Abstract
Heart failure remains a leading cause of morbidity and mortality worldwide. Although depressed pump function is common, development of effective therapies to stimulate contraction has proven difficult. This is thought to be attributable to their frequent reliance on cAMP stimulation to increase activator Ca2+. A potential alternative is nitroxyl (HNO), the 1-electron reduction product of nitric oxide (NO) that improves contraction and relaxation in normal and failing hearts in vivo. The mechanism for myocyte effects remains unknown. Here, we show that this activity results from a direct interaction of HNO with the sarcoplasmic reticulum Ca2+ pump and the ryanodine receptor 2, leading to increased Ca2+ uptake and release from the sarcoplasmic reticulum. HNO increases the open probability of isolated ryanodine-sensitive Ca2+-release channels and accelerates Ca2+ reuptake into isolated sarcoplasmic reticulum by stimulating ATP-dependent Ca2+ transport. Contraction improves with no net rise in diastolic calcium. These changes are not induced by NO, are fully reversible by addition of reducing agents (redox sensitive), and independent of both cAMP/protein kinase A and cGMP/protein kinase G signaling. Rather, the data support HNO/thiolate interactions that enhance the activity of intracellular Ca2+ cycling proteins. These findings suggest HNO donors are attractive candidates for the pharmacological treatment of heart failure.
Keywords: nitroxyl, contractility, ryanodine receptor, sarcoplasmic reticulum Ca2+-ATPase, excitation/contraction coupling
Congestive heart failure affects an estimated 5 million people in the United States and has an annual mortality rate approaching 20%. More than half of the patients have depressed cardiac function, and, although improvement in function is clearly beneficial, as revealed by heart transplantation, development of effective pharmacological therapy to safely stimulate contraction has proven problematic.1 Most such agents rely on enhancing cAMP and protein kinase A (PKA) to stimulate activator Ca2+ and increase contractility. However, this approach is less effective in failing hearts, because of downregulation of the signaling,2 and is chronically linked to toxicity and increased mortality.
We recently reported that donors of nitroxyl (HNO), the 1-electron reduction product of nitric oxide (NO),3 have novel cardiovascular effects quite different from NO. In intact in vivo hearts, the HNO donor Angeli’s salt (AS) enhances function independent of β-adrenergic blockade or stimulation and unaccompanied by changes in cGMP.4,5 Unlike most prior positive inotropes, HNO donors are similarly effective in normal and failing hearts.5 Their combined ability to enhance heart function, while reducing venous pressures, has suggested potential utility as a heart failure treatment.
The mechanisms underlying cardiac action of HNO remain unknown. HNO can stimulate ion channels such as the N-methyl-d-aspartate receptor.6,7 Recent data suggest that it also activates the skeletal muscle ryanodine receptor (RyR).8 HNO is thought to react with targeted thiols9 and, more specifically, negatively charged thiols, or thiolates. These exist in several proteins involved in Ca2+ cycling, such as the sarcoplasmic reticular (SR) Ca2+ release channel,10 SR Ca2+.pump (sarcoplasmic reticulum Ca2+-ATPase [SERCA2a]), and possibly phospholamban.11 Hence, we hypothesized that HNO activity targets heart muscle cells and directly improves contraction and relaxation by enhancing Ca2+ cycling. Our results support improvement in SR Ca2+ uptake and release that is independent of cAMP/PKA or cGMP/PKG but, rather, related to thiol modification.
Materials and Methods
Reagents
AS (Na2N2O3) was a generous gift of Dr Jon M. Fukuto and Matthew I. Jackson (University of California, Los Angeles). AS (100 mmol/L) stock solution was freshly prepared by dissolving AS in 10 mmol/L NaOH. Sodium-2-(N,N-diethylamino)-diazenolate-2-oxide (DEA/ NO) was purchased from Calbiochem (San Diego, Calif). Indo 1 acetoxymethyl ester (Indo 1-AM) was purchased from Molecular Probes/Invitrogen (Carlsbad, Calif). 1H-[1,2,4]Oxadiazolo quinoxalin 1-one (ODQ) was obtained from Tocris (Ellisville, Mo). All other compounds were purchased from Sigma Chemical Co (St Louis, Mo; Milan, Italy).
Contraction and Whole Ca2+ Transients in Mouse Ventricular Myocytes and Whole Ca2+ Transients and SR Ca2+ Load in Rat Ventricular Myocytes
Wild-type 2- to 4-month-old mice were anesthetized with intraperitoneal pentobarbital sodium (100 mg/kg IP). Heart perfusion and isolation of rat ventricular myocytes were performed as described12 (see the online data supplement, available at http://circres.ahajournals.org). Functional measurements are described in the online data supplement. The protocols were all approved by the Animal Care and Use Committee of Johns Hopkins University.
FRET Imaging
Primary cultures of cardiac ventricular myocytes from 1- to 3-dayold Sprague–Dawley rats (Charles River Laboratories, Wilmington, Mass) were prepared according to Dostal et al.13 FRET analysis was performed as described14 (see online data supplement).
Fluorescent Probes for Two-Photon Laser Scanning Microscopy and Image Acquisition
The cationic potentiometric fluorescent dye tetramethylrhodamine methyl ester (TMRM) was used to monitor changes in ΔΨm, as previously described.15 The production of the fluorescent glutathione adduct GSB from the reaction of cell permeant monochlorobimane (MCB) with reduced glutathione (GSH), catalyzed by glutathione S-transferase, was used to measure intracellular glutathione levels. Details of GSH measurements are provided in the online data supplement.
Visualization of Spontaneous Ca2+ Sparks and Measurement of Spark Frequency
Isolated mouse cardiac myocytes were loaded with the Ca2+ indicator fluo-4 acetoxymethyl ester (fluo-4/AM) (Molecular Probes, 20 µmol/L for 30 minutes). Confocal images were acquired using a confocal laser-scanning microscope (LSM510, Carl Zeiss) with a Zeiss Plan-Neofluor ×40 oil immersion objective (NA=1.3). Fluo-4/AM was excited by an argon laser (488 nm), and fluorescence was measured at >505 nm. Images were taken in the line-scan mode, with the scan line parallel to the long axis of the myocytes. Each image consisted of 512 line scans obtained at 1.92-ms intervals, each comprising 512 pixels at 0.10-µm separation. Digital image analysis used customer-designed programs coded in interactive data language and a modified spark detection algorithm.16
RyR2 Single-Channel Recordings in Planar Lipid Bilayers
Recording of single RyR2 in lipid bilayers was performed as described17 (see the online data supplement).
