Abstract
Background
Anaplastic large cell lymphoma (ALCL) is characterized by advanced disease at presentation (70-80% of pediatric cases) and accounts for 10-15% of all childhood lymphomas. Treatment strategies for pediatric ALCL vary from short pulse B-NHL chemotherapy to prolonged leukemia like therapy. The optimal treatment strategy is unknown.
Methods
CCG-5941 used a compressed aggressive multiagent T-cell lineage chemotherapy regimen consisting of a three week induction therapy (vincristine, prednisone, cyclophosphamide, daunomycin, asparaginase) followed by a three-week consolidation period (vincristine, prednisone, etoposide, 6-thioguanine, cytarabine, asparaginase, methotrexate) followed by six courses of maintenance chemotherapy at 7-week intervals (cyclophosphamide, 6-thioguanine, vincristine, prednisone, doxorubicin, asparaginase, methotrexate etoposide, cytarabine). Total therapy was 48 weeks.
Results
86 children (male 56%, female 44%) with non-localized ALCL (CD30+) were treated. The majority of tumors were positive for ALK (90%) and of T lineage (83%). Extranodal disease was common (mediastinum 35%, skin 15%, lung 14%, bone 12%, bone marrow 13%, liver 6%, and other viscera 17%). Grade 4 neutropenia occurred in 82% of patients. The 5 year EFS was 68% (95% CI of 57-78%) and the 5 year OS was 80% (95% CI of 69-87%). There were 21 relapses and 4 toxic deaths as first events. Relapse occurred early with 17 (81%) relapses occurring within 2 years of diagnosis and 12 (57%) while receiving therapy. Univariate analysis for risk factors only identified bone marrow involvement predicting lower EFS (P=0.03).
Conclusions
CCG-5941 demonstrated efficacy similar to previously reported regimens but with significant hematologic toxicity.
Keywords: Anaplastic Large Cell Lymphoma, CD30, ALK positive lymphoma, Pediatric, non-Hodgkin lymphoma, t(2;5)
Introduction
Anaplastic large cell lymphoma (ALCL) is a distinct form of non-Hodgkin lymphoma (NHL) which accounts for 10-15% of all childhood lymphomas [1;2]. First described in 1985 by Stein et al. ALCL is a T or null cell lymphoma characterized by the malignant cell expression of CD30 (Ki-1) [3]. The World Health Organization divides ALCL into a systemic form and a primary cutaneous form [4]. Systemic ALCL is more common than the cutaneous form and most frequently occurs in the first three decades of life. Clinically, systemic ALCL is characterized by advanced disease at presentation (75% of pediatric ALCL) with a high incidence of nodal involvement (>90%), frequent association with B symptoms (75%), and frequent extra-nodal involvement including skin (25%), lung (10%), bone (17%) and liver (8%) [2;5-10]. CNS involvement is rare.
Systemic ALCL is characterized by CD30 (Ki-1) positive anaplastic cells and exhibits a broad morphological spectrum (i.e. common, lymphohistiocytic, and small cell variants). The anaplastic large cell lymphoma kinase (ALK) protein has been postulated to be the pathogenesis for the majority of ALCL cases [11]. The ALK protein is a fusion protein produced by a genetic translocation most commonly t(2;5) involving the ALK gene on chromosome 2 and the nucleophosmine (NPM) gene on chromosome 5. The majority of ALCL in children is ALK positive [12], but ALK negative cases do occur. This differs from adults where ALK negative cases predominant and precludes comparison of treatment results between pediatric and adult studies [13;14].
Treatment strategies for pediatric ALCL vary from short pulse B-cell lineage NHL chemotherapy lasting a few months to prolonged 2 year therapy similar to the treatment for lymphoblastic lymphoma/leukemia [2;7-10;15]. This is a report of 86 children with non-localized ALCL treated with compressed aggressive multiagent chemotherapy designed for T-cell lineage malignancies.
