Abstract
A highly discriminative and objective genetic characterization of N. gonorrhoeae, which increases our knowledge of strain populations in different geographic areas, is crucial for the development of improved control measures. In the present study, conventional phenotypic characterization and genetic characterization by means of pulsed-field gel electrophoresis (PFGE), sequencing of the entire porB gene, N. gonorrhoeae multiantigen sequence typing (NG-MAST), and pyrosequencing of a quinolone resistance determining region (QRDR) of the gyrA gene of Swedish ciprofloxacin-resistant N. gonorrhoeae serovar IB-10 isolates (n=45) were performed. The genetic characterization identified one widely spread ciprofloxacin-resistant N. gonorrhoeae ST147 strain. In addition, isolates with slightly different genetic characteristics, which presumably reflect the ongoing evolution only, were also identified. All the isolates contained single nucleotide polymorphisms in QRDR of the gyrA gene that are highly correlated with ciprofloxacin resistance. Consequently, comprehensive characterization identified the first confirmed large domestic transmission, mainly among young heterosexuals, of one ciprofloxacin-resistant N. gonorrhoeae strain in Swedish society during 2002–2003. In conclusion, a precise, i.e. genetic, characterization for identification of individual strains is a very valuable support to the crucial active surveillance of the epidemiological characteristics and the antibiotic susceptibility of N. gonorrhoeae in the effective treatment of gonorrhoea.
Keywords: Neisseria gonorrhoeae, ciprofloxacin resistance, molecular epidemiology, porB gene, NG-MAST
Gonorrhoea remains a major sexually transmitted infection (STI) in many countries (1, 2). The mainstay in its prevention is based on the availability of effective diagnostics and antibiotic treatment, as well as regional, national, and international surveillance of the epidemiological characteristics and the antibiotic susceptibility of the etiological agent, i.e. Neisseria gonorrhoeae. Nowadays, a high level of decreased susceptibility or resistance to most traditional antibiotics used for treatment of gonorrhoea has been identified in most countries (SWEDRES 2004 http://www.strama.se (2–5)). In addition, the characteristics of circulating N. gonorrhoeae strains fluctuate over time and a highly discriminative and objective characterization, i.e. genetic typing, of the bacteria, is crucial. This genetic typing may identify the transmission of more virulent and/or antibiotic-resistant strains, information that is important for the development of improved control measures. Conventional phenotypic characterization of N. gonorrhoeae strains has several limitations (6– 10) and many genetic methods have been developed to overcome these shortcomings (11).
Since 1997 the incidence of gonorrhoea in Sweden has increased almost annually (Gonorrhoea/year and month statistics per county http://gis.smittskyddsinstitutet.se/mapapp/build/intro_eng.html (3, 8)). According to previous studies (8, 10, 12), the transmission of single strains in Swedish society may result in large domestic core groups of gonorrhoea cases and it is possible to identify these strains with a precise genetic characterization of the bacterial isolates. Since the mid-1990s, the prevalence of isolates resistant to ciprofloxacin, previously the drug of first choice for treatment of uncomplicated gonorrhoea, has been increasing in Sweden and in many other countries (SWEDRES 2004 http://www.strama.se (2–5, 13)). In N. gonorrhoeae, the ciprofloxacin resistance is mainly due to single nucleotide polymorphisms (SNPs) in the gyrA gene and the parC gene, which code for the target enzymes DNA gyrase and topoisomerase IV, respectively (14–19). The level of resistance appears to be correlated with the location and number of mutations in these genes. However, treatment failures with fluoroquinolones have been shown to be due to mutations primarily in the gyrA gene, and especially substitutions in a short quinolone resistance determining region (QRDR) of this gene (14, 19, 20). Consequently, it may be sufficient to analyze a short QRDR of the gyrA gene for identification of N. gonorrhoeae isolates with decreased susceptibility and resistance to ciprofloxacin (14–19,21,22).
In 2003, a presumed epidemiological core group comprising domestic cases of mainly young (<25 years of age) heterosexuals with urogenital gonorrhoea was identified in one Swedish county. All preserved N. gonorrhoeae isolates from these cases were assigned to serovar IB-10 and showed a high level of resistance to ciprofloxacin. During 2002 and 2003, many IB-10 isolates that showed a high level of resistance to ciprofloxacin were also identified in other geographic areas of Sweden.
