Abstract
Human speech and birdsong are shaped during a sensorimotor sensitive period in which auditory feedback guides vocal learning. To study brain activity as song learning occurred, we recorded longitudinally from developing zebra finches during the sensorimotor phase. Learned sequences of vocalizations (motifs) were examined along with contemporaneous neural population activity in the song nucleus HVC, which is necessary for the production of learned song (Nottebohm et al. [1976]: J Comp Neurol 165:457–486; Simpson and Vicario [1990]: J Neurosci 10:1541–1556). During singing, HVC activity levels increased as the day progressed and decreased after a night of sleep in juveniles and adults. In contrast, the pattern of HVC activity changed on a daily basis only in juveniles: activity bursts became more pronounced during the day. The HVC of adults was significantly burstier than that of juveniles. HVC bursting was relevant to song behavior because the degree of burstiness inversely correlated with the variance of song features in juveniles. The song of juveniles degrades overnight (Deregnaucourt et al. [2005]: Nature 433:710–716). Consistent with a relationship between HVC activity and song plasticity (Day et al. [2008]: J Neurophys 100:2956–2965), HVC burstiness degraded overnight in young juveniles and the amount of overnight degradation declined with developmental song learning. Nocturnal changes in HVC activity strongly and inversely correlated with the next day's change, suggesting that sleep-dependent degradation of HVC activity may facilitate or enable subsequent diurnal changes. Collectively, these data show that HVC activity levels exhibit daily cycles in adults and juveniles, whereas HVC burstiness and song stereotypy change daily in juveniles only. In addition, the data indicate that HVC burstiness increases with development and inversely correlates with song variability, which is necessary for trial and error vocal learning.
Keywords: critical period, sleep, top-down control, development, electrophysiology, birdsong, learning, memory, vocal, motor
INTRODUCTION
Complex sequential behaviors such as speech require dynamic guidance from sensory feedback and temporal coordination of multiple muscles. Our understanding of how neural networks control these sensorimotor behaviors is improving. The birdsong system has been used to elucidate neural mechanisms of a complex motor skill (e.g., McCasland, 1987; Scharff and Nottebohm, 1991; Perkel, 2004; Prather et al., 2008). Birdsong and human speech are both learned vocalizations that are acquired during a sensitive period of development and controlled by a series of specialized forebrain areas (Marler, 1970; Nottebohm et al., 1976; Doupe and Kuhl, 1999). Songbirds and humans learn their vocalizations by first memorizing species-typical sounds from a tutor(s) during a sensory phase and then by matching their vocalizations to this memory using auditory feedback during a sensorimotor phase (Konishi, 1965). Birdsong consists of continuous sounds known as “syllables” that are separated by silent periods. Syllables are arranged in a stereotyped sequence known as a “motif,” which is repeated to form songs. Song learning in the zebra finch can be observed motif-by-motif, syllable-by-syllable, as the bird slowly sculpts his song toward its mature form (Deregnaucourt et al., 2005).
The neural song system is a series of anatomically distinct clusters of neurons (nuclei) in the thalamus, basal ganglia, and pallium (cortex) that are dedicated to the production and plasticity of song (Nottebohm et al., 1976; Bottjer et al., 1984). HVC (this acronym is the proper name) (Jarvis et al., 2005) is a pallial song nucleus that controls song behavior (Nottebohm et al., 1976; Vu et al., 1994; Aronov et al., 2008). HVC lies at the interface of auditory and motor networks and transmits auditory signals to all downstream song nuclei (Doupe and Konishi, 1991; Vicario and Yohay, 1993), including the anterior forebrain pathway (AFP), which has roles in plasticity and the induction of song variability (Doupe and Konishi, 1991; Kao et al., 2005; Olveczky et al., 2005).
Lesioning studies have confirmed that HVC is required for the production of learned song (Nottebohm et al., 1976; Simpson and Vicario, 1990; Aronov et al., 2008), but HVC's role in song plasticity, if any, has remained unclear. Data suggest that the AFP serves to destabilize song behavior (Kao et al., 2005; Olveczky et al., 2005; Thompson et al., 2007). Recently, our lab has shown that HVC “premotor” activity during singing correlates with song stability, millisecond by millisecond (Day et al., 2008). This suggests that HVC and the AFP function antagonistically during song plasticity. In addition, HVC activity during singing changes with development (Crandall et al., 2007b) and HVC activity during sleep is positively correlated with overnight song stability in juveniles (Crandall et al., 2007a). HVC receives or generates neural signals that are selective for tutor song (Nick and Konishi, 2005b) and the bird's own song (Volman, 1993; Nick and Konishi, 2005a) depending on behavioral state (Nick and Konishi, 2005b). Collectively, these data indicate that HVC activity changes dynamically during song learning, responds selectively to tutor songs, and may stabilize the developing song.
Previously, Deregnaucourt et al. (2005) showed that song changes rapidly in the morning, stabilizes later in the day, and degrades over a night of sleep. The daily and nightly changes in behavior were dramatic, with the largest vocal changes in newly trained finches approaching 15% for some song syllables (Deregnaucourt et al., 2005). In addition, neurophysiological studies have implicated sleep in song learning (Dave and Margoliash, 2000; Crandall et al., 2007a; Shank and Margoliash, 2009). Based on these findings and recent data that show a correlation between HVC activity and song stability (Day et al., 2008), we hypothesized that premotor bursts in the juvenile HVC impede song plasticity and vary in predictable daily/nightly patterns. According to this hypothesis, HVC bursts will be weakest in the morning when song is most plastic and will become stronger later in the day as the song stabilizes. Here, we investigated daily and overnight changes in the HVC bursting activity that occurred during singing. We report that HVC “premotor” bursting activity strengthened each day and degraded each night. These results are consistent with the hypotheses that HVC bursting activity stabilizes song and that daily changes in song behavior (Deregnaucourt et al., 2005) are driven by daily changes in HVC bursting activity.
MATERIALS AND METHODS
Subjects
Forty-one juvenile (age 61–90 days; in the late sensorimotor stage of song development) and 22 adult (>100 days) male zebra finches (Taeniopygia guttata) were subjected to surgical implantation with chronic population recording electrodes. Of the juveniles, 17 had high-quality neural recordings (premotor RMS signal:noise > 2), but only 10 sang at least 100 motifs in at last 1 day and were used for this study. Of the adults, five had high-quality neural recordings and sang ≥100 motifs in 1 day. Three of the finches implanted as juveniles (Blue-70, Blue-15, and Blue-81) were also recorded after Day 90 (≥100 motifs/day) and thus included in the adult group for those days. All song recordings were made in the absence of a female (undirected). Undirected song is thought to reflect song practice (Jarvis et al., 1998). All juveniles and three of the adult finches were reared by their parents until Day 45 in our facility on a 14:10 light cycle. Two adult birds were obtained from a commercial supplier. Although experience and not age is probably the strongest predictor of song system maturity, age is correlated with experience and presents a more quantifiable parameter. None of the finches used in these experiments were ever exposed to auditory playback. The finches were allowed to hear their own vocalizations. The University of Minnesota Institutional Animal Care and Use Committee approved all procedures.