Patients and Methods
Patients
Patients newly diagnosed with non-localized ALCL (CD30+) up to the age of 21 years were eligible. All patients underwent physical exam, computed tomography (CT) scans, skeletal scintigraphy, complete blood count, bone marrow exam, cerebrospinal fluid exam, and LDH level to determine extent of disease. Patients with mediastinal or bone disease only were considered to have non-localized disease and were eligible. Patients with central nervous system (CNS) disease defined as >5 white blood cells with blasts on cytospin examination, cranial nerve palsy, or radiographic evidence of a mass lesion in the CNS were eligible except those requiring emergency radiation therapy. Emergency therapy for management of airway obstruction and/or superior vena cava syndrome was allowed provided that bone marrow and CSF exams were obtained prior to steroid therapy and that protocol therapy was begun within 72 hours. Local institutional review board approval and written informed consent was obtained prior to enrollment of all patients.
Treatment Protocol
CCG-5941 was a pilot study to estimate the feasibility of a compressed aggressive multiagent T-cell lineage chemotherapy regimen. Patients with T-cell lymphoblastic lymphoma or CD30 positive ALCL were eligible. After June 1996 PEG asparaginase was incorporated into the treatment.
The treatment protocol is shown in Table I. A three week induction therapy was followed by a three-week consolidation period. Days 0 and 16 of consolidation were dependent on neutrophil and platelet recovery with G-CSF utilized during these phases. After consolidation patients received six courses of maintenance chemotherapy at 7-week intervals. Day 0 and day 35 of each maintenance course began when the absolute neutrophil count and platelet count recovered. During maintenance, G-CSF was given only for delays in therapy secondary to myelosuppression. Patients not in complete remission after 1 course of maintenance chemotherapy were considered early failures and discontinued protocol therapy. Prophylactic craniospinal radiation was not used, though patients that were CNS positive at diagnosis received 1800 cGy cranial radiation therapy at the end of therapy.
Table I. Chemotherapy Schedule.
| Drug | Dose | Schedule |
|---|---|---|
| Induction (Days 0 – 20) | ||
| Vincristine | 1.5 mg/m2 | Days 0, 7, 14 |
| Prednisone | 60 mg/m2/day | Days 0-20 |
| Cyclophosphamide | 1200 mg/m2 | Day 0 |
| Daunomycin | 60 mg/m2 over 48 hours | Day 1 |
| PEG Asparaginase | 2000 IU/m2 | Day 3 |
| Intrathecal cytarabine | Age dependent | Day 0 |
| Intrathecal methotrexate | Age dependent | Day 14 (+ Days 7 and 21 for CNS positive) |
| G-CSF | 5 mcg/kg/day | Day 4 |
| Consolidation (Days 0-20) | ||
| Vincristine | 1.5 mg/m2 | Days 0, 7 |
| Prednisone | Taper from induction | Days 0-6 |
| Etoposide | 200 mg/m2 | Days 0, 1 |
| 6-Thioguanine | 300 mg/m2 | Days 0-3 |
| Cytarabine | 2000 mg/m2 over 3 hours | Days 0, 1 |
| PEG Asparaginase | 1750 IU/m2 | Day 9 |
| Methotrexate | 1 g/m2 over 24 hours | Day 16 |
| Intrathecal methotrexate | Age dependent | Days 14, 21 (+ Day 7 for CNS positive) |
| G-CSF | 5 mcg/kg/day | Day 4 |
| Maintenance (6 courses of 7 week cycles) | ||
| Cyclophosphamide | 1200 mg/m2 | Day 0 |
| 6-Thioguanine | 300 mg/m2 | Days 0-3 |
| Vincristine | 1.5 mg/m2 | Days 14, 21, 28 |
| Prednisone | 180 mg/m2/day | Days 14-20 |
| Doxorubicin | 30 mg/m2 over 1 hours | Day 14 |
| Methotrexate | 1 g/m2 over 24 hours | Day 28 |
| PEG Asparaginase | 1750 IU/m2 | Day 28 |
| Etoposide | 200 mg/m2 | Days 35,36 |
| Cytarabine | 2000 mg/m2 over 3 hours | Days 35,36 |
| G-CSF | 5 mcg/kg/day | As needed |
Pathology
Cases underwent central pathology review by two independent pathologists (SLP and DZ) to confirm the histopathologic diagnosis of anaplastic large cell lymphoma. Institutional phenotyping (by flow cytometry or immunohistochemical staining) was reviewed centrally as a part of confirmation of the diagnosis. In cases with insufficient phenotypic data provided by the submitting institution, an attempt was made to determine phenotype by central immunoperoxidase staining (SLP) when unstained slides were available, including staining for CD30, ALK-1 and appropriate T or B-cell lineage markers (CD20, CD3, CD43, CD45RO), as previously described [12].
