Abstract
Retinal lymphoma, the most common form of intraocular lymphoma, is a high-grade malignancy, usually of B-cell type, and is associated with a poor prognosis because of frequent central nervous system (CNS) involvement. The neoplastic B-cells of retinal lymphoma have a characteristic morphology and immunophenotype, express certain chemokines and chemokine receptors, and produce interleukins (IL), e.g. IL-10. Together with the cytological features of these tumors, the immunophenotype, presence of immunoglobulin rearrangements, and biochemical profile aid the diagnosis of retinal lymphomas. Immunophenotyping and somatic mutation analysis suggest derivation of most retinal lymphomas from an early post-germinal centre B-cell. Chromosomal translocation data would suggest, however, that a subgroup of these neoplasms may arise from germinal centre B-cells, and these could be associated with a better prognosis. Further investigations, such as gene expression profiling, are required to identify oncogenic pathways potentially involved in retinal lymphoma development, and to identify new prognostic/therapeutic markers for this tumor.
Keywords: retinal lymphoma, vitreoretinal lymphoma, vitreal lymphoma, intraocular lymphoma, IOL, PCNSL, DLBCL
INTRODUCTION
In order to understand the histogenesis of lymphomas and to define distinct lymphoma entities, the current World Health Organization (WHO) classification emphasizes an approach whereby the clinical characteristics are correlated with distinct morphological, immunophenotypical, and genotypical features of each neoplasm1 For each lymphoma entity, it has been attempted to identify or postulate a putative cell of origin. Intraocular lymphomas that originate inside the eye are heterogeneous and have been subsumed for many years under the vague title “primary intraocular lymphoma” or PIOL. In fact, intraocular lymphomas represent several distinct entities, arising in different structures of the eye and have differing clinical courses; therefore, more specific terminology is warranted (Table).2 In this review, the pathology, molecular biology and biochemical features of the most frequent type of intraocular lymphoma, namely retinal lymphoma, are summarized.
Table.
Summary of the clinical, morphological, immunophenotypical and genotypical features known to date for each of the various intraocular lymphoma subtypes
| Intraocular Lymphoma Type |
Retinal | Choroidal Primary: |
Choroidal Secondary: |
Iridal | Ciliary body |
|
|---|---|---|---|---|---|---|
| Clinical Features | 60–70 years “Floaters” Painless decrease in VA Subretinal infiltrates Often bilateral RPE changes on FA CNS involvement (70–80% of pts) |
50–60 years Blurring of vision Metamorphopsia Clear vitreous Diffuse thickening of choroids Usually unilateral Extraocular extension frequent No CNS involvement |
Previous history of systemic NHL Decrease in VA Possibly bilateral |
Pain Redness Photophobia Pseudohypopyon Usually unilateral Often ultimate systemic dissemination |
Raised IOP Ciliary body mass |
|
| Most Common Subtype(WHO) Immunoprofile |
DLBCL CD79a+ CD20+ PAX5+ BCL2+ BCL6+/− MUM1/IRF4+ CD10−/+ Ki-67 rate: high (>80%) |
EMZL CD79a+ CD20+ BCL2+ CD43 +/− IgM + CD5- CD23- CyclinD1- Low Ki-67 rate: 5–15% |
Dependent on systemic NHL Dependant on systemic NHL |
DLBCL CD20+ Ki-67 rate: high (>80%) |
TCL NOS CD3+ CD4+ Ki-67 rate: moderate (40–60%) |
EMZL CD79a+ CD20 + BCL-2 + CD43 +/− IgM + CD5- CD23- CyclinD1- Low Ki-67 rate: 5–15% |
| Genotype | High somatic IgH mutation load Few ongoing somatic mutations Chromosomal translocations: t(14;18)(q31;q21) |
Moderate somatic IgH mutation load Few ongoing somatic mutations Chromosomal abnormalities: t(11;18)(q21;q21) |
Dependant on systemic NHL |
Not known | Not known | Not known |
| Putative Cell of Origin |
2 different types?: a) Early post-germinal centre B cell = DLBCL of ABC type? b) Germinal centre cell = DLBCL of GCB type ? |
Post-germinal centre (memory) B cell |
Dependant on systemic NHL |
Not known | Peripheral T-cell |
Post-germinal centre (memory) B cell |
Key: DLBCL=diffuse large B-cell lymphoma; EMZL=extranodal marginal zone B-cell lymphoma;NHL=NonHodgkin’s lymphoma;CD=cluster of differentiation; ABC = activated B-cell type; GCB = germinal centre B-cell type; t(N1;N2) = chromosomal translocation between chromosome N1 and N2. WHO = WHO lymphoma classification system.
Retinal Lymphoma
Most intraocular lymphomas occur in the retina, usually with vitreous involvement.2,3 Retinal lymphoma can occur without visible vitreous opacities, but then, conversely, lymphomatous vitreous infiltrates can be seen in the absence of any obvious retinal disease.4 For ease of reference, these three different clinical variants of retinal and vitreal lymphoma will be described here under the term “retinal” lymphoma.2,5
Clinical features
Retinal lymphoma is a high-grade malignant neoplasm, which is often associated with cerebral disease. It may be primary or secondary to CNS lymphoma (CNSL) or may present simultaneously (Table).6–8 Exceptionally rarely, retinal lymphoma may result from systemic metastatic lymphoma.9,10 Retinal lymphoma most often affects elderly patients; however, it is also seen in younger individuals, particularly in those who are immunocompromised (e.g. with HIV infection or following organ transplantation). Inexplicably, there has been a clear increase in the incidence of retinal lymphoma worldwide, even in immunocompetent patients.11
Retinal lymphoma is usually bilateral (60–90% of patients) but is often relatively asymmetrical and sometimes appears to be unilateral at initial presentation.2 Signs and symptoms of retinal lymphoma can mimic many other intraocular conditions (“masquerade syndrome”), and therefore, it can often be difficult to diagnose clinically, requiring experience in the interpretation of ophthalmological imaging12 (Table) (Fig. 1). Confirmation of the clinical suspicion of retinal lymphoma requires ocular fluid or tissue sampling, involving morphological and immunocytological analysis by an experienced pathologist. Adjunct methods, including monoclonality studies using polymerase chain reaction (PCR) and cytokine analysis, are very useful in providing further evidence in support of the diagnosis. These methods will be discussed below.
