipic acid p-nitrophenyl ester, has been used by the Bundle group as an efficient cross-linker for preparing neoglycoproteins.18 The neutral condition for coupling reaction makes this coupling reagent attractive. Here we report that the adipic acid p-nitrophenyl ester can be used as a general and efficient coupling reagent for producing sialoside-protein conjugates containing base-sensitive groups. The method has been used for conjugating biotinylated human serum albumin (HSA) and chemoenzymatically synthesized STn analogues containing Neu5Ac, Neu5Gc, and their 9-O-acetylated derivatives. Conjugation of biotinylated human serum albumin (HSA) and chemoenzymatically synthesized α2,6-linked sialyl lactosides containing Neu5Ac, Neu5Gc, and their 9-O-acetylated derivatives has also been achieved similarly. The retaining of the O-acetyl group in the biotinylated HSA-sialoside conjugates through the coupling conditions is confirmed by sialidase hydrolasis of the conjugate followed by ESI-MS analysis.
Results and discussion
Synthesis of sialyl Tn (STn) derivatives
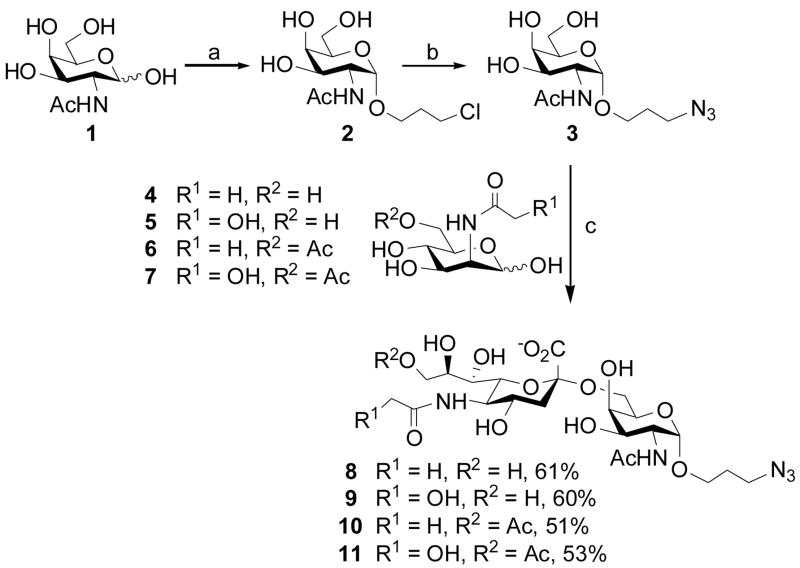
In order to prepare the sialyltransferase acceptor for chemoenzymatic synthesis of STn, 3-chloropropyl N-acetamido α-galactoside 2 was chemically synthesized by incubating N-acetylgalactosamine 1 with 3-chloropropanol at 70 °C in the presence of acetyl chloride. The chlorine group of 2 was displaced by an azide group by treating 2 with sodium azide in N,N-dimethylformamide and catalytic amount tetra-n-butylammonium iodide at 60 °C to form 3-azidopropyl N-acetamido α-galactoside 3 in quantitative yield (Scheme 1). Although 2 and 3 showed similar Rf values on TLC, they could be distinguished by 13C NMR spectra. The chemical shift of the carbon linked to the azido group in 3 was 48.29 ppm, while that of the carbon linked to chlorine in 2 was 41.52 ppm.
Scheme 1.
Chemoenzymatic synthesis of STn antigen analogs using a one-pot three-enzyme system. a) 3-chloropropanol, acetyl chloride, 70 °C, 87%; b) NaN3, NaI, DMF, 60 °C, 95%; c) 3 (20 mM), 4, 5, 6, or 7 (30 mM), pyruvate (150 mM), CTP (30 mM), MgCl2 (20 mM), a sialic acid aldolase, a CMP-sialic acid synthetase, Pd2,6ST, Tris-HCl (100 mM, pH 8.5 for 4 and 5; pH 7.5 for 6 and 7).