Statistical methods
Overall survival (OS) and event-free survival (EFS) were estimated by the Kaplan-Meier method and compared using the log-rank test. OS was defined as the time from diagnosis to death from any cause while EFS was calculated as the time from diagnosis to the first event (death, tumor progression, or second malignancy). Patients were censored at their last known follow-up date if an event had not occurred. All p values are 2-sided. A univariate analysis was utilized to evaluate for predictors of EFS.
Results
Patient Characteristics
Between June 1996 and March 2001, 152 patients were enrolled on CCG-5941. Of these, 49 were lymphoblastic lymphomas that were eligible for CCG-5941 and results for these patients are reported separately. Of the remaining 103 patients, 17 were excluded from analyses by central pathology review - Hodgkin's disease (n=2), B-cell NHL (n=3), malignant lymphoma NOS (n=1), CD30 status negative (n=4), and CD30 status unknown (n=7). CD30 positivity was confirmed centrally (SLP) for 73/86 and by the enrolling institution for 13/86. These 86 patients with non-localized ALCL (CD30+) were analyzed. The majority of tumors were positive for ALK by immunohistochemical methods (64/71 cases or 90%). Phenotyping demonstrated a predominance of T-cell phenotype (positive for CD3, CD43 and/or CD45RO in a limited analysis) in 83% of cases (64/77). Cases without definitive expression of the above T-cell markers were classified as null (17% or 13/77) with the recognition that the above immunophenotypic panel that used was limited. No cases were of B-cell lineage. The clinical features are shown in Table II. All patients had non-localized disease. Extranodal disease was common but occurred in a variety of locations (skin, lung, bone, bone marrow, and other viscera). CNS involvement was rare having been identified in only a single patient (1%).
Table II. Clinical Characteristics of 86 Children with ALCL.
| Characteristic | Number (%) (n=86) |
|---|---|
| Gender | |
| Male | 48 (56%) |
| Female | 38 (44%) |
| Age (years) | |
| < 5 | 6 (7%) |
| 5 to 9 | 29 (34%) |
| 10 to 14 | 33 (38%) |
| ≥ 15 | 18 (21%) |
| Mediastinal Mass (N=85) | 30 (35%) |
| Extranodal Involvement (N=84) | |
| Skin | 13 (15%) |
| Liver | 5 (6%) |
| Lung | 12 (14%) |
| Bone | 10 (12%) |
| Other viscera | 14 (17%) |
| Bone Marrow | 11 (13%) |
| CNS | 1 (1%) |
| Immunophenotype (n=77) | |
| T lineage | 64 (83%) |
| Null lineage | 13 (17%) |
| ALK Status (n=71) | |
| Positive | 64 (90%) |
| Negative | 7 (10%) |
| Initial LDH (n=85) | |
| Normal | 74 (86%) |
| > 2 × normal | 12 (14%) |
Outcome
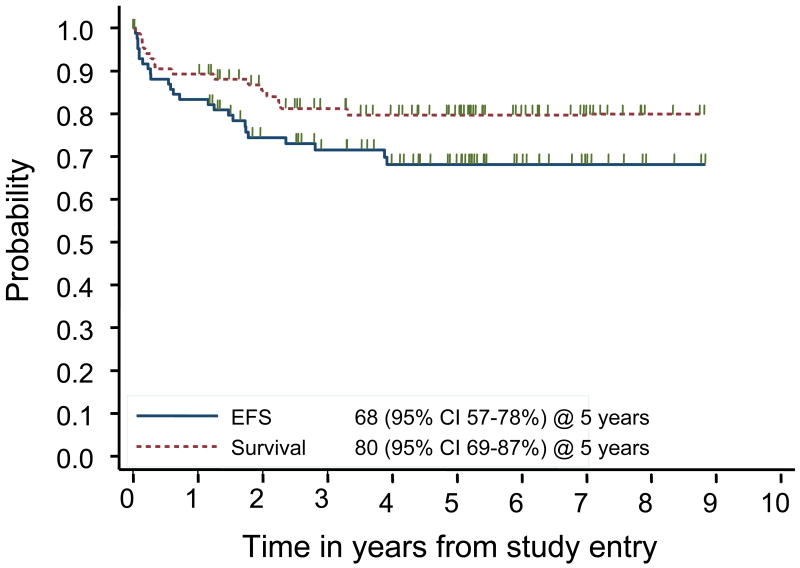
The 5 year event free survival was 68% (95% confidence interval (CI) of 57 – 78%) and the 5 year overall survival was 80% (95% CI of 69-87%) (Figure 1). There were 21 relapses and 4 toxic deaths as first events. Relapse occurred early with 17 of 21 (81%) relapses occurring within 2 years of diagnosis and 12 of 21 (57%) within the first year. Post-recurrence survival occurred in 9 of the 21 relapsed patients. Of the 4 patients who relapsed greater than 2 years from diagnosis, 3 are long term survivors.
Figure 1.
Overall and event-free survival of 86 patients with systemic ALCL treated on CCG-5941.