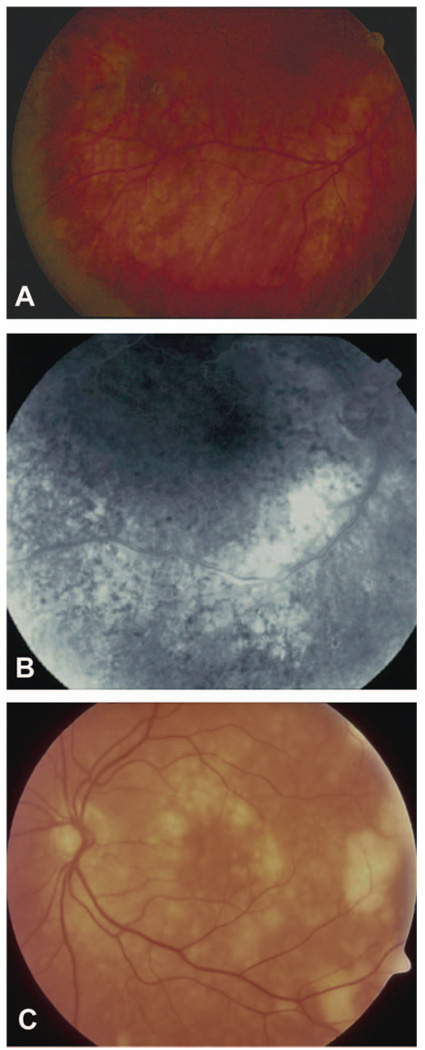
Figure 1.
(A) Color fundus photograph of the right eye of a patient with retinal lymphoma, showing some retinal pigment epithelium disturbances. (B). Late-phase fluorescein angiogram of the same patient with numerous dark spots (mask effect) corresponding to fresh tumor cells associated with the RPE disturbances. (C). Color fundus photograph of another patient, with changes suggestive of retinal lymphoma: subretinal creamy-yellow infiltrates, which appear to coalesce.
Approximately 80% of patients with retinal lymphoma subsequently develop lymphomatous involvement of the cerebral parenchyma, spinal cord or meninges (Table). This close association of retinal lymphoma and CNSL is not surprising, considering the embryological origins of the two organs. Whether oculocerebral lymphoma is consequent to direct infiltration along the optic nerve, metastatic spread or multifocal tumour development remains to be clarified. Lymphomatous involvement of the CNS can be focal and/or diffuse but usually occurs in the frontal lobe. There is commonly diffuse, leptomeningeal involvement, and seeding of lymphoma cells into the cerebral spinal fluid (CSF) has been reported in 42% of patients.13
Interestingly, retinal lymphoma and CNSL usually disseminate only within the CNS. The reason for this confinement of retinal lymphoma and CNSL is unclear; however, it may be explained to some extent by the chemokine receptor expression pattern of the neoplastic cells.14,15 As summarised below some data suggest that CNS diffuse large B-cell lymphoma (DLBCL) cells have a differing chemokine receptor profile when compared to their morphological counterparts in systemic DLBCL.16 A specific chemokine or chemokine receptor profile of tumor cells of CNSL remains, however, to be validated and identified (see below).17
Morphological features of retinal lymphoma
Histologically, the majority of retinal lymphoma are a high-grade B-cell Non-Hodgkin lymphoma (NHL), and can be subtyped as a DLBCL,7,18,19 according to the WHO lymphoma classification (Table)1 Rare subtypes have also been described, such as T-cell-rich B-cell lymphoma20 as well as T-cell lymphoma, not otherwise specified.10,21–24 Very few cases of primary T-cell retinal lymphomas have been described: compared to the retinal DLBCL, they appear to behave less aggressively, and may or may not be associated with CNS disease.
Retinal lymphomas of DLBCL subtype are characterized by pleomorphic medium-to-large sized cells with minimal cytoplasm, indented or folded nuclei, and prominent, often multiple, nucleoli (Fig. 2). Rarely, atypical mitotic figures can be seen. A background of small reactive lymphocytes, usually T-cells, and scavenger macrophages as well many necrotic cells is a typical feature of these neoplasms.6–8,25
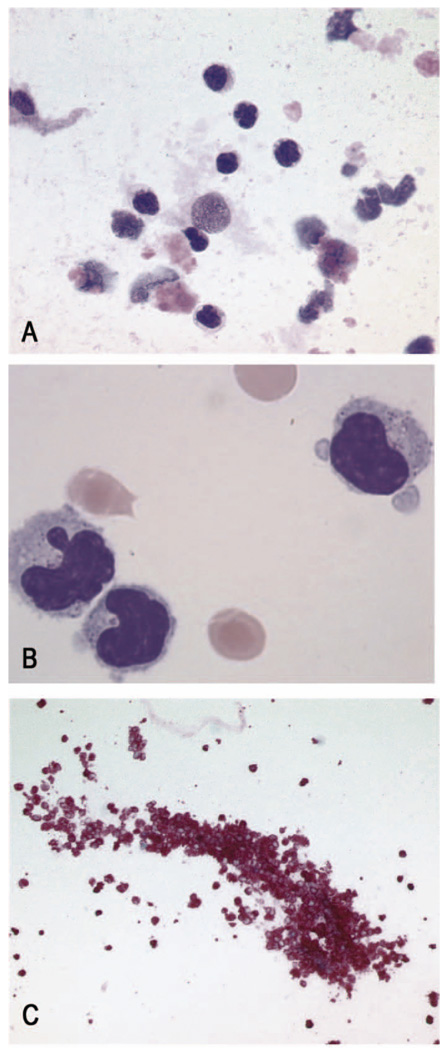
Figure 2.
(A) Vitrectomy specimen containing atypical lymphoid cells of a retinal lymphoma on a “dirty” background, consisting of lytic cells and scavenger macrophages (MGG, x60 objective). (B) Higher magnification of the atypical cells, showing the folded and irregular nuclei and the condensed chromatin of the lymphoma cells (May Grunwald Giemsa, x 100 objective). (C) Immunohistochemical staining using an antibody directed against the B-cell antigen, CD20, demonstrating another vitrectomy specimen of a patient with retinal lymphoma, consisting almost purely of atypical B-lymphocytes (Alkaline Phosphatase-Anti Alkaline Phosphatase (APAAP), x20 objective).