Chemoenzymatic sialylation of 3 was achieved using a highly efficient and convenient one-pot three-enzyme system established in our laboratory.19,21 In this system containing three recombinant bacterial enzymes including an E. coli K-12 aldolase,20 an N. meningitidis CMP-sialic acid synthetase (NmCSS),20 and a Photobacterium damsela α2,6-sialyltransferase (Pd2,6ST), N-acetylmannosamine (ManNAc), N-glycolylmannosamine (ManNGc), and their chemically synthesized 6-O-acetylated derivatives 4–719b were used as sialic acid precursors to obtain STn analogs containing Neu5Ac, Neu5Gc, 9-O-acetylated Neu5Ac, and 9-O-acetylated Neu5Gc 8–11 in yields varying from 51–61% (Scheme 1). The reactions were carried out at 37 °C in a Tris-HCl buffer (100 mM, pH 8.5 for substrates without O-acetyl groups; pH 7.5 for substrates with O-acetyl groups) containing Mg2+, pyruvate, CTP, a sialic acid precursor selected from 4–7, and 3 as a sialyltransferase acceptor. After the addition of appropriate amounts of aldolase (2.0 mg), NmCSS (0.8 mg), and Pd2,6ST (0.5 mg), the reaction was monitored by thin-layer chromatography (TLC) analysis (EtOAc:MeOH:H2O:HOAc = 5:2:1:0.1, by volume) and stained with p-anisaldehyde sugar stain. Sialoside products were purified by Bio-Gel P-2 gel filtration chromatography and the structures of all sialylated products were characterized by 1H and 13C NMR as wells as high resolution mass spectrometry (HRMS).
Synthesis of biotinylated HSA-STn conjugates
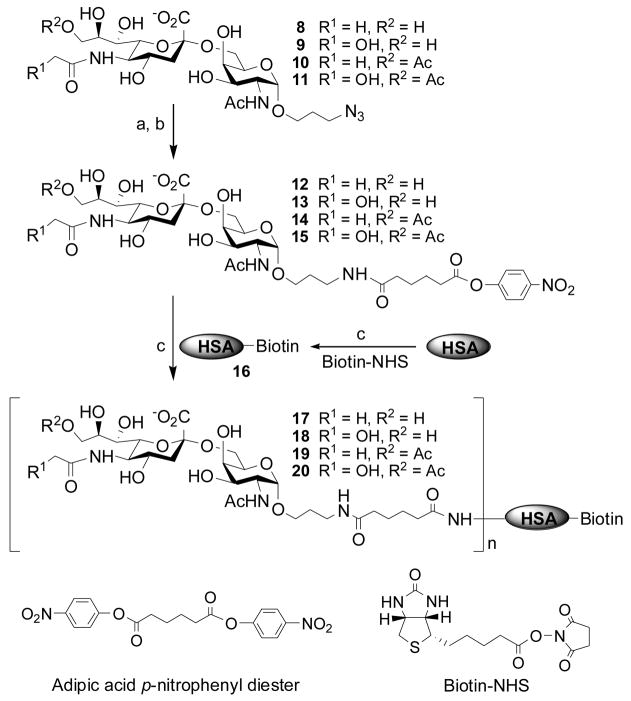
As shown in Scheme 2, biotinylated HSA was obtained by incubating one equivalent of HSA with four equivalents of N-hydroxy succinamide ester of biotin (Biotin-NHS). The biotin introduced can be used to immobilize the final conjugates to streptavidin-coated surface or to label the conjugates with streptavidin-linked molecules. In order to obtain sialoside and biotinylated HSA conjugates, half esters 12–15 were prepared by reducing the azido group in 8–11 to an amino group by catalytic hydrogenation (Pd/C in water) then reacting with an excess amount (5 equivalents) of adipic acid p-nitrophenyl diester18a in anhydrous DMF. The reaction was monitored by TLC (EtOAc:MeOH:H2O:HOAc = 4:2:1:0.1, by volume) under UV light. The disappearance of the starting material and the appearance of a UV absorbent product with a larger Rf value indicated the completion of the reaction. Products 12–15 were purified using a short silica gel column to remove the unreacted adipic acid ester. The presence of the 9-O-acetyl group at the sialic acid residue in 14 and 15 was confirmed by proton NMR, indicating the stablility of the O-acetyl group during hydrogenation and half ester formation.
Scheme 2.
Conjugation of STn antigens to human serum albumin (HSA) using adipic acid p-nitrophenyl diester coupling reagent. a) Pd/C, H2, H2O; b) adipic acid p-nitrophenyl diester, DMF, rt, > 90%; c) phosphate buffer (100 mM, pH 7.4). Average numbers of sialoside molecules incorporated were determined by MAlDI-TOF.
Finally, for coupling reactions, the purified sialoside half esters 12–15 (40 equivalents) were incubated individually with biotinylated HSA in phosphate buffer (100 mM, pH 7.4) for 24 h at room temperature before the produced conjugates were purified by HPLC using a gel filtration column. The purified conjugates were analyzed by MALDI-TOF mass spectroscopy to determine the numbers of the sialoside molecules that have been conjugated to the biotinylated HSA. The numbers obtained for the most populated conjugates are listed in Table 1. For example, MALDI-TOF data indicated a molecular ion of m/z 75,752 for glycoconjugate 17. Comparing to the m/z 67,218 for biotinylated HSA 16, one can estimate that 17 has an average of 12 sialoside molecules. The same estimation can be applied to other glycoconjugates 18–20.