Measurements of ATP-Dependent Ca2+ Uptake by Murine Cardiac SR Vesicles
Crude cardiac microsomal vesicles containing fragmented SR were prepared as described18 (see also the online data supplement). SR membrane vesicles (0.4 mg/mL) suspended in a medium containing 100 mmol/L KCl, 1 mmol/L MgCl2, 50 µmol/L arsenazo III, 5 mmol/L sodium azide, and 20 mmol/L MOPS, pH 7.4, were mixed with an equal volume of an identical medium containing 1 mmol/L Na2ATP at 24°C in a manually operated stopped-flow apparatus (Applied Photophysics Ltd). The total [Ca2+] in the uptake medium was 0.5 µmol/L, yielding a free [Ca2+] in equilibrium with the Ca/arsenazo III complex of 0.2 µmol/L (KA = 3.3×104 mol/L−1). The change in [Ca2+] was monitored at 0.1-second intervals using a single-beam UV-VIS spectrophotometer (AVIV, Model 14DS) with a monochromator setting of 650 nm. The signal change caused by vesicle light scattering was evaluated from separate measurements conducted under identical conditions at the isosbestic wavelength of 693 nm (red-shifted from 685 nm by the presence of protein). Addition of AS (250 µmol/L) to the incubation medium had no effect on the spectral characteristics of arsenazo III or its response to Ca2+. The kinetic and thermodynamic parameters for Ca2+ uptake were evaluated by fitting stopped-flow signals to 1- and 2-exponential decay functions plus a residual term using nonlinear regression. Residual plots of the difference between the fitted curve and data points were used to evaluate systematic errors in the fits and to calculate the sum-of-squares error used in selecting the best fit.
Results
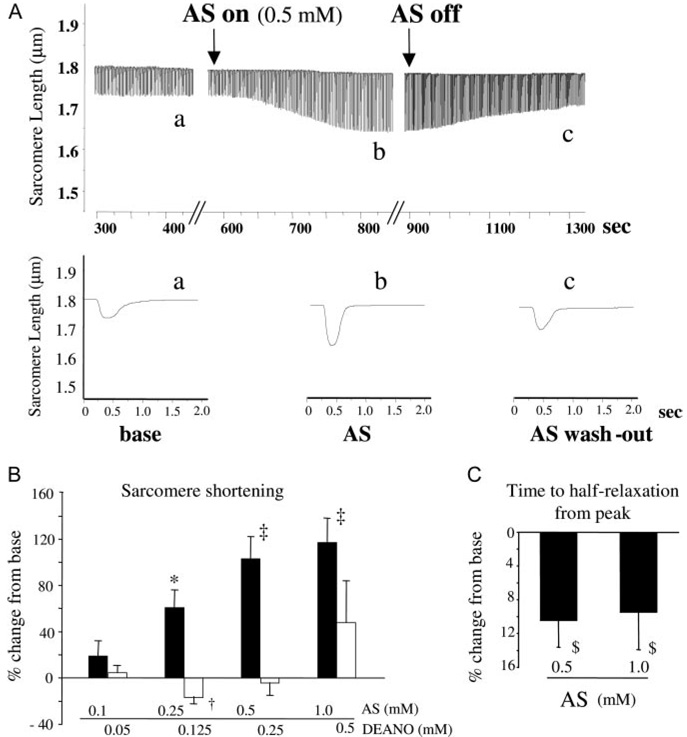
To test whether HNO directly influences myocyte function, freshly isolated adult mouse myocytes (C57/Bl6) were exposed to AS (10−6 to 10−3 mol/L), matching concentrations relevant in vivo.4,5 Myocyte contractility rose in a dose-dependent manner (Figure 1A and 1B), peaking at ≈100% at 0.5 and 1 mmol/L (both P<0.00005). Myocyte relaxation rate also improved by 10% to 20% (Figure 1C; P<0.05). These changes plateaued after ≈10 to 15 minutes and were reversible (at ≤500 µmol/L) 15 minutes after stopping exposure to AS (Figure 1A). In contrast to HNO, the NO donor DEA/NO induced slight functional depression at low doses and minimal changes at higher doses (Figure 1B).
Figure 1.
HNO increases contractility and relaxation in isolated ventricular myocytes. A, Effect of AS/HNO on sarcomere shortening in isolated mouse ventricular myocyte. B, Dose-response effect of AS/HNO and DEA/NO on cell shortening. *P<0.001 vs control, †P<0.01 vs control, ‡P<0.00005 vs control. C, AS/HNO effects on myocyte relaxation (time to 50% relengthening). $P<0.05 vs control.
At physiological pH, AS decomposes into HNO and nitrite. We therefore tested whether nitrite might contribute to the observed response. AS decomposition in the identical medium and temperature as used in the myocyte studies yielded 25% nitrite generation after ≈1000 seconds (16 minutes). Identical results were obtained with 0.1 to 1 mmol/L AS. This meant that at the time of functional analysis, 25 to 250 µmol/L NO2− was expected. However, direct exposure to such levels of NO2− (and higher and lower doses) had no effect on sarcomere shortening.
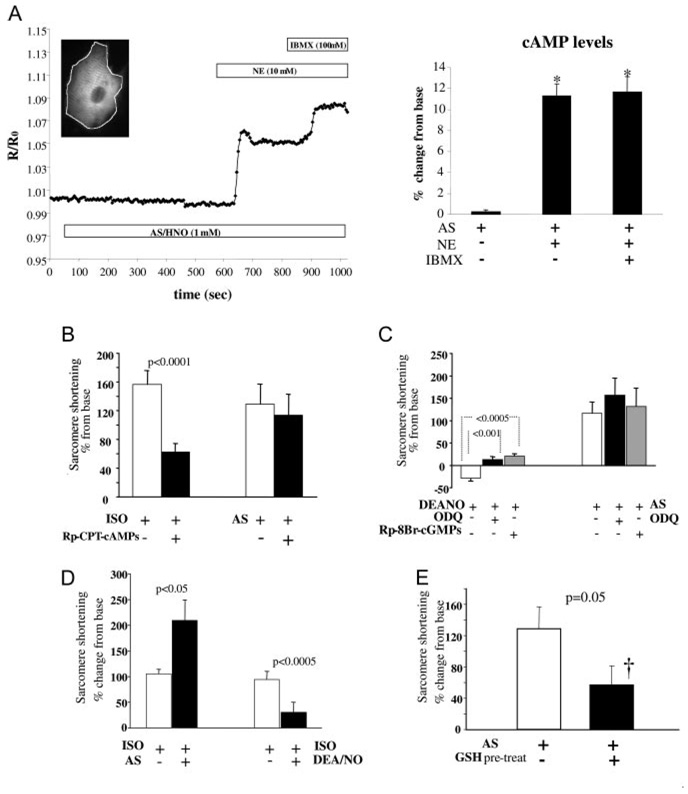
Agents that concomitantly increase myocyte contraction and accelerate relaxation are often linked to a rise in intracellular cAMP and subsequent activation of PKA.19 To test whether this applied to AS/HNO, we performed real-time imaging of cAMP on neonatal rat cardiomyocytes transfected with a cAMP FRET probe.14 On exposure to 1 mmol/L AS, the FRET signal was unchanged (0.3%±0.1%; n =23; P=NS), whereas subsequent application of norepinephrine (10 µmol/L) or phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (100 µmol/L) both increased it by 12% (P<10−6) (Figure 2A). Pretreatment of adult mouse myocytes with the PKA inhibitor Rp-CPT-cAMPs (100 µmol/L; Figure 2B) did not alter HNO-enhanced sarcomere shortening.