Chronic Physiological Recording
Basic methods have been previously described (Crandall et al., 2007b). For population recordings, finches were implanted with a set of recording electrodes: 1 or 2 50-μm nichrome-formvar electrodes in HVC or a control brain area adjacent to HVC (not plated, 1.1–1.8 MΩ, AM Systems, Carlsborg, WA), a 50-μm nichrome-formvar electrode adjacent to HVC for use as a reference electrode, and 75-μm silver wires for use as ground and electroencephalogram electrodes. The headstage and recording environment have been previously described (Schmidt and Konishi, 1998).
All data were acquired with custom-written (Datafleet, Minneapolis, MN) LabView software (National Instruments, Austin, TX) at a sampling frequency of 44.1 kHz. During recording, a light-weight operational amplifier was attached to the bird and connected to a mercury commutator via a flexible cable. HVC neural activity was amplified 1000 times and filtered 300–10,000 Hz. Song was monitored with a microphone (Earthworks, Milford, NH), high-pass in-line filtered at 100-Hz (Shure, Chicago, IL), and recorded. Localization of electrodes to HVC was confirmed with premotor activity in all cases and cresyl-violet histology in 7 of 15 finches.
Song Behavior Analysis
All data were analyzed with custom-written Matlab functions. Initial song analysis consisted of the sorting of sound data and exclusion of movement noise. Sound data were further sorted according to temporal properties. Preliminary songs were defined as sounds lasting ≥500 ms with time gaps of no more than 20 ms.
To specifically study the activity during learned song behavior, a canonical motif was identified by a skilled observer from the oldest day available from each finch and used to extract motifs and corresponding neural activity throughout the recording period. Multiple motif forms could have been selected depending on the developmental stage. However, we were most interested in how HVC activity changed once the overall vocal pattern had been established and, thus, selected the most mature motif available. Since perfusion and histology were performed as soon as the electrode recording declined, some subjects may not have achieved the final mature motif.
We focused our analysis on behaviorally relevant vocalizations (instead of, for example, movements) by band-pass filtering our vocal data (1–8 kHz). Amplitude envelopes of the canonical motif and all preliminary songs were constructed by filtering the rectified sound recording with a Savitzky-Golay smoothing filter (4th order polynomial fit; 20-ms frame size). Then, amplitude envelopes of the canonical motif and preliminary songs were cross-correlated (Crandall et al., 2007b). Sharp peaks in the cross-correlation revealed the onset of a motif that matched the canonical motif. Behavioral song motifs and corresponding neural activity were then excised and saved for further analysis.
Song was quantified based on six features: amplitude, Weiner entropy, frequency, pitch, frequency modulation, and pitch goodness (a measure of sound periodicity). These features were calculated from the raw motif in 9.3-ms bins with sliding 1-ms steps with a subprogram of Sound Analysis Pro (Tchernichovski et al., 2000), ported to Matlab by S. Saar. The variance of vocal features was calculated across 20 aligned motifs millisecond by millisecond relative to time within the motif. The mean variance across all milliseconds in the motif provided a measure of song variance that was calculated separately for the first and last 20 motifs.
Multiunit Physiology Analysis and Comparisons to Song Features
Data for analysis were limited to days during which a bird produced at least 100 identified motifs. HVC activity during motifs was digitally band-pass filtered 300–6000 Hz. To examine the levels of population spiking activity during the motif, the root mean square (RMS) was calculated across the entire motif.
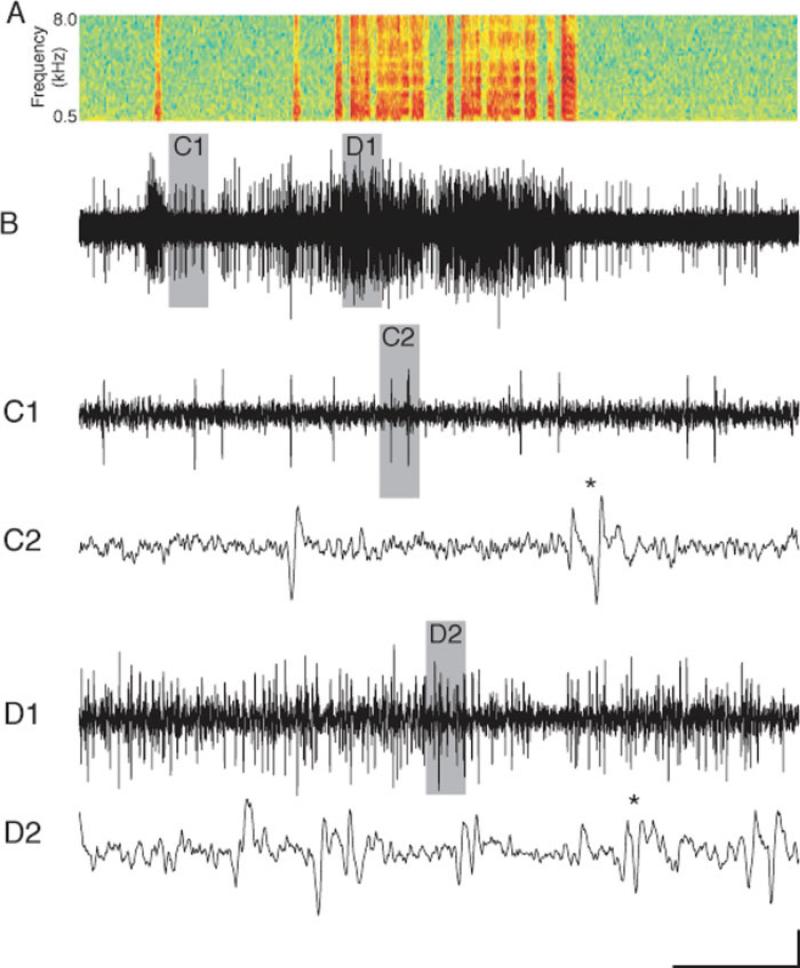
In preliminary studies, it appeared that the level of population burstiness was changing throughout the day in juveniles. Therefore, we developed a method to quantify the level of burstiness. Because of our requirement that the RMS of premotor activity be at least double the RMS of nonsinging “noise” activity, the bulk of the multiunit data presented in this article meets or exceeds a 3:1 signal:noise (Kao et al., 2008). This may not be obvious on the long-time scales of songs and motifs [Fig. 1(A,B)]. However, increasing the temporal resolution during nonsinging periods reveals large units that peak at >3 times the noise [Fig. 1(C1)]. Even higher resolution examination reveals single units that fire alone or in population bursts [Fig. 1(C2), asterisk]. This quality of data has been subjected to thresholding and detailed temporal analysis of population activity (Crandall et al., 2007b). During periods of high HVC population activity, such as during singing, the amount of activity is so great that spiking events of multiple units are consistently simultaneous or near-simultaneous [Fig. 1(D1-2)]. Smaller events can sum to exceed the threshold. Events that might normally be above threshold can be lost because they overlap temporally with other events of opposite polarity [Fig. 1(D2), asterisk]. In addition, multiple synchronous or near-synchronous events register as a single spike or no spike at all. If synchrony of activity changes during development and/or the day, thresholding methods applied to multiunit recordings will fail to detect it. Because of these concerns, we sought to analyze our data with a technique that is better at quantifying multiunit activity. We used a Savitzky-Golay filter that smoothes data in the temporal domain (4th order polynomial fit; 50-ms frame size). To measure burstiness, the peak: valley % was computed by first creating an HVC activity envelope by rectifying and smoothing the mean HVC activity amplitude. Then, the mean of the millisecond time windows that contained the maximum 25% of amplitude measurements was divided by that of the minimum 25%. This quotient was then decremented by 1 and multiplied by 100 to obtain the peak:valley %.