Table 1.
The average numbers of sialosides that have been conjugated to biotinylated HSA determined by MALDI-TOF analysis
| Conjugate | 17 | 18 | 19 | 20 | 29 | 30 | 31 | 32 | 16 (Biotin-HSA) |
|---|---|---|---|---|---|---|---|---|---|
| Peak m/z in MALDI-TOF spectrum | 75,752 | 76,826 | 77,853 | 75,971 | 76,827 | 77,079 | 75,938 | 75,871 | 67,218 |
| Average number of sialosides per conjugate | 12 | 13 | 14 | 11 | 11 | 12 | 10 | 10 | 0 |
In order to study whether the O-acetyl group is preserved throught the conjugation conditions, the conjugate 19 containing Neu5,9Ac2α2,6GalNAc was treated with commercially available Arthrobacter ureafaciens sialidase and Clostridium perfingens sialidase (both of these sialidases can hydrolyze terminal 9-O-acetylated Neu5Ac residue) in ammonium acetate buffer at pH 5.0.22 The sialidase reaction mixture was subjected to mass spectrometric analysis (ESI-MS). Only hydrolyzed Neu5,9Ac2 was detected and no signal was observed for Neu5Ac, indicating that the 9-O-acetyl group on Neu5,9Ac2α2,6GalNAc was well perserved during the conjugation process.
Synthesis of biotinylated HSA-sialyl lactoside conjugates
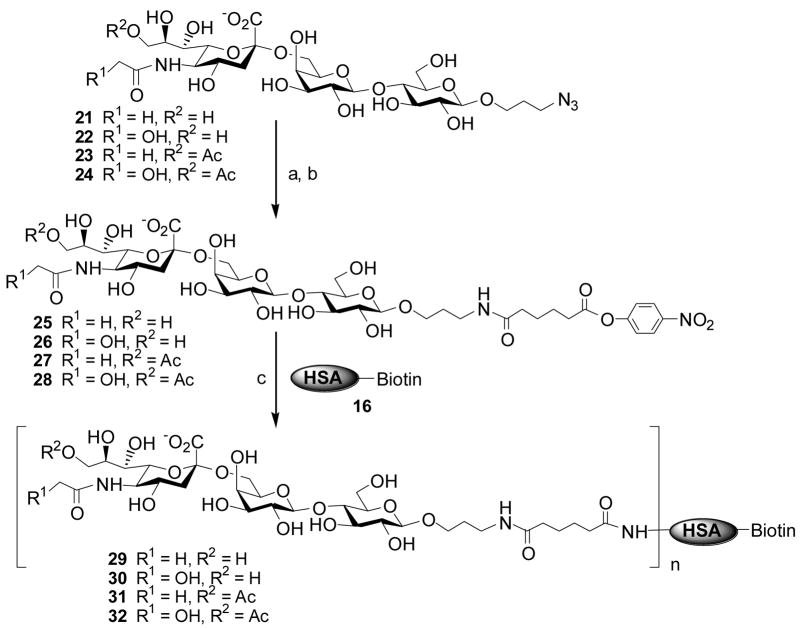
In order to evaluate the generality of applying the adipic acid p-nitrophenyl diester in carbohydrate-protein conjugation, another set of sialoside-biotinylated HSA conjugates were prepared similarly. In this case, α2,6-linked sialyl lactosides 21–24 were synthesized by the one-pot three-enzyme system using 3-azidopropyl β-D-galactopyranose-(1→4)-β-D-glucopyranoside (LacβProN3) as a sialyltransferase acceptor as described previously.19b Following the same coupling protocol as described above for the production of biotinylated HSA-STn conjugates, biotinylated HSA-sialyllactose conjugates 29–32 were successfully obtained (Scheme 3). MALDI-TOF analysis gave the average numbers of sialosides incorporated into the conjugates (Table 1).
Scheme 3.
Conjugation of sialyllactosides to human serum albumin (HSA) using adipic acid p-nitrophenyl diester coupling reagent. a) H2, Pd/C, H2O; b) adipic acid p-nitrophenyl diester, DMF, rt, > 90%; c) phosphate buffer (100 mM, pH 7.4). Average numbers of sialoside molecules incorporated were determined by MAlDI-TOF.