Univariate analysis for risk factors only identified bone marrow involvement predicting lower EFS (P=0.03). Age, gender, race, elevated LDH, mediastinal involvement were shown not to be prognostic factors for EFS. With only one patient with CNS involvement at diagnosis no analysis could be performed.
Toxicity
The Common Toxicity Criteria defined by the National Cancer Institute were used for grading toxicity. Information on toxicity was available for all 86 patients. There were 4 toxic deaths due to infection during treatment. Hematologic grade 4 toxicities were common with 82% of patients developing grade 4 neutropenia, 66% grade 4 thrombocytopenia, and 38% grade 4 anemia. In addition, 4% developed grade 4 hepatotoxicity.
Discussion
Comparison of treatment strategies for children with ALCL is limited by the varying inclusion and exclusion criteria used in the past. Many of the early trials included other large cell lymphomas, i.e. diffuse large B-cell lymphoma and Hodgkin lymphoma. An illustration of this fact is the 17% of patients excluded upon central pathology review in this report. Advances in understanding the biology of ALCL have led to a more precise pathological definition for ALCL culminating in the current World Health Organization classification [4]. Using this definition, a more informative comparison of the variety of treatment strategies for pediatric ALCL can be made for recent studies.
Among the pediatric studies reported, varying strategies for treating children with ALCL have emerged. The Berlin-Frankfort-Munster (BFM) group has utilized short (5 to 7 months) intense chemotherapy resembling that used to treat mature B-cell NHL. Stratifying treatment according to risk category, Seidemann et al. reported a 5 year EFS of 76% for 89 children with ALCL [2]. Using similar chemotherapy to the BFM group (duration of 7 to 8 months), the United Kingdom Children's Cancer Study Group (UKCCSG) group reported a 5 year EFS of 59% for 72 patients [10]. The differences in chemotherapy between the BFM and UKCCSG studies were the use of ifosfamide, dexamethosone, and longer infusion time for methotrexate in the BFM regimen. Whether the differences in outcome are related to therapy or differences in patient characteristics remains to be elucidated. Another group using B-cell NHL therapy is the French Society of Pediatric Oncology (SFOP). Adding vinblastine, etoposide, and bleomycin to the B-cell NHL regimen, Brugieres et al. reported a 3 year EFS of 66% with a 3 year EFS of 55% for patients with stage 3 and 4 disease [7]. Interestingly, the BFM and UKCCSG developed chemotherapy based on clinical stage, while the SFOP treated disease of all clinical stages uniformly, but found an inferior outcome for patients with higher clinical stage. The Italian group (AIEOP) has used a modified LSA2-L2 acute leukemia regimen (duration 2 years) resulting in a 65% 10 year EFS [9]. The majority of North American studies have not based chemotherapy on immunophenotype but rather treated all large cell NHL similarly. The most recent Pediatric Oncology Group (POG) study examined the impact of adding intermediate dose methotrexate and high dose cytarabine to a 12 month long APO regimen (doxorubicin, prednisone, vincristine, 6-mercaptopurine, and methotrexate). Finding no benefit to the additional agents, Laver et al. reported a combined 4 year EFS of 72% for 86 children with ALCL [8].