Figure 1.
The complex nature of HVC multiunit activity during singing renders thresholding methods inadequate. (A) For reference, a sonogram of recorded vocalizations is temporally aligned with B. (B) HVC population activity typically increases during singing when compared with nonsinging. In juveniles, there is often some activity immediately preceding and/or following song (Crandall et al., 2007b). Gray bars indicate time windows that are shown at higher resolution below. (C1, C2) Higher temporal resolution of activity during nonsinging reveals relatively distinct spiking events. (D1, D2) Higher resolution plotting of singing activity reveals near continuous overlap and interference of multiple units. All data are from finch Blue-46, age 66 days. Scale bar: 100 μV; A, B: 1 s; C1, D1: 55 ms; C2, D2: 3 ms. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Statistical Analysis and Data Presentation
All bars represent means and all error bars represent standard errors of the mean. Paired t-tests were used to compare data from the same finch. Unpaired t-tests were used to compare mean data across days from juveniles and adults. Significance was defined as a α = 0.05.
RESULTS
To investigate the role of the song nucleus HVC in song learning, we measured neural sensorimotor activity during singing across days, over nights, and through the end of the sensitive period for vocal plasticity. HVC population activity during stereotyped vocal sequences (motifs) was measured across multiple consecutive days. Zebra finches were maintained in the same recording chamber for many days before and during recording. Finches were only disturbed for food and water replenishment. The electrodes were never experimentally adjusted. These efforts resulted in stable recordings over many days and, in a few cases, for several weeks. For all data shown, the finch had recovered from surgery for at least 6 days, been in the recording chamber for at least 3 days, and sung in the chamber at least 1 day prior. All HVC activity in this manuscript occurred during song motifs. These points are relevant to the daily changes in HVC activity that we report later.
HVC Activity Levels During Singing Increase Daily and Decrease Overnight in Juveniles and Adults
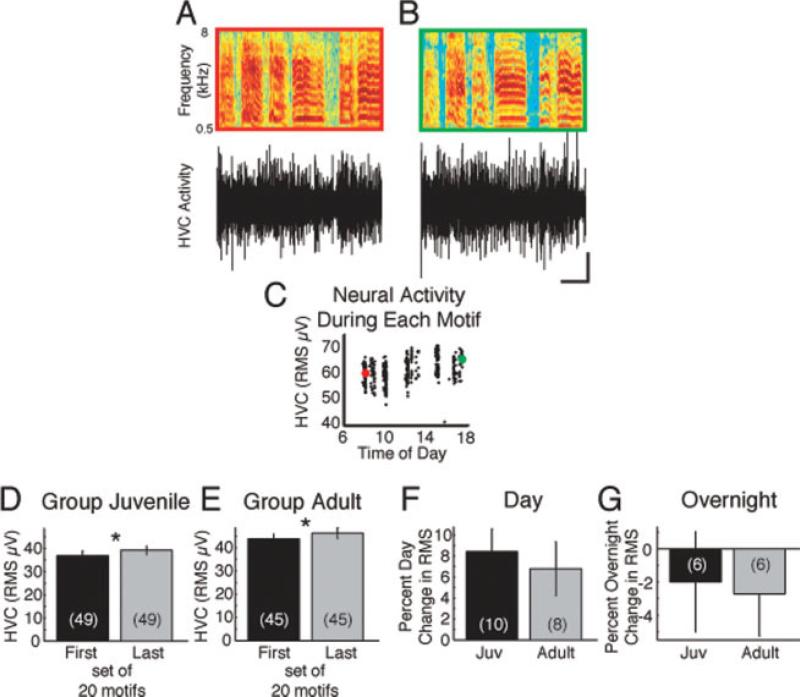
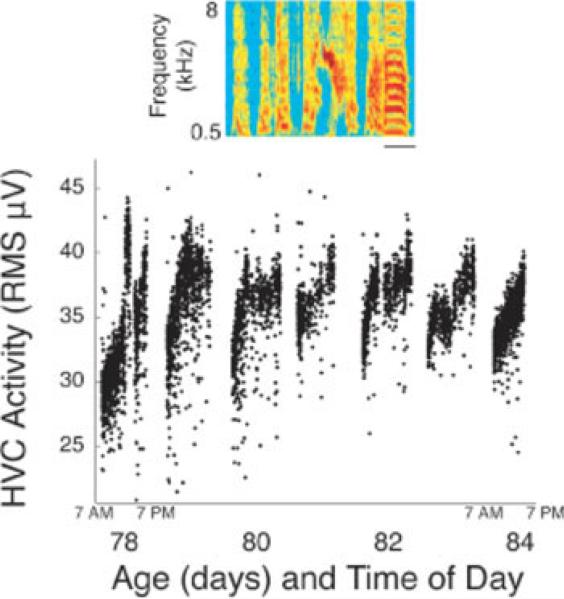
From all available song data, we extracted and temporally aligned song motifs using established methods (Crandall et al., 2007b). The RMS of HVC population activity during song motifs increased over the course of the day in juveniles and adults (Fig. 2 and Supporting Information Video; example raw traces across days and development are shown in Supporting Information Fig. 1). HVC activity during a motif that was produced early in the day [Fig. 2(A,C)] was smaller in amplitude than a motif produced later in the day [Fig. 2(B,C) and Supporting Information Figure 2]. We often noted obvious diurnal increases and nocturnal resets in HVC activity levels (Fig. 3 and Supporting Information Video). These changes in HVC activity occurred in the absence of fluctuations in recording chamber temperature (data not shown), which indicates the involvement of an intrinsic physiological process. We quantified the daily increase in HVC activity by examining 94 days of data in which each bird sang at least 100 motifs that were recognized by our motif finder program. We compared the HVC activity during the first 20 motifs with that during the last 20 motifs. HVC activity significantly increased between the first and last motifs produced each day [Fig. 2(D,E); p < 0.03, paired t-test]. The daily change was quite variable across animals and days (Supporting Information Fig. 3). The percent daily increase [Fig. 2(F)] was not different in juveniles when compared with adults. To examine the overnight change in HVC activity, we used pairs of consecutive days during which at least 100 motifs were produced. The last 20 motifs from the first day were compared with the first 20 motifs from the next day. The overnight decrease in HVC activity level was not significantly different in juveniles and adults [Fig. 2(G)]. Collectively, these data indicate that HVC activity levels during singing increase through the day and decrease overnight.