Comparing the results listed in Table 1, one can conclude that the degree of incorporation of a disaccharide STn sialoside to HSA (11–14) is similar to but a little bit higher than that for the conjugation of a trisaccharide sialyllactoside to HSA (10–12). Overall, 10 to 14 sialoside molecules were incorporated onto one molecule of HSA when 40-fold equivalents of activated STn or sialyl lactosides were used. These results are close to those reported for the coupling of oligosaccharides to bovine serum albumin (BSA) or tetanus toxiod (TT),18b,18c although higher conjugation yields were achieved for coupling monosaccharides or a lactoside disaccharide to bovine serum albumin.18a
Conclusions
In summary, we have demonstrated here that the one-pot three-enzyme system is very efficient in synthesizing STn analogs containing different forms of sialic acid. In addition, we have also proven that the adipic acid p-nitrophenyl ester is a very effective and general coupling reagent to produce carbohydrate-protein conjugates, including those with base-sensitive groups. The conjugates can now be used to study the significance of 9-O-acetyl groups in the binding properties of sialoside to various lectins and antibodies that recognize sialic acids.
Experimental
General methods
Chemicals were purchased and used without further purification. 1H NMR (300, 400, or 600 MHz) and 13C NMR (75 or 100 MHz) spectra were recorded on a Varian Mercury-300, a Varian Inova-400, or a Varian Inova-600 spectrometer. Low and high resolution electrospray ionization (ESI) mass spectra were obtained at the Mass Spectrometry Facility in the Ohio State University. Silica gel 60 Å (40–63 μm, Sorbent technologies) was used for flash chromatography. Analytical thin-layer chromatography was performed on silica gel plates 60 GF254 (Sorbent technologies) using anisaldehyde stain for detection. Gel filtration chromatography was performed using a column (100 cm × 2.5 cm) packed with BioGel P-2 Fine resins (Bio-Rad, Hercules, CA). Arthrobacter ureafaciens sialidase was purchased from MP Biomedicals. Clostridium perfingens sialidase (type Vi) was from Sigma.
Starting materials
Sodium pyruvate and ManNAc 4 were bought from Sigma. GalNAc derivative 3, ManNGc 5, 9-OAc-ManNAc 6, 9-OAc-ManNGc 7, and sialosides 21–24 were synthesized as described previously.19,20
3-Azidopropyl O-[Sodium (5-acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)]-(2→6)-O-2-acetamido-2-deoxy-α-D-galactopyranoside (8, Neu5Acα2,6GalNAcαProN3)
Yield, 61%; white foam. 1H NMR (600 MHz, D2O) δ 4.70 (d, 1H, J = 3.6 Hz), 3.97 (dd, 1H, J = 3.6 and 10.8 Hz), 3.87 (dd, 1H, J = 4.2 and 7.8 Hz), 3.83 (d, 1H, J = 3.0 Hz), 3.76–3.61 (m, 5H), 3.55–3.26 (m, 9H), 2.56 (dd, 1H, J = 4.8 and 12.0 Hz, H-3eq″), 1.87, 1.86 (2s, 2CH3), 1.74 (m, 2H), 1.51 (t, 1H, J = 12.0 Hz, H-3ax″); 13C NMR (75 MHz, D2O) δ 175.16, 174.68, 173.57, 100.49, 97.20, 72.69, 71.91, 69.74, 69.62, 68.66, 68.38, 67.61, 65.28, 63.97, 62.75, 51.99, 50.07, 48.29, 40.40, 28.06, 22.18, 22.08. HRMS (ESI) m/z calcd for C22H36N5O14Na2 (M+Na) 640.2054, found 640.2070.
3-Azidopropyl O-[Sodium (5-glycolylamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)]-(2→6)-O-2-acetamido-2-deoxy-α-D-galactopyranoside (9, Neu5Gcα2,6GalNAcαProN3)
Yield, 60 %; white foam. 1H NMR (600 MHz, D2O) δ 4.71 (d, 1H, J = 3.6 Hz), 3.97 (dd, 1H, J = 3.6 and 11.4 Hz), 3.95 (s, 2H), 3.87 (dd, 1H, J = 3.6 and 7.8 Hz), 3.83 (d, 1H, J = 3.6 Hz), 3.77–3.58 (m, 8H), 3.53–3.26 (m, 8H), 2.57 (dd, 1H, J = 4.8 and 12.6 Hz, H-3eq″), 1.87 (s, CH3), 1.74 (m, 2H), 1.53 (t, 1H, J = 12.0 Hz, H-3ax″); 13C NMR (75 MHz, D2O) δ175.97, 174.71, 173.60, 100.56, 97.25, 72.47, 72.00, 69.68, 68.71, 68.37, 68.16, 67.68, 65.35, 64.03, 62.77, 61.16, 51.73, 50.12, 48.35, 40.49, 28.10, 22.13. HRMS (ESI) m/z calcd for C22H36N5Na2O15 (M+Na) 656.2003, found 656.2015.