The aim of the present study was to thoroughly characterize all ciprofloxacin-resistant N. gonorrhoeae IB-10 isolates from an epidemiologically identified presumed core group in a Swedish county in 2003 as well as from other geographic areas of Sweden during 2002 and 2003. This was done in order to investigate the possibility of wide transmission, mainly among young (<25 years of age) heterosexuals, of one ciprofloxacin-resistant N. gonorrhoeae strain in Swedish society.
Materials and Methods
Bacterial isolates and culture conditions
In the present study, clinical ciprofloxacin-resistant N. gonorrhoeae IB-10 isolates (n=45) and clinical ciprofloxacin-susceptible N. gonorrhoeae IB-10 isolates (n=2; 165/03 and 177/03) were examined (Fig. 1). The isolates included all ciprofloxacin-resistant N. gonorrhoeae IB-10 isolates cultured and preserved in Sweden during 2002 and 2003. These were initially isolated on selective culture medium and species verified according to standard procedures at many different clinical microbiology laboratories in Sweden. Subsequently, at the Swedish Reference Laboratory for Pathogenic Neisseria, Department of Clinical Microbiology, Örebro University Hospital, all isolates were species confirmed using sugar utilisation test and Phadebact GC Monoclonal Test (Boule Diagnostics AB, Huddinge, Sweden). 19 of the isolates were collected from an epidemiologically identified presumed core group of domestic gonorrhoea cases (n=19) in Gävleborg county, 170 km north of the Swedish capital city Stockholm, during late winter and early spring 2003. One N. gonorrhoeae IB-10 isolate was cultured from an additional patient presumed to belong to the suspected core group; however, in the present study this patient was excluded because the isolate was not available. The suspected core group comprised mainly young heterosexuals (6 males and 12 females; 16/18 [89%] <25 years of age) with urogenital gonorrhoea but also a neonatal boy suffering from conjunctivitis. The remaining ciprofloxacin-resistant isolates (n=26) were cultured from gonorrhoea patients (19 males and 7 females; 16/26 [62%] <25 years of age) in 10 other cities located in both the southern and northern part of Sweden during 2002 and 2003. In addition, two clinical ciprofloxacin-susceptible (165/03 and 177/03; MIC=0.004 mg/l) N. gonorrhoeae IB-10 isolates that circulated in the Swedish society in 2003 were included for comparison. All isolates were preserved at −70°C and cultured as previously described (10).
Fig. 1.
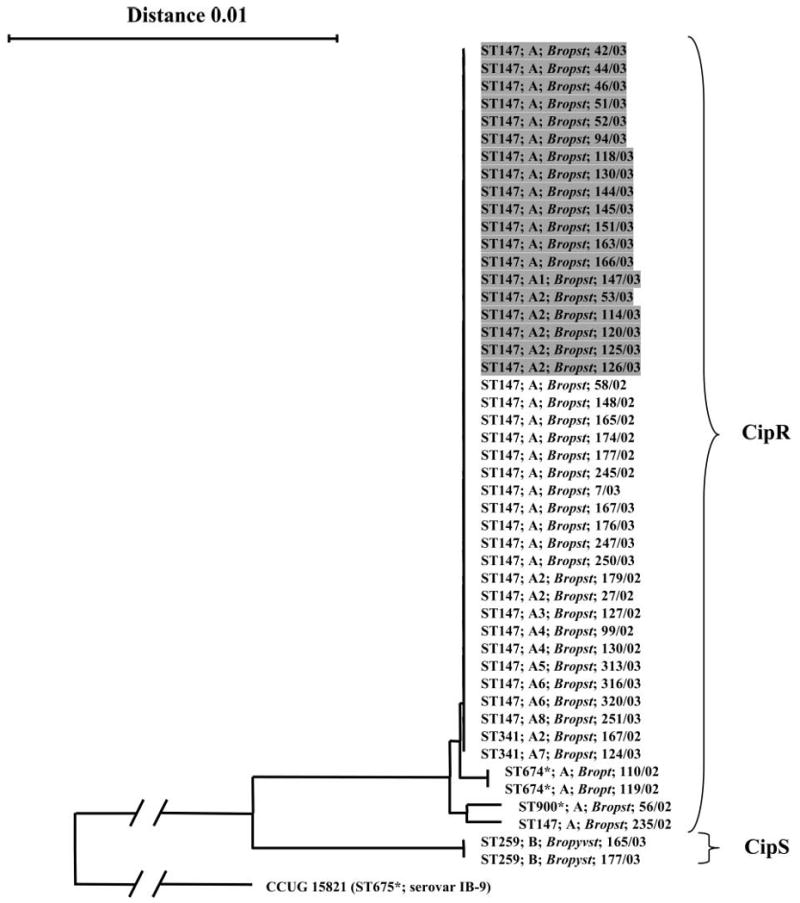
Neighbor-joining phylogenetic tree describing the evolutionary relationship of porB1b gene sequences (993 unambiguously aligned nucleotides) coding for the mature PorB (10) of clinical Neisseria gonorrhoeae serovar IB-10 isolates (n=47). The porB1b sequence of the N. gonorrhoeae IB-9 reference strain (CCUG 15821), which represents an outgroup, was used to root the tree. The length of the branches leading to the outgroup has been reduced by a factor of four. The N. gonorrhoeae multiantigen sequence typing (NG-MAST) sequence types (STs) are denoted. The pulsed-field gel electrophoresis (PFGE) types (no band difference, e.g. A) and variants (e.g. A1), i.e. less than seven bands difference in both the SpeI and the BglII fingerprints, are shown. The serovars of the isolates using Pharmacia (Ph) MAbs are indicated in italics. The designation of each isolate (laboratory number/year of isolation) and the susceptibility to ciprofloxacin (CipS, susceptible; CipR, resistant) are also shown. The isolates that originated in the domestic core group in a Swedish county in 2003 are shaded. *, indicates not previously identified ST.