Figure 2.
HVC activity levels increase each day and decrease overnight in juvenile and adult zebra finches. A motif produced early in the day (A, red) was accompanied by less HVC activity than a motif produced later in the same day (B, green). In A and B, the top panel is a sonogram of a stereotyped learned motif. The colored outlines correspond with the colored points in C. The lower panel shows HVC population activity. Scale bar: 100 ms; 100 μV. (C) The RMS of HVC population activity during singing increases during the day. The RMSs during the motifs shown in A and B are indicated by their respective colors. Exemplar data in A–C are from bird Blue-81, age 87 days. (D, E) Comparing the first 20 motifs with the last 20 motifs of each day revealed that HVC activity significantly increases in juveniles (D) and adults (E; *p < 0.03, paired t-test; day Ns in parentheses). By finch, the percent change in singing activity levels over the day (F, p = 0.63) and overnight (G, p = 0.86; finch Ns in parentheses) was not different between juveniles and adults. In general, HVC activity during singing increased during the day (F) and decreased overnight (G).
Figure 3.
Apparent cycling of HVC activity during song motifs was noted in many longitudinal recordings. Mean HVC activity during each motif for 7 consecutive days is plotted versus the time of day. HVC activity increased until approximately noon each day. Occasionally activity decreased during the day, as on Day 78. HVC activity in the morning trended to be lower than the evening before. The inset shows a sonogram of the canonical motif from bird Blue-70 that was used to extract all other motifs. All data in this figure are from Blue-70. Scale bar: 100 ms. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Simultaneous control recordings of a nonsong-related brain area immediately adjacent to HVC and within 200 μm of the HVC recording electrodes were achieved in four juveniles. In contrast to the singing-related HVC activity, which increased each day, control activity during singing decreased (HVC: +3.25 ± 6.19 RMS μV; Control: –5.83 ± 6.12 RMS μV; N = 4). The daily change in activity in HVC versus the control brain region was significantly different (p = 0.03, paired t-test).
During the Day, the Pattern of HVC Activity Becomes Burstier in Juveniles
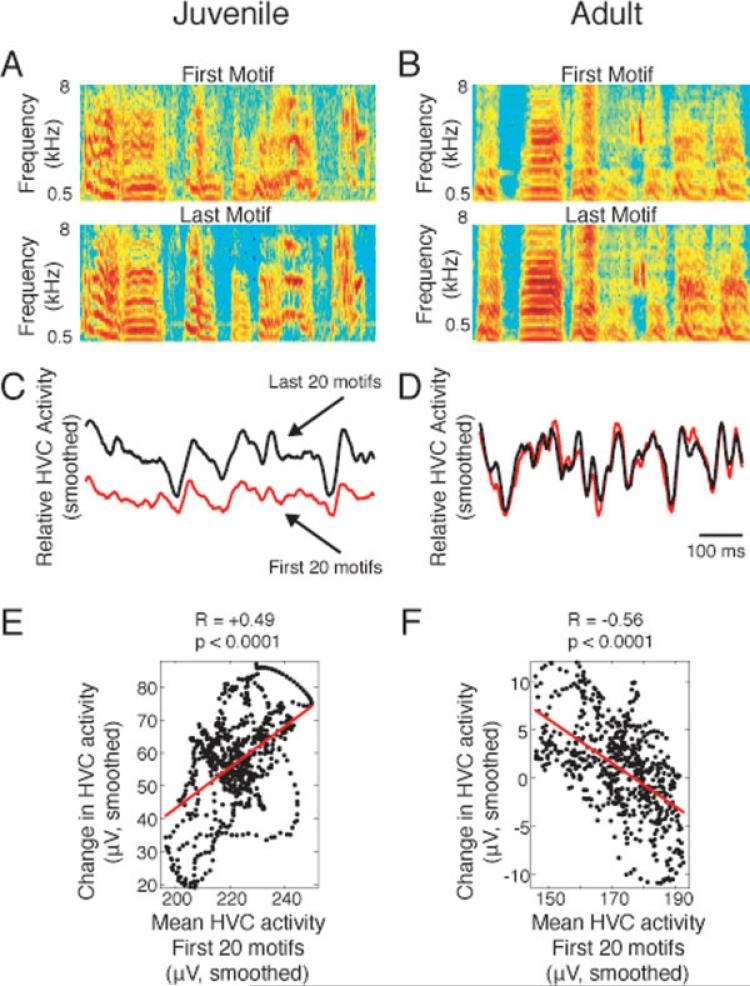
Diurnal and nocturnal changes in HVC activity levels were similar in adults and juveniles (Fig. 2). We asked whether the changes in activity were evenly distributed across the motif. We found that the peaks of activity increased more than valleys during the day in juveniles, but not in adults (Fig. 4). Peaks are upward deflections of the rectified and smoothed voltage trace [as in Fig. 4(C)], whereas valleys are downward deflections. During the day, the juvenile HVC typically concentrated more power into specific time windows that already had more power (peaks) and less power in other windows (valleys). That is, HVC became more “bursty.” The term “burst” refers to intermittent bouts of accelerated spiking activity, whereas the term “bursty” refers to the tendency to exhibit bursts of activity. The examples in Figure 4(A,B) show sonograms of the first and last motifs of a given day for a juvenile (A) and an adult (B). Note the relative variability of the juvenile motifs [Fig. 4(A)] when compared with those of the adult [Fig. 4(B)], as previously reported (Immelmann, 1969; Deregnaucourt et al., 2005). The comparison of mean smoothed and rectified HVC activity during the first and last 20 motifs reveals that peaks increased more than valleys in the juvenile [Fig. 4(C)], but not in the adult [Fig. 4(D)]. Millisecond-by-millisecond, juvenile HVC activity during the first 20 motifs correlated with the change in HVC activity during the same day, indicating that peaks preferentially increased [Fig. 4(E)]. Further analysis of the data shown in Figure 2 provides another example of increased juvenile burstiness during the day (Supporting Information Fig. 1). In the adult example, the relationship between HVC activity and the change in HVC activity that day was negative [Fig. 4(F)]. These data show that HVC burstiness increases during the day in juveniles, but not in adults.
Figure 4.