3-Azidopropyl O-[Sodium (9-O-acetyl-5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)]-(2→6)-O-2-acetamido-2-deoxy-α-D-galactopyranoside (10, Neu5,9Ac2α2,6GalNAcαProN3)
Yield, 51%; white foam. 1H NMR (600 MHz, D2O) δ 4.74 (d, 1H, J = 3.6 Hz), 4.28 (dd, 1H, J = 2.4 and 11.4 Hz), 4.06 (dd, 1H, J = 6.6 and 12.0 Hz), 4.00 (dd, 1H, J = 3.6 and 10.8 Hz), 3.97–3.47 (m, 5H), 3.40–3.29 (m, 6H), 2.59 (dd, 1H, J = 4.8 and 12.6 Hz, H-3eq″), 1.99, 1.90, 1.89 (3s, 3CH3), 1.77 (m, 2H), 1.54 (t, 1H, J = 12.0 Hz, H-3ax″); 13C NMR (75 MHz, D2O) δ 175.13, 174.69, 174.53, 173.56, 100.52, 97.21, 72.50, 69.66, 69.39, 68.67, 68.40, 67.63, 65.82, 65.31, 64.00, 51.96, 50.08, 48.30, 40.42, 28.07, 22.19, 22.07, 20.40. HRMS (ESI) m/z calcd for C24H38N5Na2O15 (M+Na) 682.2160, found 682.2174.
3-Azidopropyl O-[Sodium (9-O-acetyl-5-glycolylamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)]-(2→6)-O-2-acetamido-2-deoxy-α-D-galactopyranoside monosodium salt (11, Neu9Ac5Gcα2,6GalNAcαProN3)
Yield, 53%; white foam. 1H NMR (400 MHz, D2O) δ 4.70 (d, 1H, J = 4.0 Hz), 3.97 (d, 1H, J = 9.6 Hz), 4.04–3.97 (m, 2H), 3.95 (s, 2H), 3.93–3.26 (m, 14H), 2.56 (dd, 1H, J = 4.4 and 12.4 Hz, H-3eq″), 1.95, 1.86 (2s, 2CH3), 1.73 (m, 2H), 1.52 (t, 1H, J = 12.0 Hz, H-3ax″); 13C NMR (75 MHz, D2O) δ 175.87, 174.67, 173.52, 173.57, 100.51, 97.20, 72.21, 69.68, 69.44, 68.65, 68.20, 68.27, 67.61, 65.77, 65.30, 64.04, 61.10, 51.63, 50.07, 48.28, 40.46, 28.08, 22.07, 20.40. HRMS (ESI) m/z calcd for C24H38N5O16 (M−Na) 652.2313, found 652.2333.
General procedure for enzymatic sialylation of 3 in one-pot three-enzyme system
ManNAc 4, ManNGc 5, or their 6-O-acetylated derivatives 6 or 7 (50–100 mg, 1.5 equiv), GalNAcαProN3 3 (1.0 equiv), sodium pyruvate (7.5 equiv), CTP (1.5 equiv) were dissolved in 5 mL Tris-HCl buffer (100 mM, pH = 8.5 for 4 and 5, pH = 7.5 for 6 and 7). After the addition of E. coli K-12 sialic acid aldolase (2.0 mg), N. meningitidis CMP-sialic acid synthetase (NmCSS) (0.8 mg), and Pd2,6ST (0.5 mg), the total volume of the reaction mixture was brought to 10 mL by adding water. The reaction solution was incubated at 37 °C with agitation at 140 rpm for 2–4 hours. The reaction was monitored by TLC analysis (EtOAc:MeOH:H2O:HOAc = 5:2:1:0.1, by volume) and stained with p-anisaldehyde sugar stain. When no further product was detected, 10 mL of 95% EtOH was added to the reaction. The precipitates were removed by centrifugation. The supernatant was concentrated by rotary evaporation and purification by a Bio-Gel P-2 gel filtration column to give STn products.