Figure 2.
AS/HNO actions on myocyte function are cAMP and cGMP independent but modulated by the intracellular thiol content. A, left, Kinetics of cAM-PFRET recorded in a single living neonatal rat cardiomyocyte (inset) challenged with AS (1 mmol/L), followed by norepinephrine (NE) (10 µmol/L) and the broad-spectrum phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) (100 µmol/L). Graph depicts FRET average over the entire cell. Summary data are to the right. *P<10−6 vs control. B, PKA inhibition with 100 µmol/L Rp-CPT-cAMPs blunts ISO but not HNO inotropy. C, sGC (soluble guanylyl cyclase [ODQ]) or PKG (Rp-8Br-cGMPs) inhibition blunts NO but not HNO effects. D, NO has negative impact on concomitant β-adrenergic– stimulated contractility, whereas HNO effects are additive. E, Pretreatment with cell-permeable GSH reduces sarcomere shortening enhancement by AS/HNO. †P<0.05 vs control.
AS/HNO-stimulated contractility was also independent of cGMP/PKG. Preincubation with the soluble guanylate cyclase inhibitor ODQ (10 µmol/L×30 minutes) prevented DEA/NO-induced negative inotropy but had no impact on AS/HNO inotropy. Pretreatment with a PKG inhibitor (Rp-8Br-cGMPs, 10 µmol/L) prevented DEA/NO negative inotropy, converting it to a modest positive response, yet had no impact on AS/HNO inotropy (Figure 2C).
NO donors exert a negative effect on β-adrenergic stimulation in vitro and in vivo20; however, we previously found the opposite for HNO donors in intact hearts.5 We confirmed this in cardiomyocytes. Cells challenged with isoproterenol (ISO) (2.5 nmol/L) had a 100±27% increase in sarcomere shortening (P=0.002, n=30). This was markedly blunted by coinfusion of 0.25 mmol/L DEA/NO, whereas coapplication of 0.5 mmol/L AS/HNO doubled shortening above ISO alone (Figure 2D). Thus, AS/HNO acts in parallel with the β-adrenergic pathway.
HNO targets thiol groups on selective proteins.9 To test whether such interaction could underlie whole cell contractile effects, studies were performed in which myocyte thiol equivalents were first enhanced using a cell-permeable ester-derivative of GSH (GSH ethyl ester in Tyrode’s solution, 4 mmol/L for 3 hours). We hypothesized that by enriching the intracellular thiol content, the probability of trapping HNO before it targeted critical thiol residues related to excitation/ contraction coupling would be enhanced. Pretreatment with GSH enhanced intracellular thiol equivalents (+6±1.5% in fluorescence arbitrary units versus controls, n=40, P<0.05) determined by fluorescence assay of GSH S-bimane production using 2-photon microscopy. Pretreated cells were then exposed to AS/HNO (0.5 mmol/L), and the contractility response was substantially blunted (+57±19%; P=0.02 versus base; P=0.05 versus AS alone) (Figure 2E). This supports the targeting of HNO on SH groups to exert its cardiotropic action.
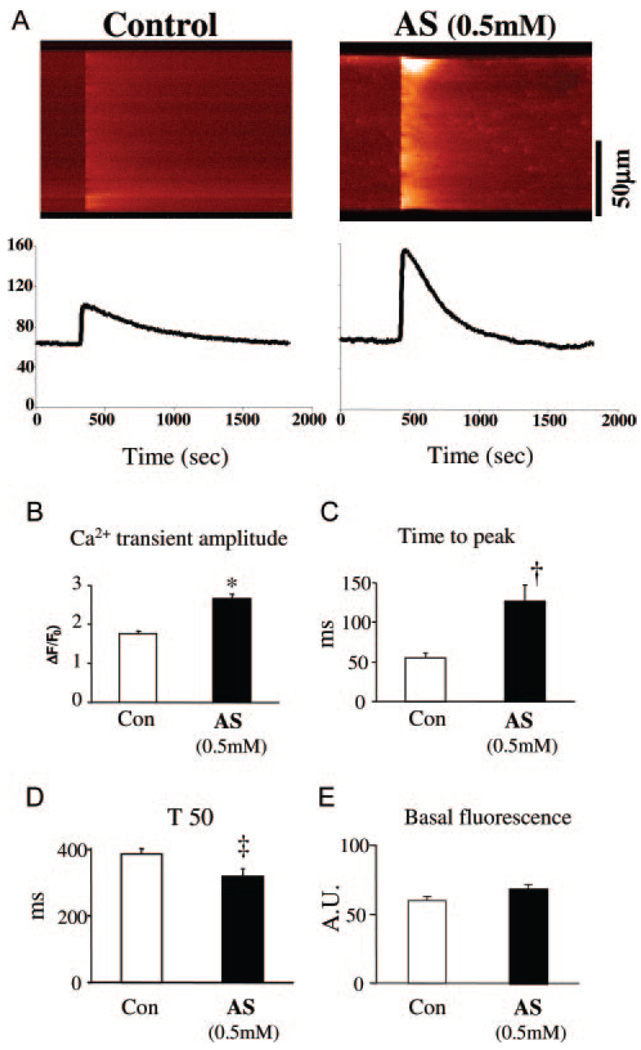
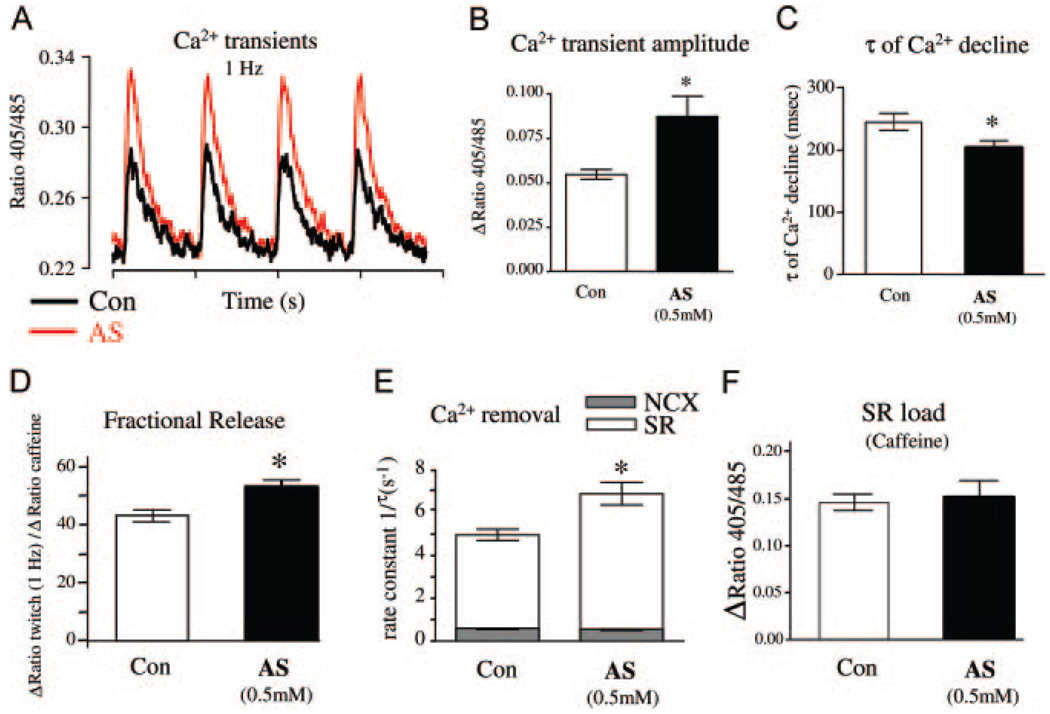