HVC singing activity becomes burstier each day in juveniles, but not in adults. In juveniles (A, C, E), peaks in neural activity during motif production typically increased more than valleys, such that the burstiness increased. In adults (B, D, F), HVC bursting activity was generally stable or declined during the day. (A) Sonograms of the first and last song motif reveal that the juvenile song (A) became more structured and complex during the day, whereas the adult song (B) was relatively stable. (C, D) HVC neural activity also changed more in the juvenile (C) than in the adult (D). (C) In the juvenile, comparison of the mean rectified and smoothed HVC activity of the first 20 motifs (red) to that of the last 20 motifs (black) reveals that the increase in neural activity during the day was focused in the peaks, with relatively little increase in activity during troughs. (D) An adult recording with an overall decrease in burstiness highlights the differences between adults and juveniles. (E, F) The change in HVC activity was directly proportional to the starting HVC activity (the first 20 motifs) during the same millisecond (relative to the motif) in the juvenile (E) and inversely proportional in the adult (F). Data are from Blue-57, age 58 days, and Orange-331, age 100 days. Two other examples similar to C and D are shown in Figure 8(A,B).
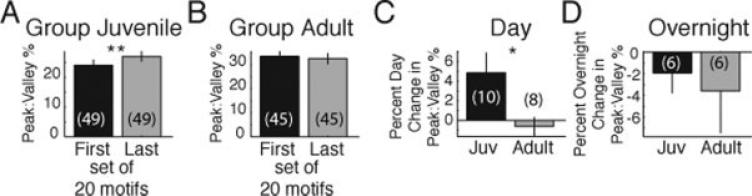
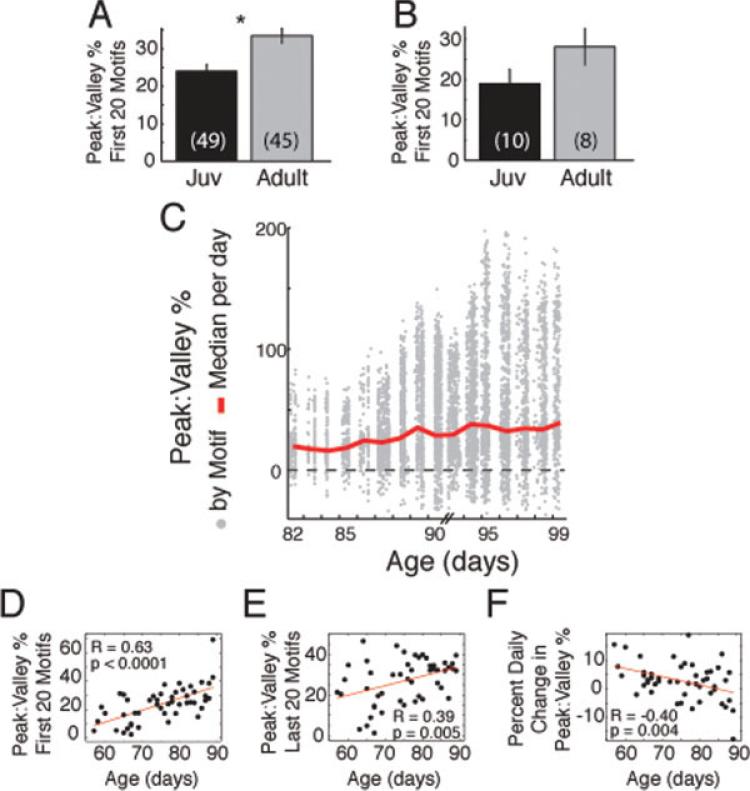
To quantify the increase in burstiness, we calculated the percent of amplitude difference between peaks and valleys of rectified, smoothed HVC activity [as shown in Fig. 4(C,D)]. As with RMS calculations, data for analysis were limited to days in which at least 100 motifs were produced. In juveniles, peak:valley % significantly increased between the first 20 and the last 20 motifs [Fig. 5(A); example RMS and peak:valley % data for 10 days and three finches are shown in Supporting Information Fig. 4]. In adults, peak:valley % trended to decrease or not change over the day, and was not significant [Fig. 5(B)]. The comparison of the mean diurnal change in peak:valley % by animal revealed that the daily change in burstiness was significantly greater in juveniles than adults [Fig. 5(C)]. In contrast to diurnal changes, HVC burstiness during singing slightly decreased between the last 20 motifs on a given day to the first 20 motifs on the next day [Fig. 5(D)]. This overnight change was rather variable and not significantly different between juveniles and adults [Fig. 5(D)]. Collectively, these data indicate that HVC burstiness increases during a day of singing in juveniles, but not in adults.
Figure 5.
As measured by peak:valley comparison, HVC singing activity becomes burstier each day in juveniles, but not in adults. Juveniles (A; **p < 0.02, paired t-test), but not in adults (B, p = 0.75), exhibit significant changes in HVC burstiness (peak:valley %) each day. (C) The percent daily change in burstiness was significantly greater in juveniles (*p < 0.04, unpaired t-test). (D) The percent overnight change in burstiness was not significantly different in juveniles versus adults (p = 0.77).
HVC Burstiness Increases with Development
These data show that juvenile HVC burstiness increases diurnally and decreases to a lesser extent nocturnally. This suggests that diurnal increases in HVC burstiness may accumulate in development. To test this hypothesis, we compared the HVC peak:valley % in juveniles and adults. Comparing day-by-day, the juvenile HVC is less bursty than that of the adult [Fig. 6(A); for this panel, each day is considered a separate data point, so individual finches may contribute more than one data point]. Collapsing data by taking the mean peak:valley % of all days for each animal revealed the same trend as the day-by-day data, but was not significant [Fig. 6(B); for this panel, each finch was only allowed to contribute one data point].
Figure 6.
HVC burstiness increases with development, whereas the degree of diurnal change in burstiness decreases with development. (A) HVC burstiness, as measured by peak:valley % across days, was greater in adults than juveniles (*p < 0.0005, unpaired t-test). (B) Analysis of data by bird yielded the same trend as the daily analysis, but the difference was not significant (p = 0.12). (C) The increase in HVC burstiness was observed day-by-day in longitudinal recordings of juveniles. The peak:valley % for each motif is shown in gray dots, and the median per day is indicated by the line. (D) Burstiness increased with age in juveniles and was most clearly observed in the first 20 motifs of each day. (E) HVC burstiness during the last 20 motifs of each day also correlated with age, but the relationship appeared more complex. (F) The percent daily change in burstiness decreased with age in juveniles. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The cumulative developmental increase in HVC burstiness could be observed in single juveniles. In Figure 6(C), the peak:valley % for all recorded motifs from a single finch from Days 82–99 are indicated by gray dots. The red line indicates the median peak:valley % for each day. Note how the red line becomes more positive, indicating that HVC becomes burstier, as the animal aged. We noted very little change in burstiness on days when this finch sang relatively little (Days 82–84), but we did not observe this correlation in every finch (data not shown). Combining all juvenile data, we found a significant positive correlation between age and the HVC burstiness during the first 20 motifs [Fig. 6(D)]. Interestingly, the correlation between age and the burstiness of last 20 motifs was less correlated [Fig. 6(E)]. Subtraction of the burstiness values for the first 20 motifs from the last 20 motifs provides a daily change metric. Since the burstiness of the first 20 motifs increased with age and this relationship was degraded during the last 20 motifs, we predicted that the relationship of the daily change in burstiness to age would be negative to account for this difference. Indeed, the degree of daily change in burstiness decreased with age in juveniles [Fig. 6(F)]. These data reveal a developmental change in HVC bursting activity that accrues daily during song learning. As the animal ages, the degree of daily change decreases and burstiness stabilizes.