Epidemiological information and demographic data
Gonorrhoea is a mandatorily notifiable disease according to the Swedish Communicable Diseases Act. In the present study, epidemiological and demographic data were collected for all gonorrhoea cases from case reports at the Department of Epidemiology, the Swedish Institute for Infectious Disease Control. In addition, for the presumed core group patient information regarding partner tracing was collected from the counsellors handling each individual case.
Phenotypic antibiotic susceptibility testing
β-lactamase production was analyzed with Nitrocefin discs (AB Biodisk, Solna, Sweden) and susceptibility to ciprofloxacin, ampicillin, cefixime, ceftriaxone, azithromycin, and spectinomycin was analyzed using the Etest method (AB Biodisk), as previously described (3).
Serological characterization
Serovar determination was performed using the co-agglutination technique (23) with the Pharmacia panel (Ph) (24) (Bactus AB, Stockholm, Sweden) and the Genetic Systems (GS) panel (25) of monoclonal antibodies (MAbs).
Pulsed-field gel electrophoresis (PFGE)
PFGE of the N. gonorrhoeae isolates, with the two restriction endonucleases SpeI and BglII used separately, was performed, documented, and analyzed as previously described (8). Isolates were considered genetically indistinguishable if no band differed in any of the fingerprints (identical PFGE type, e.g. A), closely related if 1–3 bands differed in any or both of the fingerprints (variant of the PFGE type, i.e. A1, A2, A3), possibly related if 4–6 bands differed in any or both of the fingerprints (variant of the PFGE type, i.e. A4, A5, A6, A7, A8) and different if ≥7 bands difference was documented in any of the fingerprints, e.g. PFGE type B (26) (Fig. 1).
Isolation of genomic DNA
Bacterial DNA was isolated using magnetic silica particles in a robotized system (MagNA Pure LC, Roche Molecular Biochemicals, Mannheim, Germany), as previously described (21). The DNA preparations were stored at 4 °C prior to PCR.
Real-time PCR of the entire porB gene
The entire porB gene that codes for the outer membrane porin PorB was amplified in a LightCycler System (Roche Molecular Biochemicals) using SYBR Green I fluorescence melting curve analysis for specific identification of the porB1b amplicons, with the primers PorBU and PorBL as previously described (27). The PCR products were stored at 4 °C prior to purification.
N. gonorrhoeae multiantigen sequence typing (NG-MAST)
The more variable internal segment of the porB gene and the tbpB gene that code for the β subunit of the transferring-binding protein, respectively, which are analyzed in the NG-MAST protocol (28), was PCR amplified in a LightCycler System (Roche Molecular Biochemicals) using SYBR Green I fluorescence melting curve analysis for specific identification of the amplicons. In the amplifications, the previously documented primers (28) por forward and por reverse and tbpB forward and tbpB reverse, respectively, were used. Briefly, each PCR mixture (20 μl) contained: 2 μl LightCycler – FastStart DNA Master SYBR Green I (Roche Diagnostics GmbH), 3 mM MgCl2 (Roche Diagnostics GmbH), 0.5 μM of each primer, and 2 μl of DNA template. The cycling parameters of the amplifications were as follows: an enzyme activation step at 95 °C for 10 min, followed by 30 sequential cycles of heating up to 95 °C, 58 °C (69 °C for the tbpB gene) for 10 s, and 72 °C for 30 s (24 s for the tbpB gene). The cycling parameters of the subsequent melting curve analysis were as follows: heating the PCR products up to 95 °C, cooling at 63 °C (74 °C for the tbpB gene) for 45 s, and finally slowly heating (0.1 °C/s) up to 95 °C.