Next, we examined Ca2+ cycling in adult mouse and rat cardiac myocytes. Cells were first exposed to AS/HNO for 5 to 10 minutes, then washed and loaded with Indo-1 or fluo-4 for 20 minutes. Pretreatment with AS was necessary because the drug reacted with the Ca2+ indicators (both fluo-4 and Indo-1) and altered their fluorescent properties. In mice, the Ca2+ transient amplitude assessed by confocal line-scan imaging increased by ≈40% over baseline with 0.5 mmol/L AS (n=27, P<0.001) (Figure 3A and 3B), and time to peak transient was prolonged (Figure 3C), whereas the decay time shortened (Figure 3D). Basal fluorescence (F0) was unchanged by AS pretreatment (Figure 3E). Similar results were obtained in rat myocytes (using Indo-1) for Ca2+ transient amplitude (Figure 4A and 4B) and decay time (Figure 4C). The increase in amplitude was not accompanied by an increase in diastolic Ca2+ level (ratio 405/485=0.2390±006 [control] versus 0.2430±008 [AS]; P=NS; see also Figure 3A and 3E and Figure 4A). Rapid sustained caffeine (10 mmol/L) application abruptly releases all SR Ca2+ and subsequent [Ca2+]i decline is mediated mainly via Na/Ca exchange (NCX). The amplitude and tau of decline of the caffeine-induced Ca2+ transient indicates that HNO did not alter SR Ca2+ content (Figure 4F) or NCX function (τ=2.0±0.4 versus 2.2±0.3 seconds; Figure 4E). These results indicate that the HNO-enhanced [Ca2+]i decline was attributable to increased SERCA2a function, and the HNO-enhanced Ca2+ transient amplitude was caused by enhanced fractional SR Ca2+ release (Figure 4D) with unaltered SR Ca2+ content (Figure 4F).
Figure 3.
Increase of Ca2+ transients by HNO in isolated murine myocytes. A, Line-scan confocal images of Ca2+ transients in control and AS-treated (0.5 mmol/L) mice cardiomyocytes. Cells were loaded with Ca2+ indicator fluo-4 (20 µmol/L for 20 minutes). Mean results for Ca2+ transient amplitude (ΔF/F0) (B), rising time (Time to peak) (C), time from peak to 50% relaxation (T50) (D), and basal fluorescence (E) (arbitrary units, n=27 to 28 cells from 3 hearts for each data point). *P<0.001 vs control, †P<0.01 vs control, ‡P<0.05 vs control.
Figure 4.
Increase of Ca2+ transients and Ca2+ fractional release from SR in rat cardiomyocytes. A, Representative original recordings and Ca2+ transients in untreated control (Ctr) and AS-pretreated (AS) rat myocytes. B and C, Mean results for Ca2+ transient amplitude and τ of Ca2+ decline (n=30 to 31 cells from 4 hearts). D through F, SR Ca2+ load measured via rapid application of 10 mmol/L caffeine (n=11 to 14 cells from 6 hearts). D, Twitch amplitude divided by the caffeine amplitude expressed in percentage (fractional SR Ca2+ release). E, Ca2+ removal fluxes according to the formula 1/τtwitch=1/τNCX+1/τSR; τNCX is the τ of Ca2+ decline in the presence of caffeine; relative contribution of the SR increased from 87.6% in Ctr to 91.3% in AS-pretreated cells and relative contribution of NCX decreased from 12.4% to 8.7%, respectively. Total SR load was unchanged (F). All data are means±SEM. *P<0.05 vs control.