HVC Burstiness Is Inversely Correlated with Song Variance in Developing Finches
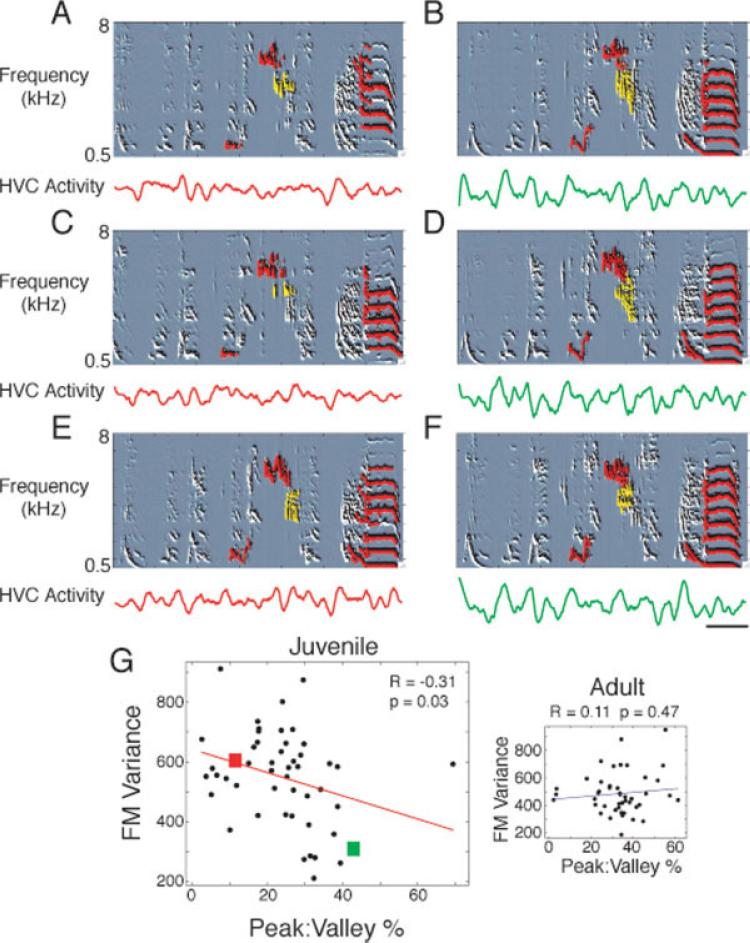
How does HVC activity impact behavior? HVC activity correlates with behavioral stability at a time point 50 ms in the future (Day et al., 2008). Higher amplitude bursts appear to finely limit song variability in discrete time windows. Correspondingly, increased HVC burstiness may enable fine song stability control, progressively locking in additional temporal fragments of the song as sensorimotor development proceeds. If so, increased HVC burstiness should predict decreased overall song variability. To calculate overall motif variability, we temporally aligned the first 20 motifs of each day and, for each millisecond of the aligned motifs, took the variance across motifs of six song features: entropy, amplitude, frequency modulation, frequency, pitch, and pitch goodness (periodicity). We then took the mean variance of each feature across all milliseconds of the motif. We found that the variance of all six song features was inversely correlated with HVC burstiness (Fig. 7, Supporting Information Fig. 5). Figure 7 shows one of the features, FM variance. The other five features can be seen in Supporting Information Figure 5. On a day when HVC had relatively little bursting activity during singing (lower panels of A,C,E), the behavior was variable (upper panels of A,C,E). Several spectrotemporal segments that were particularly variable are highlighted in red and yellow. Later in development, HVC was more bursty (lower panels of B,D,F), and the behavior was less variable (upper panels of B,D,F). This correlation was significant for data from all juveniles [Fig. 7(G)], but not in adults [Fig. 7(G), inset]. The burstiness–song variance relationship was also noted across days in individual finches [data from each finch shown in Fig. 7(G) are represented by a different colored symbol in Supporting Information Fig. 6].
Figure 7.
HVC burstiness inversely correlates with behavioral variability. A day during which behavior was relatively more variable and HVC activity was less bursty is shown in the left column (A, C, E; bird Blue-70, age 77 days) compared with a day during which behavior was more stable and HVC activity was more bursty (B, D, F; bird Blue-70, age 89 days). The top panel of each example shows spectral derivatives, which are similar to sonograms, with parts of the song spectrotemporal field labeled with red and yellow for clarity. These song segments were relatively variable in the left column when compared with the right. For example, note the leftmost red mark in each panel. Note how this sound is variable in A and C when compared with E. Note how this sound is relatively simple when compared with the corresponding and stable sound in B, D, and F. The bottom panel of each example shows rectified and smoothed HVC activity. Note the shallow peaks and valleys in the left column when compared with the right. (G) Day-by-day comparison of HVC burstiness (peak:valley %) and FM variance reveals a weak but significant correlation in juveniles. Data are from 10 birds, with multiple values reflecting multiple days from the same finch (Supporting Information Fig. 6). Exclusion of the rightmost outlier results in R = –0.39, p = 0.006. Inset: Adult HVC burstiness and song variance are not well correlated.
The Overnight Change in Burstiness Is Inversely Correlated with the Change in Burstiness the Next Day
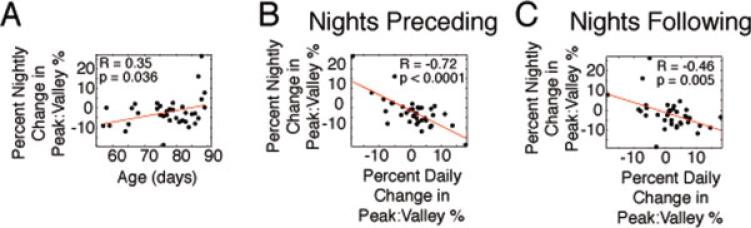
Previous work has shown that juvenile finches have less nocturnal HVC activity and that nocturnal HVC activity appears to stabilize song behavior (Crandall et al., 2007a). In addition, the total degree of overnight destabilization of song in juveniles positively correlates with how well they ultimately copy their tutor's song (Deregnaucourt et al., 2005). Collectively, these studies suggest that nocturnal changes in HVC activity may have a role in song learning. These data, combined with the data presented earlier, led us to hypothesize that HVC burstiness should degrade more overnight early in song learning, relative to later in development. This should enable destabilization of song, if HVC burstiness stabilizes behavior (Fig. 7). Consistent with this hypothesis, we found that the degree of overnight decrement in HVC burstiness became smaller with development [Fig. 8(A)]. We next asked how the overnight decrement in HVC burstiness related to daily changes in song: did daily changes in burstiness correlate more with the change in burstiness the night before or the night after? We found that the overnight change in HVC burstiness strongly and inversely correlated with the change in burstiness the next day [Fig. 8(B)]. The day's change in burstiness also correlated with the next night's change, but not as strongly [Fig. 8(C)]. These data suggest that nocturnal degradation of HVC activity enables variability in song behavior the next day and subsequent reforming of a new HVC activity pattern.