Protocol for preparing biotinylated HSA-sialoside conjugates using adipic acid p-nitrophenyl diester coupling reagent
A sialoside selected from 8–11 or 21–24 (20–30 mg, 0.03–0.05 mmol) was dissolved in water (2 mL) and the mixture was stirred under hydrogen atmosphere in the presence of 10% palladium on charcoal for overnight to afford the reduced product. Adipic acid p-nitrophenyl ester (5 mol equiv) in anhydrous DMF was added. The reaction was monitored by TLC (EtOAc:MeOH:H2O:HOAc = 4:2:1:0.1, by volume) under UV light. The disappearance of the starting material and the appearance of a UV absorbent product with a larger Rf value indicated the completion of the reaction. After stirring for overnight, the reaction mixture was concentrated and purified by flash chromatography to afford the sialoside half ester selected from 12–15 or 25–28. For the conjugation reaction, a sialoside half ester (~ 40 mol equiv) was added to a solution containing biotinylated human serum albumin (2.0 mg mL−1) in phosphate buffer (pH 7.4, 100 mM). The mixture was stirred gently at room temperature for 24 h. The resulted solution was concentrated using a centrifugal filter (Centricon Plus-20) and purified by high performance liquid chromatography (HPLC) or fast-performance liquid chromatography (FPLC) using a gel filtration column (Superdex 75 10/300 GL column) to afford biotinylated HSA-sialoside conjugate. The obtained HSA-sialoside conjugates were charactered by MALDI-TOF.
Compound 12 (p-Nitrophenyl ester of 8)
1H NMR (600 MHz, D2O) δ 8.16 (d, 2H, J = 9.0 Hz), 7.20 (d, 2H, J = 9.0 Hz), 4.70 (d, 1H, J = 3.6 Hz), 3.97-3.09 (m, 21H), 2.56-2.53 (m, 3H), 2.14 (m, 2H), 1.85 (s, 3H, NHC(O)CH3), 1.84 ((s, 3H, NHC(O)CH3), 1.65 (m, 2H), 1.54-1.46 (m, 3H).
Compound 13 (p-Nitrophenyl ester of 9)
1H NMR (600 MHz, D2O) δ 8.16 (d, 2H, J = 9.0 Hz), 7.21 (d, 2H, J = 9.0 Hz), 4.71 (d, 1H, J = 3.6 Hz), 3.97 (dd, 1H, J = 3.6 and 11.4 Hz), 3.95 (s, 2H, CH2OH), 3.85-3.10 (m, 22H), 2.58-2.55 (m, 3H), 2.15 (m, 2H), 1.86 (s, NHC(O)CH3), 1.66 (m, 2H), 1.56-1.45 (m, 3H).
Compound 14 (p-Nitrophenyl ester of 10)
1H NMR (600 MHz, D2O) δ 8.18 (d, 2H, J = 9.0 Hz), 7.22 (d, 2H, J = 8.4 Hz), 4.74 (d, 1H, J = 3.6 Hz), 4.22 (dd, 1H, J = 1.8 and 11.4 Hz), 4.03-3.11 (m, 17H), 2.58-2.53 (m, 3H), 2.16 (m, 2H), 1.95 (s, 3H, OC(O)CH3),, 1.87 (s, 3H, NHC(O)CH3)., 1.86 (s, 3H, NHC(O)CH3), 1.67 (m, 2H), 1.57-1.48 (m, 3H).
Compound 15 (p-Nitrophenyl ester of 11)
1H NMR (600 MHz, D2O) δ 8.19 (d, 2H, J = 8.4 Hz), 7.23 (d, 2H, J = 9.6 Hz), 4.70 (d, 1H, J = 4.0 Hz), 4.24-3.13 (m, 21H), 3.97 (s, 2H, CH2OH), 2.56 (m, 3H), 2.17 (m, 2H), 1.96 (s, 3H, OC(O)CH3), 1.87 (s, 3H, NHC(O)CH3), 1.67 (m, 2H), 1.60-1.50 (m, 5H).
Compound 25 (p-Nitrophenyl ester of 21)
1H NMR (600 MHz, D2O) δ 8.18 (d, 2H, J = 9.6 Hz), 7.22 (d, 2H, J = 9.0 Hz), 4.28 (d, 1H, J = 7.8 Hz), 4.23 (d, 1H, J = 8.4 Hz), 3.82-3.12 (m, 27H), 2.58-2.52 (m, 3H), 2.14 (m, 2H), 1.86 (m, 2H), 1.85 (s, 3H), 1.68-1.55 (m, 5H).
Compound 26 (p-Nitrophenyl ester of 22)
1H NMR (600 MHz, D2O) δ 8.00 (d, 2H, J = 8.4 Hz), 6.78 (d, 2H, J = 7.4 Hz), 4.30 (d, 1H, J = 7.8 Hz), 4.24 (d, 1H, J = 7.8 Hz), 3.94 (s, 2H, CH2OH), 3.81-3.01 (m, 27H), 2.55 (dd, 1H, J = 5.4 and 12.6 Hz, H-3eq″), 2.10-2.06 (m, 4H), 2.02 (m, 2H), 1.74 (m, 2H), 1.58 (t, 1H, J = 12.6 Hz, H-3ax″), 1.44-1.37 (m, 4H).