Immunophenotypic features
Compared to systemic DLBCL, knowledge about the immunophenotype of retinal lymphoma has developed slowly. This is most likely due to the rarity of this tumor, its subretinal/sub-RPE (retinal pigment epithelium) location and the small amount of tumor material obtained for evaluation via pars plana vitrectomy. Increasingly, however, combined subretinal aspirates and/or chorioretinal biopsies are being performed together with vitreous biopsies to establish or exclude the diagnosis of lymphoma.26,27 These larger tumor samples have enabled more extensive adjunctive analyses and, thereby, have provided a better understanding of the possible histiogenesis of retinal lymphomas. In particular, they have provided more information to the immunophenotype and genotype of these tumor cells (see below).
Retinal lymphomas are characterized by the following immunohistochemical expression profile: CD79a+,CD20+, BCL-2+, MUM1/IRF4+, BCL-6+(most cases), CD10−/+ (Table) (Fig. 2).28 Most retinal lymphomas demonstrate a monotypical expression for either a light (typically Ig-Kappa) and/or heavy chain of the immunoglobulin gene (usually IgM, but sometimes combined IgM and IgD). The Ki-67 growth fraction of the neoplastic B-cells is frequently high (i.e., about 90%), reflecting the high-grade malignancy of this lymphoma. Should the neoplastic cells not be monotypical on immunohistochemistry, clonality assessment can be performed on DNA extracted from retinal lymphoma cells using PCR with primers directed against the immunoglobulin heavy and light chains in B-cell lymphomas (IgH-PCR and IgL-PCR, respectively).29 Rarely, PCR directed against the T-cell receptor (TCR-γ-PCR) is necessary in the case of the suspected intraocular T-cell lymphoma.10,21
Genotypic features
Before discussing the genotypic features of retinal lymphoma, it is first necessary to review the developments in our understanding of systemic DLBCL. Until recently, DLBCL represented a large heterogeneous group of lymphomas, which shared some vaguely similar morphological and immunophenotypical features, but which showed considerable variation with respect to clinical course. In 2000, Alizadeh and co-workers used lymphochip complementary DNA microarrays to perform gene expression profiling analysis of untreated de novo DLBCL.30 They subdivided DLBCL into 3 groups, on the basis of genes expressed in a particular stage of the B-cell differentiation pathway or during a particular biologic response.31,32 These subgroups are: activated B-cell DLBCL (ABC type); germinal centre DLBCL (GCB type); and primary mediastinal (thymic large) B-cell DLBCL (type 3).
The main feature of ABC type DLBCL is the dysregulation of the NF-kB gene, resulting in an uncontrolled proliferation of lymphocytes.33 Other chromosomal abnormalities of ABC type DLBCL include gains in chromosome 3q and 18q21–q22, and deletions in 6q21–q22 as well as inactivation of the PRDM1/BLIMP1 gene.34–36 In contrast, GCB type DLBCL are characterized by the translocation t(14;18)(q32;q21) and a gain in chromosome 12q1237–39 Type 3 DLBCL have overlapping features of both ABC and GCB-type DLBCL but like ABC DLBCL appear to show constitutive activation of the NF-kB pathway.40 These findings suggest that the types of DLBCL arise from different stages of normal B-cell development, perhaps representing distinct entities. This molecular subdivision of DLBCL has been shown to have clinical relevance with respect to prognosis.41,42 Patients with ABC subtype DLBCL have a considerably worse prognosis than those with GCB lymphomas.41,43
Gene expression microarray analysis is not yet feasible in routine diagnostic practice, particularly as it requires good quality RNA, which may be difficult to obtain from formalin-fixed material. For this reason, surrogate immunohistochemical markers using algorithms such as the “Hans classifier” have been proposed,44 to identify GCB-type and ABC subgroups of DLBCL. These include CD10 and BCL-6 as GCB B-cell markers, and MUM1/IRF4 as a non-GCB B-cell marker. Usage of the “Hans classifier” in routine practice and in larger research studies has demonstrated its limitations.45 Despite this, the Lunenburg Lymphoma Biomarker Consortium have concluded that “semiquantitative immunohistochemistry for prognostic stratification of DLCBL is feasible (if performed) in a reproducible way. . . ”.45
When trying to understand the cellular origin of retinal lymphoma and its relationship to either the ABC or GCB types, one can only gather the thin strands of evidence, which are presented in the literature. To date, no gene expression profiling studies have been performed on retinal lymphomas. Therefore, we have to rely on indirect evidence provided by genotypic and immunophenotypic studies, knowing, however, that this evidence is likely to be wanting.
The only chromosomal translocation reported to occur in retinal lymphoma is t(14;18), which involves the bcl-2 gene, with rearrangements being reported in up to 67% of cases.29,46 This results in overexpression of BCL-2 protein, a mitochondrial outer membrane protein that protects cells from apoptosis. The presence of this mutation and consequent overexpression of this protein in some retinal lymphoma could suggest that they are DLBCL of GCB type, as this translocation is seen most commonly in this type of DBLCL. The incidence of this particular translocation, however, seems to be considerably higher than in peripheral DLBCL (approx. 20–30%).47,48 Further studies are needed to validate these data and to determine the exact prevalence in retinal lymphomas.
Possibly contradicting the above data and raising the suggestion that some retinal lymphoma are DLBCL of ABC type are data obtained from sequencing of the variable region of the immunoglobulin heavy chain gene (VH) of retinal lymphoma cells and from their immunophenotyping. Two independent groups of investigators have demonstrated an intermediate to large number of somatic mutations (average 37) in retinal lymphoma cells with no evidence of antigen selection or significant intraclonal heterogeneity.18,49 Interestingly, a similar high mutation frequency was reported for VH region genes in PCNSL.50–52 The findings of a high somatic mutations load in the VH genes of retinal lymphoma, together with the tumor cell immunophenotype (MUM1/IRF4+, BCL-6+/−, CD10-), suggest that retinal lymphoma is a DLBCL of ABC subtype, i.e. they are derived from mature B-cells, which have undergone a prolonged interaction in the microenvironment of the germinal center and are either at the late germinal center stage of differentiation or are early post-germinal center B-cells.