Figure 8.
Nocturnal decrements in burstiness decrease with development and inversely correlate with the next day's change in burstiness. (A) Overnight decrements in HVC burstiness decreased with age in juveniles. (B) The overnight change in burstiness strongly and inversely correlated with the degree of change in burstiness the next day. (C) The change in burstiness during the day correlated with the overnight change in bursting the following night. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
This study investigated the role of the song control nucleus HVC in vocal learning. It provides the first evidence that HVC activity levels change on a daily cycle. In juveniles and adults, HVC activity levels increased during the day and decreased at night. Burstiness increased each day in juveniles, but not in adults. These observed changes in burstiness are relevant to behavior: HVC activity levels inversely correlate with song variability, on the timescales of milliseconds (Day et al., 2008), days, and development (current study). In addition, burstiness degrades overnight in juveniles, which may underlie a daily cycle of song learning and stabilization during the day and destabilization at night.
Daily Cycling of HVC Activity
We have found that HVC activity increases daily. In juveniles, the oscillatory burst pattern of HVC activity typically becomes stronger during the day [Fig. 9(A)]. In adults, overall HVC levels increase, but burst dynamics are relatively unchanged [Fig. 9(B)]. Figure 9(C) presents a simple representation of our results in juveniles. In the morning, lower HVC activity levels and bursting correlate with plasticity in song behavior (upper right three sonograms). Later in the day, increased HVC activity correlates with increased song stereotypy, particularly during parts of the song that correspond to bursts in HVC activity (Day et al., 2008). As development proceeds, burstiness continues to increase, which may enable tight temporal control of song in the adult. The correlation between HVC burstiness and behavioral variance on the timescales of days and development could indirectly result from processes that coincidentally affect both (e.g., circadian rhythms). However, another study found the same inverse relationship between HVC bursts and song variance on a millisecond timescale (Day et al., 2008), which suggests that daily and developmental processes alone cannot explain the predictive capacity of HVC activity on song variability. In addition, neural activity recorded outside of the song system does not show the same pattern of increased activity during the day as it does in HVC (see Results section).
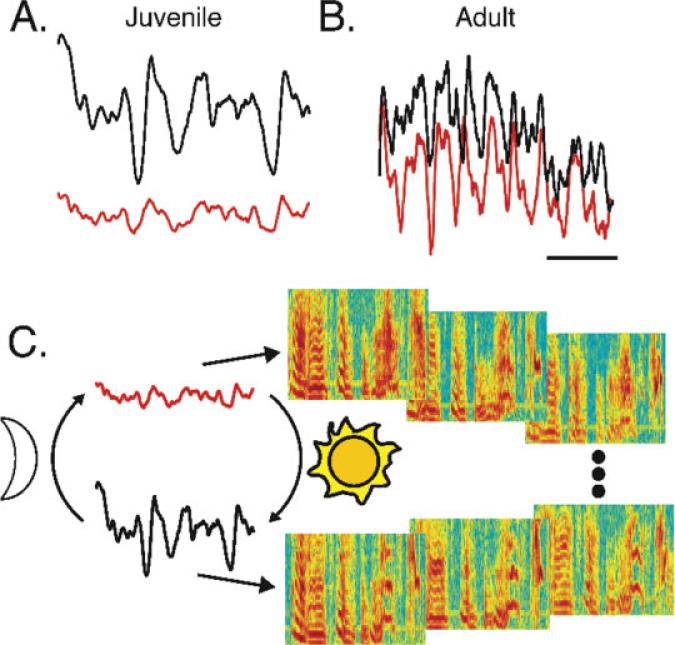
Figure 9.
A model of daily cycling of motor control during song learning. Nightly decrement of HVC activity levels and bursting may allow song variability and subsequent rearrangement and/or strengthening of HVC activity pattern via activity dependent plasticity. In turn, activity-dependent plasticity may result in daily increment in HVC activity levels and bursting. (A) Mean juvenile HVC activity for the first 20 motifs (red) was greater than that of the last 20 (black) motifs on the same day. (B) Mean adult HVC activity for the first 20 also increased relative to the last 20 motifs on the same day. HVC activity levels cycled in both juveniles (A) and adults (B). In contrast, the relative burstiness increased during the day in juveniles (A), but was relatively stable in adults (B). For A and B, y-axis: relative voltage; x-axis: time relative to the motif; scale bar: 200 ms. (C) We propose that increasing HVC activity increases the entrainment of projection neurons and stabilizes behavior. In the morning, juvenile HVC population activity is low in amplitude with low oscillatory power (red). Lack of correlated interneuron bursting in juveniles may allow variability in the firing of projection neurons and consequent variability in song behavior (the three motif sonograms in the upper right). Later in the day, HVC interneuron population activity is higher amplitude (black) and concentrated in more compact bursts. The hypothesis predicts that putative interneuron bursts entrain the activity of projection neurons and thereby decrease behavioral variability (the three motif sonograms in the lower right).
Why Cycle Premotor Activity?
Avian and mammalian sleep share fundamental characteristics such as slow-wave activity (Jones et al., 2008; Low et al., 2008). The synaptic homeostasis hypothesis (Tononi and Cirelli, 2006) posits that sleep serves in a homeostatic fashion to downscale synaptic strengths that are enhanced during waking. Sleep-dependent downscaling may increase the signal-to-noise ratio by decreasing the probability that a neuron will fire in response to a given input. The model proposes that slow-wave sleep increases the energetic efficiency of the brain by universally downscaling all synaptic strengths without regard to previous experience-dependent change. Our data are consistent with this model. We observe the following: (1) HVC activity potentiates during waking, which may reflect synaptic potentiation (this study); (2) increased burst strength correlates with stability of song 50-ms into the future, suggesting underlying mechanisms of activity-dependent plasticity (Day et al., 2008); (3) HVC activity levels typically decrease after sleep regardless of developmental status or learning state, which may reflect nonspecific synaptic downscaling (this study). We have found that levels of HVC singing activity in adult finches increase each day and decrease each night, but oscillatory bursting and song stability do not appear to cycle. In contrast, juveniles cycle all three parameters: HVC activity levels, burstiness, and song stereotypy. These data are consistent with a synaptic homeostasis model in which, throughout the day, synapses are selectively strengthened during specific song time windows (during bursts), perhaps due to an auditory-driven instructive signal (Nick and Konishi, 2005b). According to this model, relative differences in strengths of adult synapses are already large, as evidenced by strong bursts during the first 20 motifs, relative to juveniles. Thus, further synaptic strengthening has little effect on the burst pattern. In contrast, juveniles have relatively weak bursts, a subset of which becomes stronger as the day progresses. In this model, during sleep, both adults and juveniles would experience nonspecific synaptic decrement, which would be reflected in a decrease in activity levels. The decreases in activity levels need not be reflected in a decrease in measured burstiness and a change in burst pattern in animals with already strong HVC bursts (i.e., adults). Since HVC bursts predict song stability (Day et al., 2008), adults may exhibit increased song stereotypy because they have a strong and stable HVC burst pattern that is resistant to the effects of sleep-dependent synaptic downscaling.