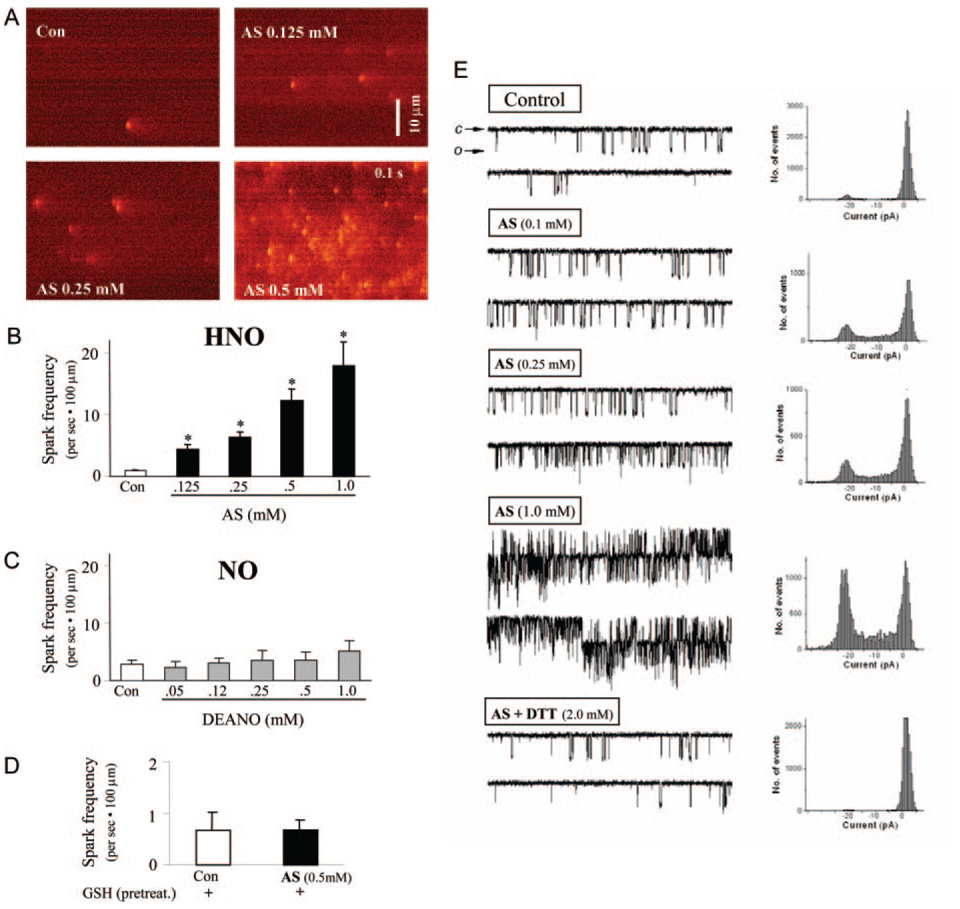
Given evidence for enhanced SR Ca2+ reuptake and release, with no net gain in total SR Ca2+ content, we next examined direct effects of AS/HNO on the ryanodine-sensitive release channel (RyR2). In intact myocytes, AS enhanced RyR2 opening probability, as revealed by an increased frequency of Ca2+ sparks assessed by line-scan confocal microscopy (Figure 5A), in a dose-dependent manner (Figure 5B; 18-fold rise in spark frequency at 1 mmol/L AS, n=10 to 24, P<0.001). Conversely, DEA/NO had no effect on spark generation (Figure 5C). Individual spark amplitude, rise time, and spatial width were unaltered by AS, indicating a primary effect on RyR2 activation. SR Ca2+ store depletion by thapsigargin (10 µmol/L, 30 minutes) or ryanodine exposure (10 µmol/L) abolished Ca2+ sparks in control and AS (0.5 mmol/L, data not shown). The influence of AS/HNO on Ca2+ sparks was thiol sensitive. Preincubating cells with reduced glutathione (4 mmol/L for 3 hours) before AS exposure prevented increased spark frequency (Figure 5D), indicating that increased intracellular thiol content effectively quenched HNO action.
Figure 5.
AS/HNO increase RyR2 function in a thiol-sensitive manner. A, Line-scan images of Ca2+ sparks in intact murine myocytes in control conditions and after increasing concentrations of AS/HNO. B, Dose-dependent effect of AS/HNO on Ca2+ spark frequency (*P<0.001 vs control). C, Neutral effect of DEA/NO on Ca2+ spark frequency. D, Pretreatment with GSH abolishes AS-induced increase in Ca2+ spark frequency. E, Representative original tracings of single-channel recordings in RyR2 from murine myocytes. Cardiac RyR2 channels were reconstituted into planar lipid bilayers and activated by 3 µmol/L (cis) cytosolic Ca2+. From the top to the bottom, RyR2 single recordings in control conditions and after the exposure to increasing concentration of AS/HNO, showing dose-dependent increase in Po with increasing doses of AS/HNO. In the lowest trace, the AS-induced increase in RyR2 open probability is almost fully reversed by the addition of the thiol-reducing agent dithiothreitol (DTT) to the cytosolic side.
To further test whether HNO directly interacted with RyR2 proteins to increase open probability, purified reconstituted RyR2 were expressed in planar lipid bilayers and steady-state activity recorded with or without AS/HNO. The cis (cytosolic) solution contained 10 µmol/L activating Ca2+, and recordings were made at positive 30-mV holding potential. AS (0.1 to 1 mmol/L) produced a dose-dependent rapid increase in frequency and the mean time of open events without altering unitary channel conductance (Figure 5E). The probability of the channel being open (Po) increased from an average 0.16±0.03 without AS/HNO to 0.46±0.07 at 0.3 mmol/L AS added to the cytoplasmic side of the channel (n=4). This was reversible on addition of 2 mmol/L dithiothreitol (0.11±0.04). These findings support direct HNO/ RyR2 interaction likely via a reversible reaction with thiol groups in the protein.