As the majority of ALCL have a T cell phenotype, our approach in this trial was to treat all patients uniformly with chemotherapy based on that used for T lineage lymphoma. The regimen was intensified and shortened to 11 months in duration. Using this strategy, we achieved a 5 year EFS of 68% which is similar to that found in the best previously published regimens. In addition, the intensification treatment strategy utilized here achieved a comparable EFS rate while treating only patients with non-localized disease. Thus, chemotherapy intensification may be necessary in the treatment of non-localized ALCL.
We identified bone marrow involvement as the only clinical variable to be an adverse prognostic factor. While bone marrow involvement is rare in some studies [2;10], others have shown it to be a poor prognostic factor [16;17]. A report from Damm-Welk et al. demonstrated that minimal disease in bone marrow found by RT-PCR at diagnosis identified a group of patients with a cumulative incidence of relapse of 71% [17]. In addition, they showed that bone marrow involvement at diagnosis continued to be highly prognostic in multivariate analysis while other clinical features were not.
While there are multiple reports of ALK negative tumors having a negative impact on survival, this is irrelevant in pediatric studies as virtually all tumors are ALK positive, with the ALK-negative tumors being primarily localized skin disease. We have previously shown in a large sample that 98% of non-localized pediatric ALCL were ALK-positive by use of either immunohistochemical staining or fluorescent in-situ hybridization (FISH) [12]. The “European Intergroup Study for ALCL” combined data on 235 patients from the BFM, UKCCSG, and SFOP studies [5]. In multivariate analysis, they found that mediastinal involvement (relative risk of failure, 2.1), visceral involvement defined as lung, liver, or spleen involvement (relative risk of failure, 2.1) and skin involvement (relative risk of failure, 1.9) were all prognostic. Based on this model, they found a high risk group (any one of the 3 sites involved) to have a 5 year EFS of 61% while the low risk group (none of the 3 sites involved) had a 5 year EFS of 89%. The only adverse prognostic factor identified in our study was bone marrow involvement. Our inability to demonstrate prognostic value of other factors might be explained in two ways: the dose intensification used in this protocol nullifies the increased risk associated with these characteristics or the number of patients enrolled in our study was too few to accurately define the prognostic significance of any single variable. In order to validate prognostic variables a large number of patients would need to be treated uniformly likely requiring an international collaboration.
Our dose intensification strategy did result in a number of hematologic toxicities. The large percentage of patients experiencing grade 4 neutropenia increased the risk of complications reflected in the death of 4 patients due to infectious causes. As with other studies, the majority of relapses occurred within 2 years of initial diagnosis. Although numbers are too small to make any conclusions, it is of interest that 3 of the 4 patients who had later relapses are long term survivors.
In conclusion, CCG-5941 demonstrated efficacy similar to the previously reported regimens in treating children with non-localized ALCL. Therefore the question of intensity and duration of therapy for pediatric patients with non-localized ALCL remains unanswered. Incorporation of vinblastine, which is known to have good activity even as monotherapy in relapsed disease [18], is currently being evaluated in randomized trials. However, evaluation of novel agents such as anti-CD30 monoclonal antibodies and kinase inhibitors is warranted. Minimal residual disease for risk stratification requires further evaluation and due to the rarity of ALCL, international collaboration is needed to define the optimal treatment strategy for children with ALCL.