A positive control (DNA of N. gonorrhoeae reference strain CCUG 15821) and a negative control (distilled water) were included in each PCR run. The PCR products were stored at 4 °C prior to purification.
gyrA PCR
The QRDR of the gyrA gene was amplified using the primers GYRA2-1 and GYRA2-2 (biotin-labeled for single-strand separation) as previously described (21). The PCR products were stored at 4 °C prior to sequencing using Pyrosequencing technology.
Conventional Sanger-dideoxy sequencing
The amplified porB1b allele was cycle sequenced using the primers PorBU, PorBL, PorB1bU, and PorB1bL (10) and the more variable segments of the porB gene and the tbpB gene, analyzed in the NG-MAST protocol (28), were cycle sequenced with the same primers as used in the PCR as previously described (29). The purification of the PCR products and the sequence extension products as well as the sequence determination with an ABI PRISM™ 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) were performed as previously documented (29). The sequence of each strand of each compiled sequence was determined.
gyrA sequencing by Pyrosequencing technology
The Pyrosequencing method is a real-time DNA sequencing-by-synthesis technology (21, 27, 30). As previously described (21), single-strand DNA sample preparation prior to sequencing was performed semi-automatically on a Vacuum Prep Workstation (Biotage, Uppsala, Sweden) using streptavidin sepharose beads (Amersham Biosciences, Uppsala, Sweden). The single-strand prepared PCR products from the isolates were sequenced, with the primer GYRA2–3, using the PSQ 96 SNP reagent kit (Biotage) and an automated plate-based bench-top PSQ™ HS96A System (Biotage), as previously documented (21). Result analysis was performed automatically with SNP PSQ™ HS96A 1.2 software and by visual inspection. The wild-type gyrA gene in the ciprofloxacin-susceptible N. gonorrhoeae reference strain FA 1090 (ATCC 700825) was used for evaluation of the results.
Sequence analysis and phylogenetic inference
Multiple-sequence alignments of the entire porB gene segments encoding the mature PorB (10), the more variable regions of the porB gene and the tbpB gene analyzed in the NG-MAST protocol (28), and the sequences of the QRDR in the gyrA gene were performed with BioEdit (version 5.0.9) software and by manual adjustment. Construction of the phylogenetic tree of the entire porB gene sequences was performed with TREECON (version 1.3b) software (31), as previously described (9). The porB gene sequence of an N. gonorrhoeae serovar IB-9 reference strain (CCUG 15821) was used as an outgroup to root the tree.
The more variable segments of the porB gene (490 bp) and the tbpB gene (390 bp), analyzed in the NG-MAST protocol (28), in all isolates were compared to identify polymorphisms. By using the NG-MAST website (available at http://www.ng-mast.net), each isolate was assigned to a sequence type (ST), which is defined by the allelic profile present at the two different loci.
Evaluation of data
All data regarding patients and isolate characteristics were compiled and evaluated in an Excel database.
Results
Characterization of ciprofloxacin-resistant N. gonorrhoeae IB-10 isolates from gonorrhoea cases from a suspected domestic core group in a Swedish county in 2003 (n=19)
The IB-10 isolates (n=19) were all assigned to serovar Bropst with Ph MAbs (Fig. 1). In addition, all isolates were β-lactamase negative, highly resistant to ciprofloxacin (MIC≥4 mg/l), showed decreased susceptibility to ampicillin (MIC≤0.125 mg/l), and full susceptibility to cefixime (MIC≤0.064 mg/l), ceftriaxone (MIC≤0.064 mg/l), azithromycin (MIC≤0.5 mg/l), and spectinomycin (MIC≤32 mg/l). Furthermore, all isolates showed similar MIC values, with differences within ±1 log2.