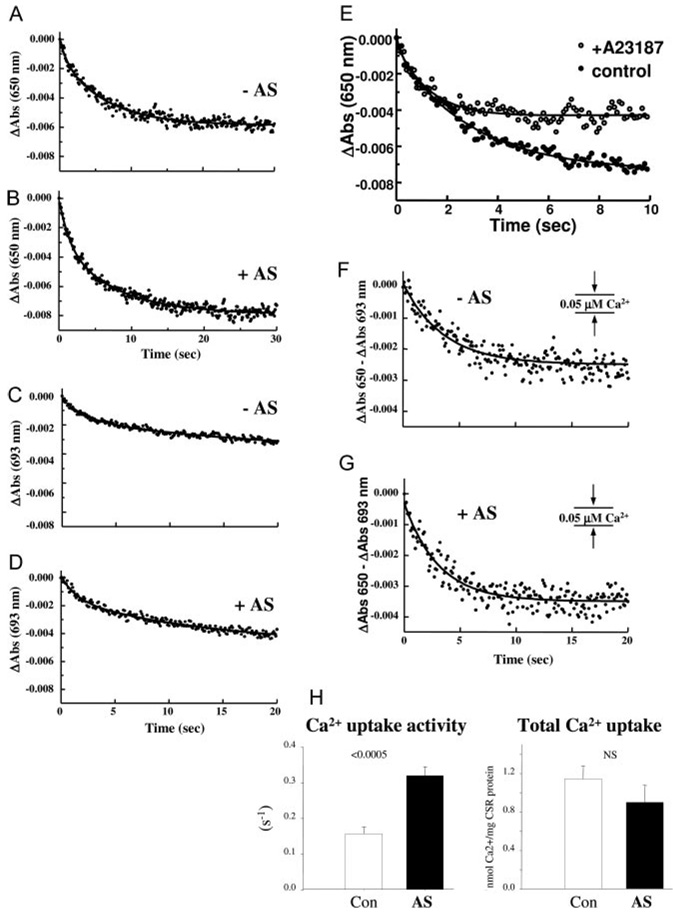
We investigated whether HNO directly enhances SR Ca2+ uptake by studying its effects on SR membrane vesicles isolated from pooled mouse hearts. Crude SR microsomal vesicles were incubated with 250 µmol/L AS before measuring ATP-dependent Ca2+ uptake by stopped-flow mixing at 24°C. Arsenazo III was used to monitor Ca2+ removal from the extravesicular compartment and buffer the free [Ca2+] at a level producing half-saturation of the Ca2+ pump (≈0.2 µmol/L). The time course of Ca2+ accumulation monitored at 650 nm was biphasic (Figure 6A), likely reflecting different vesicle populations associated with the light and heavy fractions of SR.21 Incubation with 250 µmol/L AS for 15 minutes increased the activity of the fast (0.047 versus 0.64 sec−1; P<0.05) and the slow (0.069 versus 0.136 sec−1; P<0.0005; n=6) uptake phases (Figure 6B; Table), without affecting total Ca2+ uptake (Table). Ca2+ uptake activity was abolished by preincubation with 10 µmol/L thapsigargin (not shown), whereas exposure to the Ca2+ ionophore A23187 (5 µg/mg SR protein) diminished total Ca2+ uptake by ≈50% (Figure 6E). Stopped-flow signals acquired at the isosbestic wavelength of 693 nm were also biphasic (Figure 6C and 6D). The decrease in absorbance at 693 nm, representing scattered light associated with Ca2+ sequestration and osmotic vesicle swelling, was subtracted from the 650 nm signal before analysis. After subtraction, Ca2+ accumulation exhibited a monophasic time course with >90% of uptake occurring within the initial 20s (Figure 6F and 6G).
Figure 6.
HNO increases ATP-dependent Ca2+ uptake in murine sarcoplasmic reticulum (SR) vesicles. A and B, Representative stopped-flow recordings of active Ca2+ accumulation monitored by arsenazo III at 650 nm in the absence (−AS) and presence (+AS) of 250 µmol/L AS. The downward deflection of the signal represents Ca2+ uptake from the extravesicular medium. The initial absorbance reading on the y-axis was normalized to 0 by subtracting the absorbance at t=0 from each of the absorbance readings. The solid curve through the data points represents the best fit of the data to a biexponential plus residual equation. C and D, Representative changes in light-scattering measured by stopped-flow mixing at the isosbestic wavelength of 693 nm in the absence (−AS) and presence (+AS) of 250 µmol/L AS. Reaction conditions were identical to those in A and B above. E, Representative stopped-flow recordings of active Ca2+ accumulation monitored at 650 nm in the absence (control) and presence of the Ca2+ ionophore (A23187). F and G, Representative time course of active Ca2+ uptake in SR vesicles determined by subtraction of a stopped-flow trace acquired at 693 nm from a trace acquired at 650 nm (ΔAbs 650 nm−ΔAbs 693 nm). The traces were normalized as described above before subtraction. H, Effect of AS/HNO on (left) Ca2+ uptake activity (sec−1) and (right) total Ca2+ uptake evaluated from the 650 to 693 nm signal.
Effect of AS/HNO on Kinetic Parameters for Ca2+ Uptake by Cardiac SR Vesicles
| Absorbance | Without AS | With AS | P |
|---|---|---|---|
| 650 nm | |||
| k1 | 0.4717±0.0419 | 0.6402±0.0898 | <0.05 |
| k2 | 0.0696±0.078 | 0.1362±0.0120 | <0.0005 (n=6) |
| A1+A2 | 0.006395±0.0005 | 0.005696±0.0006 | NS |
| 693 nm | |||
| k1 | 0.4669±0.0167 | 0.6326±0.0418 | <0.05 (n=4) |
| k2 | 0.0398±0.0020 | 0.0753±0.0070 | 0.011 |
| A1+A2 | 0.0051±0.0004 | 0.0046±0.0008 | NS |
| 650–693 nm | |||
| k | 0.1563±0.0204 | 0.3204±0.0244 | <0.0005 (n=6) |
| A | 0.00257±0.0003 | 0.00202±0.0004 | NS |
k1 indicates rate constant for fast phase of Ca2+ uptake; k2, rate constant for slow phase of Ca2+ uptake; A1, amplitude of fast phase of Ca2+ uptake; A2, amplitude of slow phase of Ca2+ uptake; k and A, rate constant and amplitude for Ca2+ uptake for 650–693 nm signal, respectively.
AS/HNO exposure increased the rate constant for Ca2+ uptake by 104% based on exponential analysis of the 650 to 693 nm signal (0.1563 versus 0.3204 sec−1; P<0.0005; n=6) (Figure 6H, left). The difference between total Ca2+ uptake at equilibrium before and after exposure to AS/HNO was not significant (Figure 6H, right; P=NS; n=6), indicating that activation by HNO increases the catalytic efficiency of the Ca2+ pump without changing its thermodynamic efficiency. The enhanced SERCA2a function and unaltered net SR Ca2+ uptake in these vesicle experiments are consistent with the acceleration of the decay of the [Ca2+]i transient by AS in intact cardiac myocytes (Figure 4C through 4F and Figure 5).
Discussion
In the physiological setting, cardiac contractile force and rate of force decay are enhanced via cAMP/PKA-coupled mechanisms that trigger activator Ca2+ to stimulate the myofilaments and SR uptake to hasten relaxation. Yet, altered cAMP/PKA signaling can contribute to chronic remodeling and failure. Therapies mimicking these pathways have generally proven ineffective for long-term treatment of cardiac failure. Here we reveal that HNO acts very differently on the heart muscle cell, augmenting contractility and accelerating relaxation, independent of cAMP/PKA, by enhancing the Ca2+ transient by increasing both SR Ca2+ uptake and release. These 2 counterbalancing effects likely explain why diastolic Ca2+ does not rise and total SR Ca2+ load remains unchanged. Moreover, this direct effect is redox sensitive and reversible and is very different to the effects produced by NO.