Compound 27 (p-Nitrophenyl ester of 23)
1H NMR (400 MHz, D2O) δ 8.19 (d, 2H, J = 9.2 Hz), 7.22 (d, 2H, J = 8.8 Hz), 4.28 (d, 1H, J = 7.6 Hz), 4.07-3.11 (m, 28H), 2.57-2.50 (m, 3H), 2.15 (m, 2H), 1.95 (s, 3H, OC(O)CH3), 1.86 (s, 3H, NHC(O)CH3), 1.74 (m, 2H), 1.67-1.52 (m, 5H).
Compound 28 (p-Nitrophenyl ester of 24)
1H NMR (600 MHz, D2O) δ 8.18 (d, 2H, J = 9.0 Hz), 7.22 (d, 2H, J = 9.0 Hz), 4.27 (d, 1H, J = 7.8 Hz), 4.24-4.22 (m, 2H), 4.02 (dd, 1H, J = 6.0 and 12.0 Hz), 3.95 (s, 2H, CH2OH), 3.93 (m, 1H), 3.85-3.10 (m, 24H), 2.56 (m, 3H), 2.15 (m, 2H), 1.96(s, 3H, OC(O)CH3), 1.94 (m, 2H), 1.67-1.55 (m, 5H).
Sialidase treatment of biotinylated HSA-sialoside conjugate 19 and MS analysis
To confirm the presence of O-acetylated sialosdies in HSA conjugates, conjugate 19 (1.6 mg/mL) was incubated in a final volume of 15 μL with Arthrobacter ureafaciens sialidase (0.75 mU) and Clostridium perfingens sialidase (15 mU) for 1 h at 37 °C in ammonium acetate buffer (pH 5.0, 100 mM). Mass spectrometric analysis (ESI-MS) of the reaction solution was carried out immediate and showed the mass ion of Neu5,9Ac2 at 350.20 a.m.u (M-H). No signal for de-O-acetylated Neu5Ac was observed in the MS spectrum.
Supplementary Material
Acknowledgments
This work was supported by NIH R01GM076360 and the start-up funds to X. C. from the Regents of the University of California.
Footnotes
Electronic Supplementary Information (ESI) available: NMR spectra for the compounds 8–15, 25–28.
References
- 1.(a) Varki A. Glycobiology. 1993;3:97. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Koeller KM, Wong CH. Nat Biotechnol. 2000;18:835. doi: 10.1038/78435. [DOI] [PubMed] [Google Scholar]
- 2.(a) Angata T, Varki A. Chem Rev. 2002;102:439. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]; (b) Schauer R. Glycoconj J. 2000;17:485. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilatte Y, Bignon J, Lambre CR. Glycobiology. 1993;3:201. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kelm S, Schauer R. Int Rev Cytol. 1997;175:137. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Varki A. Glycobiology. 1992;2:25. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakomori S. Adv Exp Med Biol. 2001;491:369. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 6.Sewell R, Backstrom M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, Gatgens J, Clausen H, Hansson GC, Burchell J, Taylor-Papadimitriou J. J Biol Chem. 2006;281:3586. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 7.(a) Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Cancer. 1990;66:1960. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]; (b) Nakagoe T, Sawai T, Tsuji T, Jibiki MA, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K, Ishikawa H. Anticancer Res. 2002;22:451. [PubMed] [Google Scholar]
- 8.Holmberg LA, Sandmaier BM. Expert Rev Vaccines. 2004;3:655. doi: 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ragupathi G, Koide F, Sathyan N, Kagan E, Spassova M, Bornmann W, Gregor P, Reis CA, Clausen H, Danishefsky SJ, Livingston PO. Cancer Immunol Immunother. 2003;52:608. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keding SJ, Danishefsky SJ. Proc Natl Acad Sci USA. 2004;101:11937. doi: 10.1073/pnas.0401894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Iijima H, Ogawa T. Carbohydr Res. 1988;172:183. doi: 10.1016/s0008-6215(00)90853-x. [DOI] [PubMed] [Google Scholar]; (b) Schwarz JB, Kuduk SD, Chen XT, Sames D, Glunz PW, Danishefsky SJ. J Am Chem Soc. 1999;121:2662. [Google Scholar]; (c) Wu J, Guo ZW. Bioconjug Chem. 2006;17:1537. doi: 10.1021/bc060103s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SK, Schwientek T, Holm B, Reis CA, Clausen H, Kihlberg J. J Am Chem Soc. 2001;123:11117. doi: 10.1021/ja015570t. [DOI] [PubMed] [Google Scholar]