According to the genetic characterization, all the isolates comprised identical porB gene sequences and were assigned to ST147 in the NG-MAST (Fig. 1). The 19 isolates comprised two distinguishable PFGE fingerprints with use of BglII and three with SpeI. However, 18 (95%) of the isolates showed indistinguishable fingerprints with use of BglII and 13 (68%) with SpeI. In addition, all fingerprints differed by a maximum of two bands. Consequently, 13 (68%) of the isolates were indistinguishable (PFGE-type A) and the remaining six isolates were closely related, i.e. interpreted as two variants of the same PFGE-type (A1 and A2), when the results of both enzymes were combined (Fig. 1).
Characterization of ciprofloxacin-resistant N. gonorrhoeae IB-10 isolates from other geographic areas of Sweden in 2002-2003 (n=26)
24 (92%) of the IB-10 isolates (n=26) were assigned to Ph serovar Bropst and two were assigned to Ph serovar Bropt, i.e. differed by the reactivity of one MAb only (Fig. 1). Nine (35%) of the isolates were β-lactamase negative, highly resistant to ciprofloxacin (MIC≥1.5 mg/l), showed decreased susceptibility to ampicillin, and full susceptibility to cefixime, ceftriaxone, azithromycin, and spectinomycin. The remaining 17 isolates (65%) produced β-lactamase. However, except for this difference, all of the isolates showed MICs similar to those of the β-lactamase-negative core group isolates to the other antibiotics.
According to the genetic characterization, the isolates (n=26) possessed four slightly different porB gene sequences (differed by one to two nucleotides) and were assigned to four different STs (ST147, ST341, ST674, and ST900; Fig. 1). However, 22 (85%) of the isolates revealed a porB gene sequence identical to that of the core group isolates mentioned above (Fig. 1). In addition, 21 (81%) of the isolates were assigned to ST147, i.e. the same ST as the core group isolates, and three of the remaining five isolates only differed by a maximum of one nucleotide in the porB segment and comprised identical sequences in the tbpB segment, i.e. had closely related STs (ST674 and ST900; Fig. 1). However, the last two isolates, i.e. 167/02 and 124/03, that were assigned to ST341 had an identical porB gene sequence but the tbpB segment differed by 77 nucleotides (Fig. 1). The 26 isolates possessed six distinguishable PFGE patterns with use of BglII and three with SpeI, but 18 (69%) of the isolates were indistinguishable from any of the core group isolates. In addition, the fingerprints of all the 26 isolates differed by less than 7 bands (both enzymes used) from the fingerprints of the core group isolates, i.e. were possibly related. Consequently, these fingerprints were interpreted as PFGE variants of the same type (Fig. 1).
The two Swedish ciprofloxacin-susceptible IB-10 isolates from 2003 (165/03 and 177/03; Fig. 1), which were included for comparison, had similar antibiotic susceptibility patterns, with the exception of ciprofloxacin, to those of the ciprofloxacin-resistant IB-10 isolates. However, these isolates differed substantially in the entire porB gene, were assigned to the highly divergent ST259, and the PFGE fingerprints differed significantly from those of the core group isolates, i.e. by more than seven bands with both enzymes (Fig. 1).
Pyrosequencing of QRDR of the gyrA gene in N. gonorrhoeae IB-10 isolates (n=47)
All the phenotypically determined ciprofloxacin-resistant N. gonorrhoeae IB-10 isolates (n=45) contained two nonsynonymous SNPs in the examined QRDR of the gyrA gene, i.e. in the Ser91 and the Asp95 codons of the wild-type gyrA gene sequence (Table 1). However, two different polymorphic variants of the Asp95 codon were identified. Consequently, 43 of the isolates contained Gly95 while the two remaining isolates contained Ala95 (Table 1). The two ciprofloxacin-susceptible IB-10 isolates, included for comparison, comprised the wild-type QRDR of the gyrA gene (Table 1).
TABLE 1. DNA sequences encoding amino acid position 90–96 in the quinolone resistance determining region (QRDR) of the gyrA gene in N. gonorrhoeae IB-10 isolates (n=47).
| Amino acid position in GyrA | |||||||
|---|---|---|---|---|---|---|---|
| 90 | 91 | 92 | 93 | 94 | 95 | 96 | |
| Ciprofloxacin-susceptible isolates (n=2)a | GAT | TCC | GCA | GTT | TAC | GAC | ACC |
| Ciprofloxacin-resistant isolates (n=45)b | (Asp) | (Ser) | (Ala) | (Val) | (Tyr) | (Asp) | (Thr) |
| Variant 1 (n=43) | GAT | TTC | GCA | GTT | TAC | GGC | ACC |
| (Asp) | (Phe) | (Ala) | (Val) | (Tyr) | (Gly) | (Thr) | |
| Variant 2 (n=2)c | GAT | TTC | GCA | GTT | TAC | GCC | ACC |
| (Asp) | (Phe) | (Ala) | (Val) | (Tyr) | (Ala) | (Thr) | |
The ciprofloxacin-susceptible isolates had MICs of 0.004 and comprised a wild-type gyrA gene segment, i.e. identical to the one in the ciprofloxacin-susceptible N. gonorrhoeae reference strain FA 1090 (ATCC 700825).