Taken together, the genotypic observations suggest that there are possibly at least two different types of retinal lymphoma: i.e. DLBCL of ABC and GCB types. The only way to test this hypothesis is to perform gene expression profiling studies using RNA extracted from retinal lymphoma cells. To date, such analyses have not been possible, mainly due to the poor quality and quantity of the RNA obtained from retinal lymphoma specimens. Current technologies for RNA amplification and/or new methods that allow expansion of malignant B cell populations53 and/or amplification of RNA may facilitate future studies. Animal models of retinal lymphoma54–58 do not allow us to answer this question as yet. Data from gene expression profiling studies of PCNSL provide some information that could be extrapolated with caution to retinal lymphoma. The first gene expression profiling studies of PCNSL by Rubenstein and co-workers59,60 demonstrated that there was an equal distribution of ABC, GCB and Type 3 DLBCL in this location. Using quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and hierarchical clustering of gene expression data, Courts et al. revealed two distinct subgroups of PCNSL, which were characterized by significantly different transcriptional levels of BCL10, REL, and IAP-1.61 However, these subtypes are not identical with the ABC- and GCB-subtype of DLBCL. More recent studies using immunohistochemistry62,63 and gene expression profiling64 suggest that PCNSL can be attributed to both ABC and GCB groups.
The Role of IL-10 in Retinal Lymphoma and DLBCL
Biochemical analysis of ocular specimens (e.g. vitreous biopsy or aqueous humor tap) for interleukin ratios may support the diagnosis of retinal lymphoma. Malignant B-cells often express relatively high levels of IL-10 whereas inflammatory cells produce higher levels of IL-6.65–69 A high IL-10 to IL-6 ratio on ELISA (enzyme-linked immunosorbent assay) may be, therefore, suggestive of B-cell lymphoma.70,71 However, it is by no means diagnostic of the disease, and as indicated above must be considered in the context of the available cytological, immunohistological and clinical data.
Interleukin-10, originally identified as an immunoregulatory Th2 cytokine able to inhibit Th1 cytokine, is highly homologous to viral IL-10 or BCRF-1, an open reading frame in EBV genome72 IL-10 is a B-cell autocrine growth factor and can promote B-cell lymphoma development and proliferation.73 While normal B-lymphocytes produce IL-10,74 malignant B-cells frequently produce much higher levels of this cytokine. These high levels of IL-10 can promote tumor cell survival in a number of ways. For example, IL-10 can interfere with the immune response against tumor cells through inhibition of Th1 cytokines,.75 and thus inhibit cytotoxic T-cell effects directed against neoplastic B-cells. In addition, IL-10 enhances Bcl-2 expression on B-cells and prevents apoptosis.76
More than 10 years ago, elevations of IL-10 levels were reported to be present in the serum of patients with B-cell lymphomas, such as Burkitt’s lymphoma,77 AIDS-associated lymphomas,78 and Epstein Barr Virus (EBV)-positive Hodgkin’s disease.79 At a similar time, high IL-10 levels were also detected in vitreous humor samples,67–71 and later in the aqueous80 of patients with retinal lymphomas.
Vitreous humor samples are usually obtained by pars plana vitrectomy, and they are often diluted. Thus, the absolute level of IL-10 in vitreous biopsies is difficult to assess. A ratio of IL-10 to IL-6, however, will allow for this dilution effect, as each cytokine will be diluted equally.70 A ratio of IL-10 to IL-6 greater than 1.0 suggests an intraocular manifestation of a B-cell lymphoma. In a study of 35 retinal lymphoma and 64 uveitic patients, the cutoff made at 1.0 for differential diagnosis was correct when compared with the cytological, immunohistological and clinical findings in 74.7% of cases.71 This study had a sensitivity of 74.3% and specificity of 75%. This dilution effect is not so problematic with aqueous samples: a cutoff of an absolute value of 50 pg/mL IL-10 in the aqueous has been demonstrated to be associated with a sensitivity of 89% and specificity of 93% for the diagnosis of 51 patients with retinal lymphoma.80
Therefore, high IL-10 levels or high IL-10:IL-6 ratios in ocular fluids may be helpful in the diagnosis of retinal lymphoma of DLBCL subtype. It remains to be determined whether they are also useful in the extremely rare retinal lymphomas of T-cell type. Vitreous humor IL-10 and IL-6 levels may be used to predict the responses to chemotherapy in retinal lymphoma.81,82 Interestingly, both high serum IL-10 and IL-6 levels are reported to correlate with clinical and pathological features and prognosis in patients with peripheral DLBCL.83 Recently, IL-10 gene polymorphisms have been associated with the risk of development and outcome of DLBCL.84–86 For example, IL-10-7400DelDel or the haplotype TCA (IL-10-6752T-6208C-3538A) could be a risk factor for poor clinical outcome in patients with aggressive non-Hodgkin’s lymphoma.85 It remains to be determined whether such polymorphisms are significant for the prognosis of retinal or CNSL.
Expression of Chemokines in Retinal Lymphoma and PCNSL
Since the normal CNS contains no lymphoid collections, why retinal lymphoma and PCNSL are confined to the CNS is a perplexing question that remains unanswered. Over 20 years ago, Hochberg andMiller87 suggested that one explanation for this confined localization might be the “homing” of a malignant clone of B cells to the brain and/or eye. Recently, B cell lymphoid chemokines have been investigated as potential homing signals on retinal and cerebral vascular endothelium.