References
- 1.Sandlund JT, Downing JR, Crist WM. Non-Hodgkin's lymphoma in childhood. N Engl J Med. 1996;334:1238–48. doi: 10.1056/NEJM199605093341906. [DOI] [PubMed] [Google Scholar]
- 2.Seidemann K, Tiemann M, Schrappe M, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97:3699–706. doi: 10.1182/blood.v97.12.3699. [DOI] [PubMed] [Google Scholar]
- 3.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58. [PubMed] [Google Scholar]
- 4.Jaffe ES, Harris NL, Stein H, Vardiman JWE. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 5.Le Deley MC, Reiter A, Williams D, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008;111:1560–6. doi: 10.1182/blood-2007-07-100958. [DOI] [PubMed] [Google Scholar]
- 6.Sandlund JT, Pui CH, Roberts WM, et al. Clinicopathologic features and treatment outcome of children with large-cell lymphoma and the t(2;5)(p23;q35) Blood. 1994;84:2467–71. [PubMed] [Google Scholar]
- 7.Brugieres L, Deley MC, Pacquement H, et al. CD30(+) anaplastic large-cell lymphoma in children: analysis of 82 patients enrolled in two consecutive studies of the French Society of Pediatric Oncology. Blood. 1998;92:3591–8. [PubMed] [Google Scholar]
- 8.Laver JH, Kraveka JM, Hutchison RE, et al. Advanced-stage large-cell lymphoma in children and adolescents: results of a randomized trial incorporating intermediate-dose methotrexate and high-dose cytarabine in the maintenance phase of the APO regimen: a Pediatric Oncology Group phase III trial. J Clin Oncol. 2005;23:541–7. doi: 10.1200/JCO.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 9.Rosolen A, Pillon M, Garaventa A, et al. Anaplastic large cell lymphoma treated with a leukemia-like therapy: report of the Italian Association of Pediatric Hematology and Oncology (AIEOP) LNH-92 protocol. Cancer. 2005;104:2133–40. doi: 10.1002/cncr.21438. [DOI] [PubMed] [Google Scholar]
- 10.Williams DM, Hobson R, Imeson J, Gerrard M, McCarthy K, Pinkerton CR. Anaplastic large cell lymphoma in childhood: analysis of 72 patients treated on The United Kingdom Children's Cancer Study Group chemotherapy regimens. Br J Haematol. 2002;117:812–20. doi: 10.1046/j.1365-2141.2002.03482.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 12.Perkins SL, Pickering D, Lowe EJ, et al. Childhood anaplastic large cell lymphoma has a high incidence of ALK gene rearrangement as determined by immunohistochemical staining and fluorescent in situ hybridisation: a genetic and pathological correlation. Br J Haematol. 2005;131:624–7. doi: 10.1111/j.1365-2141.2005.05808.x. [DOI] [PubMed] [Google Scholar]
- 13.Tilly H, Gaulard P, Lepage E, et al. Primary anaplastic large-cell lymphoma in adults: clinical presentation, immunophenotype, and outcome. Blood. 1997;90:3727–34. [PubMed] [Google Scholar]
- 14.Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93:3913–21. [PubMed] [Google Scholar]
- 15.Sandlund JT, Pui CH, Santana VM, et al. Clinical features and treatment outcome for children with CD30+ large-cell non-Hodgkin's lymphoma. J Clin Oncol. 1994;12:895–8. doi: 10.1200/JCO.1994.12.5.895. [DOI] [PubMed] [Google Scholar]
- 16.Mussolin L, Pillon M, d'Amore ES, et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia. 2005;19:1643–7. doi: 10.1038/sj.leu.2403888. [DOI] [PubMed] [Google Scholar]
- 17.Damm-Welk C, Busch K, Burkhardt B, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2007;110:670–7. doi: 10.1182/blood-2007-02-066852. [DOI] [PubMed] [Google Scholar]
- 18.Brugieres L, Quartier P, Le Deley MC, et al. Relapses of childhood anaplastic large-cell lymphoma: treatment results in a series of 41 children--a report from the French Society of Pediatric Oncology. Ann Oncol. 2000;11:53–8. doi: 10.1023/a:1008352726155. [DOI] [PubMed] [Google Scholar]