In the context of the synaptic homeostasis hypothesis, the sleep “replay” or “rehearsal” of song-related neural patterns (Dave and Margoliash, 2000; Shank and Margoliash, 2009) represents a trace of synaptic enhancement that occurred during waking (Tononi and Cirelli, 2006). If the activity pattern that we observe during singing is a neural prediction or expectation that is strengthened after each of thousands of motifs, then the synaptic trace pattern should be very strong in adults and weaker in juveniles. Consistent with this hypothesis, previous work has shown that putative sleep replay activity is significantly less in juveniles when compared with adults (Crandall et al., 2007a).
Pressing Questions
This study has raised many new questions regarding birdsong learning. In terms of percentages, are the small changes in activity levels behaviorally significant? Since daily cycling of neural activity levels during a specific behavior has never been reported and never shown to correlate with behavioral variability (as we observe), no scale exists with which to judge the size of the effect. Is sleep required for the resetting of HVC activity, or does it result from a circadian rhythm? Developmental analysis of song behavior has revealed that sleep can reset the song entropy variance (Deregnaucourt et al., 2005; Shank and Margoliash, 2009), which suggests that sleep may be involved in the resetting of HVC activity that is closely tied to behavioral variance (this study, Day et al., 2008). Does auditory feedback affect the oscillatory burst strength of HVC population activity? Auditory signals increase HVC population activity during singing (Sakata and Brainard, 2008), consistent with the hypothesis that an instructive signal strengthens HVC bursts (Nick and Konishi, 2005b). Does HVC entrain the activity of the AFP? Fast-spiking putative interneurons dominate multielectrode single-unit recordings (Crandall et al., 2007b) and may become more synchronous with development (Day et al., unpublished observations). Thus, the strong oscillatory bursting activity characteristic of the adult HVC (Crandall et al., 2007b) may be due to the synchronous firing of interneurons. Synchronous activity in HVC interneurons may entrain the activity of Area X-projecting HVC neurons and thus entrain the activity of the AFP. Alternatively, HVC interneurons may directly entrain the HVC neurons that project down a pathway directly involved in producing song (the neurons that project to the robust nucleus of the arcopallium). What stabilizes the neural oscillation at the end of vocal learning and decreases song variability in adults? Other studies have found that perineuronal nets may close critical periods and stabilize developing networks (Sur et al., 1988; Pizzorusso et al., 2002). Perineuronal nets appear in the song system during the sensorimotor phase (Balmer et al., unpublished observations). Answering these questions will illuminate the role of HVC in vocal learning and, perhaps, reveal fundamental mechanisms of sensorimotor learning.
Supplementary Material
Acknowledgments
The authors thank S. Crandall, T. Balmer, V. Carels, A. Craig, J. Frisch, S. Kerrigan, and L. Onikoro for technical assistance. They also thank M. Chafee, M. Coleman, T. Ebner, R. Poppele, S. Saar, O. Tchernichovski, and D. Vicario for critically reviewing the preliminary drafts of this article.
Contract grant sponsor: National Institute on Deafness and Other Communication Disorders; contract grant numbers: R01-DC007384, K02-DC008521.
Contract grant sponsor: National Institute of Neural Disease and Stroke; contract grant number: R01-NS050436.
Contract grant sponsor: National Institute of General Medical Sciences; contract grant number: NI5T32-GM008471-15.
Contract grant sponsors: John Merck Scholars Program, Minnesota Medical Foundation, University of Minnesota Graduate School, University of Minnesota Undergraduate Research Opportunities Program.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Aspects of this study have previously appeared in abstract form (Nick et al., 2007).
REFERENCES
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Bottjer S, Miesner E, Arnold A. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Adam M, Kinnischtzke AK, Nick TA. HVC neural sleep activity increases with development and parallels nightly changes in song behavior. J Neurophysiol. 2007a;98:232–240. doi: 10.1152/jn.00128.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall SR, Aoki N, Nick TA. Developmental modulation of the temporal relationship between brain and behavior. J Neurophysiol. 2007b;97:806–816. doi: 10.1152/jn.00907.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Day N, Kinnischtzke AK, Adam M, Nick TA. Top-down regulation of plasticity in the birdsong system: “Premotor” activity in the nucleus HVC predicts song variability better than it predicts song features. J Neurophys. 2008;100:2956–2965. doi: 10.1152/jn.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra PP, Feher O, Pytte CL, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci USA. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: Common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrilid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge University Press; Cambridge: 1969. pp. 61–74. [Google Scholar]
- Jarvis ED, Gunturkun O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, et al. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: Context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jones SG, Vyazovskiy VV, Cirelli C, Tononi G, Benca RM. Homeostatic regulation of sleep in the white-crowned sparrow (Zonotrichia leucophrys gambelii). BMC Neurosci. 2008;9:47. doi: 10.1186/1471-2202-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- Low PS, Shank SS, Sejnowski TJ, Margoliash D. Mammalian-like features of sleep structure in zebra finches. Proc Natl Acad Sci USA. 2008;105:9081–9086. doi: 10.1073/pnas.0703452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Birdsong and speech development: Could there be parallels? Am Sci. 1970;58:669–673. [PubMed] [Google Scholar]
- McCasland JS. Neuronal control of bird song production. J Neurosci. 1987;7:23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Kinnischtzke AK, Adam M. Eighth International Congress of Neuroethology. Vancouver, BC: 2007. Rhythmic spiking in HVC during singing increases during the day and decreases after a night of sleep. p. 112. [Google Scholar]
- Nick TA, Konishi M. Neural auditory selectivity develops in parallel with song. J Neurobiol. 2005a;62:469–481. doi: 10.1002/neu.20115. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol. 2005b;62:231–242. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:902–909. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel DJ. Origin of the anterior forebrain pathway. Ann NY Acad Sci. 2004;1016:736–748. doi: 10.1196/annals.1298.039. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. J Neurosci. 2008;28:11378–11390. doi: 10.1523/JNEUROSCI.3254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavior deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–77. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Frost DO, Hockfield S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci. 1988;8:874–882. doi: 10.1523/JNEUROSCI.08-03-00874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Wu W, Bertram R, Johnson F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. J Neurosci. 2007;27:12308–12320. doi: 10.1523/JNEUROSCI.2853-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Vicario DS, Yohay KH. Song-selective auditory input to a forebrain vocal control nucleus in the zebra finch. J Neurobiol. 1993;24:488–505. doi: 10.1002/neu.480240407. [DOI] [PubMed] [Google Scholar]
- Volman SF. Development of neural selectivity for birdsong during vocal learning. J Neurosci. 1993;13:4737–4747. doi: 10.1523/JNEUROSCI.13-11-04737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo Y-C. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.