The lymphoid—or homeostatic—chemokines are chemoattractant cytokines that are expressed in secondary lymphoid organs, where they control cell migration and organize the structure of these organs (reviewed by Cyster)88 B and T cells express relevant cell surface receptors making them responsive to different chemokines. CCL21 (secondary lymphoid tissue chemokine, SLC), expressed by high endothelial venules, draws naïve CCR7-positive T and B cells into secondary organs. The T cells move to T cell areas in response to the local secretion of CCL21 and CCL19 (EBV-induced molecule 1 ligand chemokine, ELC). Follicular dendritic cells produce CXCL13 (B-cell attracting chemokine 1, BCA-1), which attracts the B cells into follicles. Memory lymphocytes are retained in crypts due to the expression of CXCL12 (stromal derived growth factor 1, SDF-1) and CCL20 (macrophage inflammatory protein-3α, MIP-3α) by crypt epithelium.89 Although originally described in relation to homeostasis, the lymphoid chemokines have subsequently been implicated in the development of inflammatory diseases and malignancies by multiple groups. In particular, several laboratories have investigated the expression of B cell lymphoid chemokines in retinal lymphoma or PCNSL.14–17,90
Brain biopsy specimens are more readily obtained than retinal tissue and have been used for most studies of B cell chemokine expression in PCNSL and retinal lymphoma (Fig. 3). Since genotyping has established that malignant cells from intraocular and intracranial sites in the same patient are identical, it is reasonable to assume that any homing signal(s) expressed in vascular beds of the retina and brain are similar. In one relatively early study of 24 cases of PCNSL,14 expression of CXCL13 - but not CCL21 or CCL19 - protein was identified in all cases by immunohistochemical testing of brain biopsy material. Expression of CXCL13 by both vascular endothelium and malignant B cells was observed. Interestingly, the vascular endothelium did not appear to synthesize the chemokine, since in situ hybridization detected CXCL13 transcript in B cells only. This finding led the authors to postulate that endothelial expression occurred through the process of transcytosis, and to conclude that although expressed in PCNSL, BCA-1 was unlikely to be a primary homing signal. In an independent study of three ocular specimens, CXCL13 expression was detected in the RPE.90
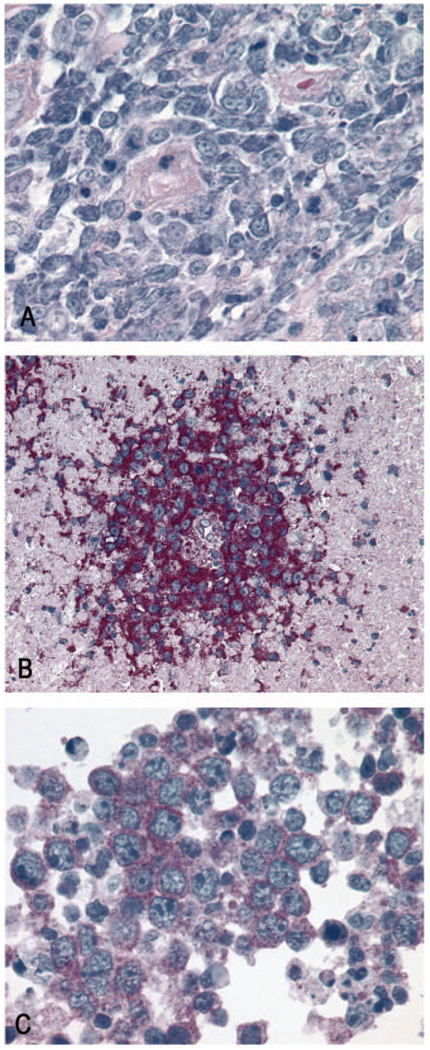
Figure 3.
(A) Stereotactic biopsy of a PCNSL, with a dense predominantly perivascular infiltration of medium-sized atypical lymphocytes with minimal cytoplasm, large nuclei and discrete nucleoli. Mitotic figures and scattered apoptotic bodies can be seen (Giemsa, x40 objective). (B) Cytoplasmic and possibly membranous positivity of the CNSL cells for CXCR4 (APAAP, x 20 objective). (C) Predominantly cytoplasmic immunoreactivity of the CNSL cells for CXCR5 (APAAP, x60 objective).
Both normal and malignant B cells respond differently to chemokines depending on their status of maturation and chemokine receptor profile. A recent examination of the Ig transcription factors suggests that malignant B cells in retinal lymphoma and PCSNL are mature B cells that have undergone germinal center reactions18 This finding suggested that research efforts should be concentrated on the role of CXCL12 and CCL20. In one immunohistochemical analysis of brain biopsy samples from 40 patients,15 expression of CXCL12 was localized to resident cells, including neurons, meningeal cells and endothelial cells. Interestingly in 80% of the cases of CNSL, expression of CXCL12 by the malignant B cells was observed. In contrast, CCL20 was not detected in the samples. The malignant cells also expressed the CXCL12 receptor, CXCR4 (Fig. 3). The study of the 3 ocular specimens cited in the previous paragraph, also reported that tumor cells expressed CXCR4.90 However, CXCR4 is also expressed on malignant cells in systemic DLBCL. Consequently, although CXCL12 might attract tumor cells into the CNS, it would be unlikely to be the sole factor responsible for migration.
Perhaps the most interesting observation in PCNSL is the expression of CXCL13 and CXCL12, and their respective receptors, CXCR5 and CXCR4, by the neoplastic B cells: this is of interest since normal B cells are not a source for these B cell chemokines (Fig. 3). This finding has been reported by two independent groups.14,15,17 The function of tumor-derived CXCL12 and CXCL13 in the pathogenesis of PCNSL can only be speculated. It is possible that they promote anti-tumor responses, or conversely, that they promote neoplastic B-cell proliferation and/or stimulate angiogenesis within the tumor. However, the situation is not straightforward: another group has reported that although cells may express CXCR4 and CXCR5, this expression appears to be restricted to the cytoplasm and nucleus. This cellular distribution would limit the ability of tumor cells to respond directly to the chemokines. To move forward with functional studies that investigate the roles of B cell lymphoid chemokines in retinal lymphoma and PCSNL, it will be necessary to procure large numbers of neoplastic B-cells. A recent publication describes a cell culture system in which human endothelial cells express HIV genes, Vpu and Tat.53 This system allows outgrowth of CNSL cells from CSF samples of patients with this tumor, and may provide favorable conditions for such studies.
SUMMARY
Retinal lymphoma is a high-grade B-cell malignancy, characterized by typical morphological, immunophenotypical, molecular and biochemical characteristics. Data suggests that the putative cell of origin is either a late germinal-centre or an early post-germinal centre B-cell. It demonstrates a preferential dissemination pattern within the CNS system, exceptionally rarely spreading to the peripheral lymphatic or blood circulation. This may be due to the chemokine and chemokine receptor profile of the neoplastic B-cells; however, genetic and microenvironmental factors may also play a role. Due to the cerebral involvement and to the inherent aggressive nature of retinal lymphoma, patients tend to have a poor prognosis.