Increased SR Ca2+ release with unaltered total SR Ca2+ content suggests HNO modifies RyR2 function rather than induces a leak by increasing intra-SR Ca2+ stores.22 These effects are quite different from that exerted by NO donors, β agonists, and caffeine. NO donors are reported to enhance23,24 or inhibit RyR2,25 but not alter basal Ca2+ spark frequency.26 β Agonists stimulate RyR2 open probability via PKA-mediated phosphorylation,27 and Ca2+ spark frequency can increase by this mechanism and further by phosphorylation of phospholamban, which enhances SR Ca2+ load.28 In transgenic mice overexpressing human β2 receptors, Ca2+ sparks are larger and more frequent than in nontransgenic cells, despite having resting cytosolic Ca2+ and Ca2+ SR load similar to controls.28 This suggests that β-mediated cAMP-PKA activation alters not only RyR2 sensitivity to Ca2+ but also Ca2+ release-linked RyR2 inactivation,29 potentially changing SR stability. In contrast, HNO increases spark frequency without altering individual spark characteristics or adversely impacting Ca2+ stability. The action of HNO on RyR2 is also different from that of caffeine, which increases the frequency of spontaneous Ca2+-release events (Ca2+ waves), an effect that persists even after discontinuing the drug,30 leading to a substantial decrease in SR Ca2+ content.
The unique action of HNO on RyR2 may relate to its thiophilic chemistry.3,9 HNO effects were rapidly reversed by reducing equivalents, suggesting real-time competition for HNO between free thiols and critical thiol residues on the RyR2. The data showing that a 6% increase in intracellular GSH blunts 57% of HNO effects on sarcomere shortening suggests HNO targets selective thiolate residues rather than having a generalized interaction.9 Identification of these specific targets awaits subproteome analysis of cysteine modification, with site mutagenesis, to confirm the functional importance of particular targets. Selective thiophilic action of HNO3 might suggest that it is an in vivo signaling molecule, 31,32 although this remains speculative as methods to measure in vivo synthesis are currently unavailable.
To sustain cardiac inotropy in the presence of HNO-induced increase in the fractional release of Ca2+ from RyR2, the velocity of Ca2+ reuptake into the SR should increase during relaxation.33 This latter process is slowed in the failing heart, and recent efforts to stimulate it by gene modulation (eg, manipulation of phospholamban34,35 or increased SERCA2a expression36) highlight the therapeutic attractiveness of this target. AS/HNO stimulated Ca2+ uptake in both myocytes and isolated cardiac SR, supporting direct action on SERCA2a. The mechanism remains unknown but could involve direct targeting of SERCA2a by HNO, or releasing some of the inhibition of SERCA2a by phospholamban.37
Although we did not assess whether HNO alters the phosphorylation of various EC coupling proteins (eg, RyR2, phospholamban) as a mechanism for inotropy, several lines of evidence suggests such changes are unlikely and/or separate from HNO modulation. First, both PKG and PKA blockade had no effect on HNO inotropy. Second, HNO did not alter cAMP. Third, HNO effects were rapidly reversible by adding thiol-reducing agents, which would not be observed if a primary phosphorylation mechanism was involved. Fourth, the RyR2 studies were performed in reconstituted membranes without kinases to stimulate phosphorylation, and the responses in this preparation were highly concordant with those observed by Ca2+sparks in intact cells. Lastly, HNO inotropic response in myocytes was shown to be additive to β agonists, suggesting that HNO and β-adrenergic pathways act in parallel.
Our data provide important new insights into our prior intact animal studies4,5 that first revealed HNO donors improve function in the failing heart, independent of β-adrenergic blockade, and additive to β-adrenergic agonists. Initial studies had first suggested a possible role of HNO in stimulating calcitonin gene–related peptide (CGRP) release4; however, subsequent studies confirmed this effect was sympathostimulatory, inhibited by β blockers, and not mediated by direct myocyte CGRP effects.38 The current data reveal a direct enhancement of myocyte Ca2+ cycling. However, changes in Ca2+ handling are not the sole mechanisms as other recent data from our laboratory have found AS/HNO also enhances maximal Ca2+-activated force without altering diastolic Ca2+ levels in isolated rat trabeculae. Thus, HNO also acts as a myofilament Ca2+ sensitizer at systolic Ca2+ levels (T. Dai, Y. Tian, C. G. Tocchetti, T. Katori, D. A. Kass, N. Paolocci, W. Gao, manuscript submitted for publication). This factor would appear to work in concert with increased Ca2+ cycling revealed in the current study.
Several study limitations should be noted. First, cells from healthy hearts were studied, and the observed effects of HNO may not directly translate to myocytes from failing ventricles. However, in prior in vivo studies, we observed a similar efficacy of HNO on cardiac function in normal and failing hearts.5 Second, we did not examine the coupling between L-type calcium current and RyR2 activation (coupling gain), or determine whether the L-type current itself is altered by HNO. However, enhanced SR calcium uptake and release was demonstrated in isolated SR and reconstituted RyR2, where the gain interaction would not be relevant. Regarding the latter, the lack of change in Ca2+ extruded by the NCX and in total SR Ca2+ content suggests L-type Ca2+ current was unlikely to be altered.
The present data suggest an intriguing potential for the use of HNO donors to treat depressed heart function, particularly in light of prior work confirming efficacy in intact large animals with heart failure. Although an agent that increased SR Ca2+ release might raise concerns of proarrhythmia,39 the manner by which HNO achieves this effect is novel, and thus its consequences may be as well. Importantly, the current data show increased Ca2+ fractional release counterbalanced by improved uptake so that SR Ca2+ load and diastolic Ca2+ levels are unchanged.
Future studies examining HNO responses in myocytes from failing hearts, longer-term exposure studies, and, ultimately, clinical studies will be needed to prove HNO efficacy and safety for the treatment of decompensated hearts, but the present data provide a valuable starting point for such investigations.
Supplementary Material
Acknowledgments
We thank Dr J.M. Fukuto (University of California, Los Angeles) for critically reviewing the manuscript. Matthew I. Jackson and Christopher Pavlos are gratefully acknowledged for preparing Angeli’s salt and for technical assistance in performing Angeli’s salt decomposition studies, respectively.