The ciprofloxacin-resistant isolates showed MICs of 1.5–16. All of these isolates comprised nonsynonymous nucleotide substitutions that are highly correlated with ciprofloxacin resistance in N. gonorrhoeae, i.e. in amino acid codon 91 and 95. These substitutions are indicated by underlined, boldface capital letters.
Variant 2 was identified in one of the isolates originating in the Swedish core group (163/03; see Fig. 1) and in one isolate from 2002 (179/02; see Fig. 1).
The encoded amino acids are shown in parentheses.
Discussion
In the present study, thorough genetic characterization together with phenotypic and epidemiological information identified a transmission of one ciprofloxacin-resistant N. gonorrhoeae serovar IB-10 ST147 strain in Swedish society during 2002 and 2003. This was the first confirmed large domestic spread of one ciprofloxacin-resistant N. gonorrhoeae strain in Sweden. In 2003, transmission of the strain caused the gonorrhoea cases in a domestic core group comprising mainly young (<25 years of age) heterosexuals in one Swedish county. The majority of these patients showed epidemiological connections with each other according to the partner notification investigations. The strain was also identified in several other Swedish counties during 2002 and 2003, in a total of 10 different cities located in both the southern and northern part of Sweden. Minor phenotypic and genetic diversities of some isolates of the strain were identified. For instance, some isolates seemed to have acquired a β-lactamase plasmid and detailed genetic analysis revealed some minor polymorphisms in the porB1b gene, the tbpB gene, the QRDR of the gyrA gene, and the PFGE fingerprints. This limited variation probably reflects ongoing evolution of the same strain only, which also the phylogenetic analysis of the porB1b gene sequences suggested. Most interesting, many isolates of ciprofloxacin-resistant N. gonorrhoeae ST147 were also identified in 2000–2004 in London, England (32, 33) and in 2002 in Scotland (34), which may indicate import of this strain to Sweden. In London, England, the transmission of the ciprofloxacin-resistant ST147 isolates was predominantly by homosexual men (32), whereas in Scotland mainly heterosexual transmission occurred (34). However, none of the Swedish patients reported a sexual contact in England or Scotland.
Overall, the results of the sequencing of the entire porB gene, NG-MAST, and PFGE showed congruence but not complete identity, which is in concordance with previous studies (10, 35–37). All these genetic methods exhibited a high discriminatory ability and excellent typeability, which also agree with previous studies (8–10, 28, 34–38). However, when characterizing N. gonorrhoeae for epidemiological purposes, a sequence-based genetic typing seems to be the best single choice of method. This is due to the fact that sequence data are also objective, highly reproducible, and portable for comparison between laboratories. In comparison with porB gene sequencing, NG-MAST has the advantage of analyzing segments of two different genes. Consequently, NG-MAST can identify that highly divergent sequences in one of the genetic loci may be due to a single genetic event, i.e. by genetic exchange and/or recombination, and, if there are identical sequences in the other locus, isolates may in fact still be closely related. Gel-based genetic typing such as PFGE and opa typing (39) is laborious, time-consuming, subjectively interpreted, and requires pronounced standardization, especially for interlaboratory comparisons. However, PFGE and opa typing may reveal a somewhat higher discrimination between isolates compared to sequencing of the entire porB gene and NG-MAST (present study, 28, 36). Consequently, in some situations, especially involving short-term epidemiology, these methods may provide a useful complement to sequence-based approaches for characterization. In the present study, the slightly divergent PFGE fingerprints of some of the isolates probably reflect that point mutations or recombination have occurred causing a distinct evolutionary history in the porB1b gene and/or the tbpB gene in comparison to other parts of the genome. No restriction sites for the PFGE enzymes SpeI and BglII existed in the porB1b genes or the tbpB genes of the examined isolates. Thus, the results of the present study as well as numerous previous studies (8–10, 12, 28, 34–38) emphasize that a highly discriminative, reproducible, and objective characterization of N. gonorrhoeae is crucial for understanding gonorrhoea epidemiology. A precise method, i.e. genetic, for short-term epidemiological characterization of N. gonorrhoeae is a necessary tool for conducting efficient contact tracing, identification of core groups or clusters of gonorrhoea cases, performance of precise epidemiological surveillance, and identification of the spread of, for example, more virulent and/or antibiotic-resistant strains. This information is important for development of improved control measures.