In order to better understand the histiogenesis of retinal lymphoma, with a view to identifying biomarkers, signaling pathways, and ultimately improving therapy, additional molecular biological techniques, such as gene expression profiling and array-based comparative genomic hybridisation, are required. These may be applied in conjunction with cell culture or animal models. For this, concentrated efforts are required to collect sufficient tumor material in ocular oncology and uveitis specialist centers, in order to establish Biobanks allowing for collaborative research.
ACKNOWLEDGMENTS
Dr Justine Smith acknowledges support from Research to Prevent Blindness (unrestricted grant to Casey Eye Institute) and the Schnitzer-Novack Foundation. Dr Chi-Chao Chan acknowledges support from the ENI Intramural Research Program. Dr Sarah Coupland would like to acknowledge the generous support of the Eye Tumour Research Fund, Royal Liverpool University Hospital, UK. She would also like to express her gratitude to Professor Bertil Damato (Royal Liverpool University Hospital) for the discussions about the manuscript. All authors would also like to thank Dr Nathalie Cassoux and Professor Phuc LeHoang (Department of Ophthalmology, Pitié-Salpétrière Hospital, University Paris VI, Paris, France) for the provision of the clinical photographs.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Dr Sarah E. Coupland, Department of Cellular and Molecular Pathology, University of Liverpool, Liverpool, England.
Chi Chao Chan, Immunopathology Section, Laboratory of Immunology, National Eye Institute, National Institutes of Health, Bethesda, Maryland, USA.
Justine Smith, Casey Eye Institute and Department of Cell & Developmental Biology, Oregon Health & Science University, Portland, OR.
REFERENCES
- 1.Swerdlow SH, Campo E, Harris NL, et al. In: WHO classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, editor. IARC; 2008. [Google Scholar]
- 2.Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Experiment Ophthalmol. 2008;36(6):564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan CC, Gonzales J. Primary Intraocular Lymphoma. Singapore, London, Hackensack NJ: World Scientific Publishing Co; 2007. [Google Scholar]
- 4.Smith JR, Rosenbaum JT. Neurological concomitants of uveitis. Br J Ophthalmol. 2004;88(12):1498–1499. doi: 10.1136/bjo.2003.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan CC, Sauer TC. Ocular imaging in primary retinal lymphoma. Am J Ophthalmol. 2009;147(5):764–765. doi: 10.1016/j.ajo.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coupland SE, Heimann H. Primary intraocular lymphoma. Ophthalmologe. 2004;101(1):87–98. doi: 10.1007/s00347-003-0855-6. [DOI] [PubMed] [Google Scholar]
- 7.Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma:a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004;242(11):901–913. doi: 10.1007/s00417-004-0973-0. [DOI] [PubMed] [Google Scholar]
- 8.Davis JL. Diagnosis of intraocular lymphoma. Ocul Immunol Inflamm. 2004;12(1):7–16. doi: 10.1076/ocii.12.1.7.28072. [DOI] [PubMed] [Google Scholar]
- 9.Coupland SE, Damato B. Lymphomas involving the eye and the ocular adnexa. Curr Opin Ophthalmol. 2006;17(6):523–531. doi: 10.1097/ICU.0b013e328010948d. [DOI] [PubMed] [Google Scholar]
- 10.Levy-Clarke GA, Greenman D, Sieving PC, et al. Ophthalmic manifestations, cytology, immunohistochemistry, and molecular analysis of intraocular metastatic T-cell lymphoma:report of a case and review of the literature. Surv Ophthalmol. 2008;53:285–295. doi: 10.1016/j.survophthal.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy-Clarke GA, Chan CC, Nussenblatt RB. Diagnosis and management of primary intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19(3):739–749. doi: 10.1016/j.hoc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Fardeau C, Lee CP, Merle-Béral H, et al. P. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin primary intraocular lymphoma. Am J Ophthalmol. 2009;147(5):886–894. doi: 10.1016/j.ajo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Feiden W, Milutinovic S. Primary CNS lymphomas. Morphology and diagnosis. Pathologe. 2002;23(4):284–291. doi: 10.1007/s00292-002-0539-z. [DOI] [PubMed] [Google Scholar]
- 14.Smith JR, Braziel RM, Paoletti S, et al. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101(3):815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 15.Smith JR, Falkenhagen KM, Coupland SE, et al. Malignant B cells from patients with primary central nervous system lymphoma express stromal cell-derived factor-1. Am J Clin Pathol. 2007;127(4):633–641. doi: 10.1309/NUQHJ79BHWYD9TAF. [DOI] [PubMed] [Google Scholar]
- 16.Jahnke K, Coupland SE, Na IK, et al. Expression of the chemokine receptors CXCR4, CXCR5, and CCR7 in primary central nervous system lymphoma. Blood. 2005;106(1):384–385. doi: 10.1182/blood-2005-01-0324. [DOI] [PubMed] [Google Scholar]
- 17.Brunn A, Montesinos-Rongen M, Strack A, et al. Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol. 2007;114(3):271–276. doi: 10.1007/s00401-007-0258-x. [DOI] [PubMed] [Google Scholar]
- 18.Coupland SE, Hummel M, Muller HH, Stein H. Molecular analysis of immunoglobulin genes in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46(11):3507–3514. doi: 10.1167/iovs.05-0401. [DOI] [PubMed] [Google Scholar]
- 19.Coupland SE, Hummel M, Stein H, et al. Demonstration of identical clonal derivation in a case of "oculocerebral" lymphoma. Br J Ophthalmol. 2005;89(2):238–239. doi: 10.1136/bjo.2004.047001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings TJ, Stenzel TT, Klintworth G, Jaffe GJ. Primary intraocular T-cell-rich large B-cell lymphoma. Arch Pathol Lab Med. 2005;129(8):1050–1053. doi: 10.5858/2005-129-1050-PITLBL. [DOI] [PubMed] [Google Scholar]
- 21.Coupland SE, Anastassiou G, Bornfeld N, et al. Primary intraocular lymphoma of T-cell type:report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2005;243(3):189–197. doi: 10.1007/s00417-004-0890-2. [DOI] [PubMed] [Google Scholar]
- 22.Goldey SH, Stern GA, Oblon DJ, et al. Immunophenotypic characterization of an unusual T-cell lymphoma presenting as anterior uveitis. A clinicopathologic case report. Arch Ophthalmol. 1989;107(9):1349–1353. doi: 10.1001/archopht.1989.01070020419047. [DOI] [PubMed] [Google Scholar]