Sources of Funding
This work was supported by the Italian Society of Cardiology and the American Heart Association (to C.G.T.); by Telethon Italy (TCP00089 and GGP05113) and Fondazione Compagnia di San Paolo (the HFSPO RGP1/2005) (to M.Z.); NIH grants HL30077 (to D.M.B.) and HL47511, PO1HL077180, and PO1HL59408 (to D.A.K.); and NIH grant HL075265 and an American Heart Association Scientist Development Grant (to N.P.).
Footnotes
Disclosures
None.
References
- 1.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 3.Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The physiological chemistry and biological activity of nitroxyl (HNO): the neglected, misunderstood, and enigmatic nitrogen oxide. Chem Res Toxicol. 2005;18:790–801. doi: 10.1021/tx0496800. [DOI] [PubMed] [Google Scholar]
- 4.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proc Natl Acad Sci U S A. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colton CA, Gbadegesin M, Wink DA, Miranda KM, Espey MG, Vicini S. Nitroxyl anion regulation of the NMDA receptor. J Neurochem. 2001;78:1126–1134. doi: 10.1046/j.1471-4159.2001.00509.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim WK, Choi YB, Rayudu PV, Das P, Asaad W, Arnelle DR, Stamler JS, Lipton SA. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO- Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 8.Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. doi: 10.1016/j.ceca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Lopez BE, Rodriguez CE, Pribadi M, Cook NM, Shinyashiki M, Fukuto JM. Inhibition of yeast glycolysis by nitroxyl (HNO): mechanism of HNO toxicity and implications to HNO biology. Arch Biochem Biophys. 2005;442:140–148. doi: 10.1016/j.abb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Hidalgo C, Donoso P, Carrasco MA. The ryanodine receptors Ca2+ release channels: cellular redox sensors? IUBMB Life. 2005;57:315–322. doi: 10.1080/15216540500092328. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Akin BL, Stokes DL, Jones LR. Cross-linking of C-terminal residues of phospholamban to the Ca2+ pump of cardiac sarcoplasmic reticulum to probe spatial and functional interactions within the transmembrane domain. J Biol Chem. 2006;281:14163–14172. doi: 10.1074/jbc.M601338200. [DOI] [PubMed] [Google Scholar]
- 12.Bassani RA, Bers DM. Na-Ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. J Mol Cell Cardiol. 1994;26:1335–1347. doi: 10.1006/jmcc.1994.1152. [DOI] [PubMed] [Google Scholar]
- 13.Dostal DE, Rothblum KN, Conrad KM, Cooper GR, Baker KM. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. Am J Physiol. 1992;263:C851–C863. doi: 10.1152/ajpcell.1992.263.4.C851. [DOI] [PubMed] [Google Scholar]
- 14.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 15.Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 18.Froehlich JP, Lakatta EG, Beard E, Spurgeon HA, Weisfeldt ML, Gerstenblith G. Studies of sarcoplasmic reticulum function and contraction duration in young adult and aged rat myocardium. J Mol Cell Cardiol. 1978;10:427–438. doi: 10.1016/0022-2828(78)90364-4. [DOI] [PubMed] [Google Scholar]
- 19.Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac beta(2)-adrenergic signal transduction. Circ Res. 1999;85:1092–1100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- 20.Massion PB, Pelat M, Belge C, Balligand JL. Regulation of the mammalian heart function by nitric oxide. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:144–150. doi: 10.1016/j.cbpb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Antipenko A, Spielman AI, Kirchberger MA. Kinetic differences in the phospholamban-regulated calcium pump when studied in crude and purified cardiac sarcoplasmic reticulum vesicles. J Membr Biol. 1999;167:257–265. doi: 10.1007/s002329900490. [DOI] [PubMed] [Google Scholar]
- 22.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 25.Zahradnikova A, Minarovic I, Venema RC, Meszaros LG. Inactivation of the cardiac ryanodine receptor calcium release channel by nitric oxide. Cell Calcium. 1997;22:447–454. doi: 10.1016/s0143-4160(97)90072-5. [DOI] [PubMed] [Google Scholar]
- 26.Ziolo MT, Katoh H, Bers DM. Positive and negative effects of nitric oxide on Ca(2+) sparks: influence of beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2295–H2303. doi: 10.1152/ajpheart.2001.281.6.H2295. [DOI] [PubMed] [Google Scholar]
- 27.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YY, Song LS, Lakatta EG, Xiao RP, Cheng H. Constitutive beta2-adrenergic signalling enhances sarcoplasmic reticulum Ca2+ cycling to augment contraction in mouse heart. J Physiol. 1999;521(pt 2):351–361. doi: 10.1111/j.1469-7793.1999.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sham JS, Song LS, Chen Y, Deng LH, Stern MD, Lakatta EG, Cheng H. Termination of Ca2+ release by a local inactivation of ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci U S A. 1998;95:15096–15101. doi: 10.1073/pnas.95.25.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balasubramaniam R, Chawla S, Grace AA, Huang CL. Caffeine-induced arrhythmias in murine hearts parallel changes in cellular Ca(2+) homeostasis. Am J Physiol Heart Circ Physiol. 2005;289:H1584–H1593. doi: 10.1152/ajpheart.01250.2004. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt HH, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. No .NO from NO synthase. Proc Natl Acad Sci U S A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adak S, Wang Q, Stuehr DJ. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J Biol Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 33.Diaz ME, Graham HK, O’Neill SC, Trafford AW, Eisner DA. The control of sarcoplasmic reticulum Ca content in cardiac muscle. Cell Calcium. 2005;38:391–396. doi: 10.1016/j.ceca.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez P, Kranias EG. Phospholamban: a key determinant of cardiac function and dysfunction. Arch Mal Coeur Vaiss. 2005;98:1239–1243. [PubMed] [Google Scholar]
- 35.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, Wang Y, Ross J, Jr, Chien KR. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 36.del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res. 2001;88:e66–e67. doi: 10.1161/hh1101.092004. [DOI] [PubMed] [Google Scholar]
- 37.Kirchberger MA, Tada M, Katz AM. Adenosine 3′:5′-monophosphatedependent protein kinase-catalyzed phosphorylation reaction and its relationship to calcium transport in cardiac sarcoplasmic reticulum. J Biol Chem. 1974;249:6166–6173. [PubMed] [Google Scholar]
- 38.Katori T, Hoover DB, Ardell JL, Helm RH, Belardi DF, Tocchetti CG, Forfia PR, Kass DA, Paolocci N. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res. 2005;96:234–243. doi: 10.1161/01.RES.0000152969.42117.ca. [DOI] [PubMed] [Google Scholar]
- 39.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.