In the present study, the QRDR of the gyrA gene in all isolates was successfully sequenced using Pyrosequencing technology. All phenotypically determined ciprofloxacin-resistant isolates comprised SNPs that are highly correlated with ciprofloxacin resistance, i.e. in the Ser91 and the Asp95 codons of the wild-type gyrA gene sequence (14–19, 21, 22). This method basedon Pyrosequencing technology is rapid, high-throughput, sensitive, accurate, and cost-effective for identification of ciprofloxacin resistance in N. gonorrhoeae. However, a few N. gonorrhoeae isolates with decreased susceptibility or resistance to ciprofloxacin that lack substitutions in the QRDR of the gyrA gene as well as in the parC gene have previously been described (40–42). These very rare isolates result in discrepancies between phenotypic and genetic ciprofloxacin susceptibility testing and may be due to polymorphisms in other regions of the gyrA gene or parC gene, or polymorphisms in other genes such as the porB gene (20, 40, 43–45). Whilst culture remains the gold standard for diagnosis of N. gonorrhoeae, an increasing number of laboratories are using molecular methods for diagnosis. Consequently, effective molecular methods for determination of the antibiotic susceptibility of N. gonorrhoeae and for precise epidemiological characterization of N. gonorrhoeae from clinical samples need to be developed.
With the exception of ciprofloxacin, the disseminated strain identified in the present study did not show any resistance to antibiotics currently used for treatment of gonorrhoea. In Sweden, mainly ceftriaxone, cefotaxime, spectinomycin, cefixime, azithromycin—or in some cases still ciprofloxacin—are used for treatment of gonorrhoea. Previously, ciprofloxacin was the drug of first choice, but resistance to this antibiotic has rapidly increased (SWEDRES 2004 http://www.strama.se (3, 12)). In addition, domestic transmission of azithromycin-resistant N. gonorrhoeae strains has recently been described (12). Consequently, at present ceftriaxone, spectinomycin, or cefixime can be recommended as the drug of first choice for treatment of gonorrhoea in Sweden in situations when the result of antibiotic susceptibility testing is still pending.
In conclusion, a thorough genetic characterization together with phenotypic and epidemiological information identified the first confirmed large domestic transmissionofone ciprofloxacin-resistant N. gonorrhoeae strain in Sweden during 2002 and 2003. Whilst minor phenotypic and genetic diversities of some isolates of the strain were identified, this presumably only reflects the ongoing evolution. The present study emphasizes that a precise, i.e. genetic, characterization of N. gonorrhoeae for identification of individual strains is a very valuable support to the crucial active surveillance of the epidemiological characteristics and the antibiotic susceptibility of the bacteria.
Acknowledgments
We are grateful to Angela Steen and Betina Colucci for their technical assistance with the serological characterization, epidemiologist Torsten Berglund, and counsellor Elisabet Eriksson for the epidemiological information. The present study was supported by grants from the Research Committee of Örebro County, the Örebro University Hospital Research Foundation and the National Institute for Public Health, Sweden.