- 23.Lobo A, Larkin G, Clark BJ, et al. Pseudo-hypopyon as the presenting feature in B-cell and T-cell intraocular lymphoma. Clin Experiment Ophthalmol. 2003;31(2):155–158. doi: 10.1046/j.1442-9071.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- 24.Saenz AD, Amador A, Ruiz BM, et al. Cytofluorographic and molecular identification of a CD8-positive, TCR-alpha/beta-negative intraocular T cell lymphoma:a case report and review of the literature. J Med Case Reports. 2007;1:114. doi: 10.1186/1752-1947-1-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaldivar RA, Martin DF, Holden JT, Grossniklaus HE. Primary intraocular lymphoma:clinical, cytologic, and flow cytometric analysis. Ophthalmology. 2004;111(9):1762–1767. doi: 10.1016/j.ophtha.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Coupland SE, Bechrakis NE, Anastassiou G, et al. Evaluation of vitrectomy specimens and chorioretinal biopsies in the diagnosis of primary intraocular lymphoma in patients with Masquerade syndrome. Graefes Arch Clin Exp Ophthalmol. 2003;10(10):860–870. doi: 10.1007/s00417-003-0749-y. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27(4):241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coupland SE, Loddenkemper C, Smith JR, et al. Expression of immunoglobulin transcription factors in primary intraocular lymphoma and primary central nervous system lymphoma. Invest Ophthalmol Vis Sci. 2005;46(11):3957–3964. doi: 10.1167/iovs.05-0318. [DOI] [PubMed] [Google Scholar]
- 29.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- 30.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large cell B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 31.Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105(5):1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 32.Staudt LM. Molecular diagnosis of the hematologic cancers. N Engl J Med. 2003;348(18):1777–1785. doi: 10.1056/NEJMra020067. [DOI] [PubMed] [Google Scholar]
- 33.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194(12):1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bea S, Colomo L, Lopez-Guillermo A, et al. Clinicopathologic significance and prognostic value of chromosomal imbalances in diffuse large B-cell lymphomas. J Clin Oncol. 2004;22(17):3498–3506. doi: 10.1200/JCO.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Bea S, Zettl A, Wright G, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood. 2005;106(9):3183–3190. doi: 10.1182/blood-2005-04-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasqualucci L, Compagno M, Houldsworth J, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203(2):311–317. doi: 10.1084/jem.20052204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang JZ, Sanger WG, Greiner TC, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99(7):2285–2290. doi: 10.1182/blood.v99.7.2285. [DOI] [PubMed] [Google Scholar]
- 38.Iqbal J, Sanger WG, Horsman DE, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165(1):159–166. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dybkaer K, Iqbal J, Zhou G, Chan WC. Molecular diagnosis and outcome prediction in diffuse large B-cell lymphoma and other subtypes of lymphoma. Clin. Lymphoma. 2004;5(1):19–28. doi: 10.3816/clm.2004.n.006. [DOI] [PubMed] [Google Scholar]
- 40.Feuerhake F, Kutok JL, Monti S, et al. NFkappaB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood. 2005;106(4):1392–1399. doi: 10.1182/blood-2004-12-4901. [DOI] [PubMed] [Google Scholar]
- 41.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Eng J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 42.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome predicted by gene-expression profiling and supervised machine learning. Nat Med. 2002;8(1):68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 43.Wright G, Tan B, Rosenwald A, et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(17):9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 45.de Jong D, Rosenwald A, Chhanabhai M, et al. Immuno-histochemical prognostic markers in diffuse large B-cell lymphoma:validation of tissue microarray as a prerequisite for broad clinical applications—a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25(7):805–812. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
- 46.Wallace DJ, Shen D, Reed GF, et al. Detection of the bcl-2 t(14;18) translocation and proto-oncogene expression in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2006;47(7):2750–2756. doi: 10.1167/iovs.05-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson JO, Wilkes BM, Kwaiatkowski DJ, et al. bcl-2 rearrangements in de novo diffuse large cell lymphoma. Association with distinctive clinical features. Cancer. 1993;72(1):231–236. doi: 10.1002/1097-0142(19930701)72:1<231::aid-cncr2820720141>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 49.Malumbres R, Davis J, Ruiz P, Lossos IS. Somatically mutated immunoglobulin IGHV@ genes without intraclonal heterogeneity indicate a postgerminal centre origin of primary intraocular diffuse large B-cell lymphomas. Br J Haematol. 2007;138(6):749–755. doi: 10.1111/j.1365-2141.2007.06744.x. [DOI] [PubMed] [Google Scholar]
- 50.Montesinos-Rongen M, Kuppers R, Schluter D, et al. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol. 1999;155(6):2077–2086. doi: 10.1016/S0002-9440(10)65526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pels H, Montesinos-Rongen M, Schaller C, et al. VH gene analysis of primary CNS lymphomas. J Neurol Sci. 2005;228(2):143–147. doi: 10.1016/j.jns.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 52.Thompsett A, Ellison D, Stevenson F, Zhu D. V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood. 1999;94(5):1738–1746. [PubMed] [Google Scholar]
- 53.Smith JR, Henderson WW, Rosenbaum JT, et al. Cultured human endothelial cells expressing HIV-1 Vpu and Tat support the expansion of malignant B cells from primary central nervous system lymphoma. Br J Ophthalmol. 2008;92(2):297–299. doi: 10.1136/bjo.2007.119461. [DOI] [PubMed] [Google Scholar]
- 54.Chan CC, Fischette M, Shen D, et al. Murine model of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46(2):415–419. doi: 10.1167/iovs.04-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Mahesh SP, Shen de F, et al. Eradication of tumor colonization and invasion by a B cell-specific immunotoxin in a murine model for human primary intraocular lymphoma. Cancer Res. 2006;66(21):10586–10593. doi: 10.1158/0008-5472.CAN-06-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Touitou V, Daussy C, Bodaghi B, et al. Impaired th1/tc1 cytokine production of tumor-infiltrating lymphocytes in a model of primary intraocular B-cell lymphoma. Invest Ophthalmol Vis Sci. 2007;48(7):3223–3229. doi: 10.1167/iovs.07-0008. [DOI] [PubMed] [Google Scholar]