- 12.Blixt O, Allin K, Pereira L, Datta A, Paulson JC. J Am Chem Soc. 2002;124:5739. doi: 10.1021/ja017881+. [DOI] [PubMed] [Google Scholar]
- 13.Teo CF, Hwang TS, Chen PH, Hung CH, Gao HS, Chang LS, Lin CH. Adv Synth Catal. 2005;347:967. [Google Scholar]
- 14.(a) Meikle PJ, Bundle DR. Glycoconj J. 1990;7:207. [Google Scholar]; (b) Kondejewski LH, Kralovec JA, Blair AH, Ghose T. Bioconjug Chem. 1994;5:602. doi: 10.1021/bc00030a016. [DOI] [PubMed] [Google Scholar]
- 15.(a) Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, Harris C, Glunz PW, Livingston PO, Danishefsky SJ. Proc Natl Acad Sci USA. 2002;99:13699. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wan Q, Chen JH, Chen G, Danishefsky SJ. J Org Chem. 2006;71:8244. doi: 10.1021/jo061406i. [DOI] [PubMed] [Google Scholar]; (c) Ni J, Singh S, Wang LX. Bioconjug Chem. 2003;14:232. doi: 10.1021/bc025617f. [DOI] [PubMed] [Google Scholar]
- 16.(a) Tietze LF, Arlt M, Beller M, Glusenkamp KH, Jahde E, Rajewsky MF. Chem Ber. 1991;124:1215. [Google Scholar]; (b) Tietze LF, Schroter C, Gabius S, Brinck U, Goerlach-Graw A, Gabius HJ. Bioconjug Chem. 1991;2:148. doi: 10.1021/bc00009a003. [DOI] [PubMed] [Google Scholar]; (c) Kamath VP, Diedrich P, Hindsgaul O. Glycoconj J. 1996;13:315. doi: 10.1007/BF00731506. [DOI] [PubMed] [Google Scholar]; (d) Bergh A, Magnusson BG, Ohlsson J, Wellmar U, Nilsson UJ. Glycoconj J. 2001;18:615. doi: 10.1023/a:1020639603070. [DOI] [PubMed] [Google Scholar]; (e) Johansson SM, Arnberg N, Elofsson M, Wadell G, Kihlberg J. ChemBioChem. 2005;6:358. doi: 10.1002/cbic.200400227. [DOI] [PubMed] [Google Scholar]; (f) Blixt O, Norberg T. Carbohydr Res. 1999;319:80. doi: 10.1016/s0008-6215(99)00135-4. [DOI] [PubMed] [Google Scholar]; (g) Owen RM, Carlson CB, Xu J, Mowery P, Fasella E, Kiessling LL. ChemBioChem. 2007;8:68. doi: 10.1002/cbic.200600339. [DOI] [PubMed] [Google Scholar]; (h) Chernyak A, Oscarson S, Turek D. Carbohydr Res. 2000;329:309. doi: 10.1016/s0008-6215(00)00189-0. [DOI] [PubMed] [Google Scholar]; (i) Chernyak A, Karavanov A, Ogawa Y, Kovac P. Carbohydr Res. 2001;330:479. doi: 10.1016/s0008-6215(01)00018-0. [DOI] [PubMed] [Google Scholar]
- 17.Graminski GF, Carlson CL, Ziemer JR, Cai F, Vermeulen NM, Vanderwerf SM, Burns MR. Bioorg Med Chem Lett. 2002;12:35. doi: 10.1016/s0960-894x(01)00659-x. [DOI] [PubMed] [Google Scholar]
- 18.(a) Wu X, Ling CC, Bundle DR. Org Lett. 2004;6:4407. doi: 10.1021/ol048614m. [DOI] [PubMed] [Google Scholar]; (b) Wu X, Bundle DR. J Org Chem. 2005;70:7381. doi: 10.1021/jo051065t. [DOI] [PubMed] [Google Scholar]; (c) Rich JR, Wakarchuk WW, Bundle DR. Chem Eur J. 2006;12:845. doi: 10.1002/chem.200500518. [DOI] [PubMed] [Google Scholar]
- 19.(a) Yu H, Chokhawala H, Karpel R, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J Am Chem Soc. 2005;127:17618. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]; (b) Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Angew Chem Int Ed Engl. 2006;45:3938. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Chokhawala HA, Huang S, Chen X. Nat Protoc. 2006;1:2485. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chokhawala HA, Yu H, Chen X. ChemBioChem. 2007;8:194. doi: 10.1002/cbic.200600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.