- 57.Hochman J, Assaf N, Deckert-Schluter M, et al. Entry routes of malignant lymphoma into the brain and eyes in a mouse model. Cancer Res. 2001;61(13):5242–5247. [PubMed] [Google Scholar]
- 58.Mineo JF, Scheffer A, Karkoutly C, et al. Using human CD20-transfected murine lymphomatous B cells to evaluate the efficacy of intravitreal and intracerebral rituximab injections in mice. Invest Ophthalmol Vis Sci. 2008;49(11):4738–4745. doi: 10.1167/iovs.07-1494. [DOI] [PubMed] [Google Scholar]
- 59.Kadoch C, Treseler P, Rubenstein JL. Molecular pathogenesis of primary central nervous system lymphoma. Neurosurg Focus. 2006;21(5):E1. doi: 10.3171/foc.2006.21.5.2. [DOI] [PubMed] [Google Scholar]
- 60.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107(9):3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Courts C, Montesinos-Rongen M, Martin-Subero JI, et al. Transcriptional profiling of the nuclear factor-kappaB pathway identifies a subgroup of primary lymphoma of the central nervous system with low BCL10 expression. J Neuropathol Exp Neurol. 2007;66(3):230–237. doi: 10.1097/01.jnen.0000248553.45456.96. [DOI] [PubMed] [Google Scholar]
- 62.Camilleri-Broet S, Criniere E, Broet P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas:analysis of 83 cases. Blood. 2006;107(1):190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 63.Lin CH, Kuo KT, Chuang SS, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12(4):1152–1156. doi: 10.1158/1078-0432.CCR-05-1699. [DOI] [PubMed] [Google Scholar]
- 64.Montesinos-Rongen M, Brunn A, Bentink S, et al. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia. 2008;22(2):400–405. doi: 10.1038/sj.leu.2405019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blay JY, Favrot M, Rossi JF, Wijdenes J. Role of interleukin-6 in paraneoplastic thrombocytosis. Blood. 1993;82(7):2261–2262. [PubMed] [Google Scholar]
- 66.Bost KL, Bieligk SC, Jaffe BM. Lymphokine mRNA expression by transplantable murine B lymphocytic malignancies. Tumorderived IL-10 as a possible mechanism for modulating the anti-tumor response. J Immunol. 1995;154(2):718–729. [PubMed] [Google Scholar]
- 67.Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol. 1995;120(5):671–673. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- 68.Cassoux N, Merle-Beral H, Lehoang P, et al. Interleukin-10 and intraocular-central nervous system lymphoma. Ophthalmology. 2001;108(3):426–427. doi: 10.1016/s0161-6420(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 69.Merle-Beral H, Davi F, Cassoux N, et al. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124(4):469–473. doi: 10.1046/j.1365-2141.2003.04800.x. [DOI] [PubMed] [Google Scholar]
- 70.Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115(9):1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- 71.Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology. 2003;110(8):1671–1672. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- 72.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beatty PR, Krams SM, Martinez OM. Involvement of IL-10 in the autonomous growth of EBV-transformed B cell lines. J Immunol. 1997;158(9):4045–4051. [PubMed] [Google Scholar]
- 74.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 75.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93(1):424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benjamin D, Knobloch TJ, Dayton MA. Human B-cell interleukin-10:B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt’s lymphoma constitutively secrete large quantities of interleukin-10. Blood. 1992;80(5):1289–1298. [PubMed] [Google Scholar]
- 78.Emilie D, Touitou R, Raphael M, et al. In vivo production of interleukin-10 by malignant cells in AIDS lymphomas. Eur J Immunol. 1992;22(11):2937–2942. doi: 10.1002/eji.1830221127. [DOI] [PubMed] [Google Scholar]
- 79.Herbst H, Foss HD, Samol J, et al. Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin’s disease. Blood. 1996;87(7):2918–2929. [PubMed] [Google Scholar]
- 80.Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48(7):3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sou R, Ohguro N, Maeda T, et al. Treatment of primary intraocular lymphoma with intravitrealmethotrexate. Jpn J Ophthalmol. 2008;52(3):167–174. doi: 10.1007/s10384-008-0519-9. [DOI] [PubMed] [Google Scholar]
- 82.Sen HN, Chan CC, Byrnes G, et al. Intravitreal methotrexate resistance in a patient with primary intraocular lymphoma. Ocul Immunol Inflamm. 2008;16(1):29–33. doi: 10.1080/09273940801899764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nacinovic-Duletic A, Stifter S, Dvornik S, et al. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol. 2008;30(3):230–239. doi: 10.1111/j.1751-553X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 84.Wang SS, Cozen W, Cerhan JR, et al. Immune mechanisms in non-Hodgkin lymphoma:joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors. Cancer Res. 2007;67(10):5042–5054. doi: 10.1158/0008-5472.CAN-06-4752. [DOI] [PubMed] [Google Scholar]
- 85.Kube D, Hua TD, von Bonin F, et al. Effect of interleukin-10 gene polymorphisms on clinical outcome of patients with aggressive non-Hodgkin’s lymphoma:an exploratory study. Clin Cancer Res. 2008;14(12):3777–3784. doi: 10.1158/1078-0432.CCR-07-5182. [DOI] [PubMed] [Google Scholar]
- 86.Park YH, Sohn SK, Kim JG, et al. Interaction between BCL2 and interleukin-10 gene polymorphisms alter outcomes of diffuse large B-cell lymphoma following rituximab plus CHOP chemotherapy. Clin Cancer Res. 2009;15(6):2107–2115. doi: 10.1158/1078-0432.CCR-08-1588. [DOI] [PubMed] [Google Scholar]
- 87.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68(6):835–853. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- 88.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286(5447):2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 89.Casamayor-Palleja M, Mondiere P, Amara A, et al. Expression of macrophage inflammatory protein-3alpha, stromal cell-derived factor-1, and B-cell-attracting chemokine-1 identifies the tonsil crypt as an attractive site for B cells. Blood. 2001;97(12):3992–3994. doi: 10.1182/blood.v97.12.3992. [DOI] [PubMed] [Google Scholar]
- 90.Chan CC, Shen D, Hackett JJ, et al. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110(2):421–426. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]