References
- 1.Fenton KA, Lowndes CM, European Surveillance of Sexually Transmitted Infections (ESSTI) Network Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80:255–63. doi: 10.1136/sti.2004.009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapsall J. Antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization (WHO) report 2001. WHO/CDS/CSR/DSR/2001.3. [Google Scholar]
- 3.Berglund T, Unemo M, Olcén P, Giesecke J, Fredlund H. One year of Neisseria gonorrhoeae isolates in Sweden: the prevalence study of antibiotic susceptibility shows relation to the geographic area of exposure. Int J STD AIDS. 2002;13:109–14. doi: 10.1258/0956462021924730. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men – United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:335–8. [PubMed] [Google Scholar]
- 5.Martin IM, Hoffmann S, Ison CA. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J Antimicrob Chemother. 2006;58:587–93. doi: 10.1093/jac/dkl265. [DOI] [PubMed] [Google Scholar]
- 6.Ison CA, Whitaker L, Renton A. Concordance of auxotype/serovar classes of Neisseria gonorrhoeae between sexual contacts. Epidemiol Infect. 1992;109:265–71. doi: 10.1017/s0950268800050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng LK, Carballo M, Dillon JAR. Differentiation of Neisseria gonorrhoeae isolates requiring proline, citrulline, and uracil by plasmid content, serotyping, and pulsed-field gel electrophoresis. J Clin Microbiol. 1995;33:1039–41. doi: 10.1128/jcm.33.4.1039-1041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unemo M, Berglund T, Olcén P, Fredlund H. Pulsed-field gel electrophoresis as an epidemiologic tool for Neisseria gonorrhoeae; identification of clusters within serovars. Sex Transm Dis. 2002;29:25–31. doi: 10.1097/00007435-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Unemo M, Olcén P, Albert J, Fredlund H. Comparison of serologic and genetic porB-based typing of Neisseria gonorrhoeae: Consequences for future characterization. J Clin Microbiol. 2003;41:4141–7. doi: 10.1128/JCM.41.9.4141-4147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unemo M, Olcén P, Berglund T, Albert J, Fredlund H. Molecular epidemiology of Neisseria gonorrhoeae: Sequence analysis of the porB gene confirms presence of two circulating strains. J Clin Microbiol. 2002;40:3741–9. doi: 10.1128/JCM.40.10.3741-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredlund H, Falk L, Jurstrand M, Unemo M. Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions. APMIS. 2004;112:771–84. doi: 10.1111/j.1600-0463.2004.apm11211-1205.x. [DOI] [PubMed] [Google Scholar]
- 12.Lundbäck D, Fredlund H, Berglund T, Wretlind B, Unemo M. Molecular epidemiology of Neisseria gonorrhoeae – identification of the first presumed Swedish transmission chain of an azithromycin resistant strain. APMIS. 2006;114:67–71. doi: 10.1111/j.1600-0463.2006.apm_332.x. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous. WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the World Health Organization Western Pacific Region, 2003. Commun Dis Intell. 2005;29:62–4. [Google Scholar]
- 14.Deguchi T, Saito I, Tanaka M, Sato K, Deguchi K, Yasuda M, et al. Fluoroquinolone treatment failure in gonorrhea. Emergence of a Neisseria gonorrhoeae strain with enhanced resistance to fluoroquinolones. Sex Transm Dis. 1997;24:247–50. doi: 10.1097/00007435-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Deguchi T, Yasuda M, Asano M, Tada K, Iwata H, Komeda H, et al. DNA gyrase mutations in quinolone-resistant clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1995;39:561–3. doi: 10.1128/aac.39.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deguchi T, Yasuda M, Nakano M, Ozeki S, Ezaki T, Saito I, et al. Quinolone-resistant Neisseria gonorrhoeae: correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob Agents Chemother. 1996;40:1020–3. doi: 10.1128/aac.40.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles JA, Falconio J, Yuenger JD, Zenilman JM, Dan M, Bash MC. Quinolone resistance-determining region mutations and por type of Neisseria gonorrhoeae isolates: resistance surveillance and typing by molecular methodologies. J Infect Dis. 2004;189:2085–93. doi: 10.1086/386312. [DOI] [PubMed] [Google Scholar]
- 18.Lindbäck E, Rahman M, Jalal S, Wretlind B. Mutations in gyrA, gyrB, parC, and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS. 2002;110:651–7. doi: 10.1034/j.1600-0463.2002.1100909.x. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Sagiyama K, Haraoka M, Saika T, Kobayashi I, Naito S. Genotypic evolution in a quinolone-resistant Neisseria gonorrhoeae isolate from a patient with clinical failure of levofloxacin treatment. Urol Int. 1999;62:64–8. doi: 10.1159/000030344. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Nakayama H, Haraoka M, Nagafuji T, Saika T, Kobayashi I. Analysis of quinolone resistance mechanisms in a sparfloxacin-resistant clinical isolate of Neisseria gonorrhoeae. Sex Transm Dis. 1998;25:489–93. doi: 10.1097/00007435-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Gharizadeh B, Akhras M, Unemo M, Wretlind B, Nyrén P, Pourmand N. Detection of gyrA mutations associated with ciprofloxacin resistance in Neisseria gonorrhoeae by rapid and reliable pre-programmed short DNA sequencing. Int J Antimicrob Agents. 2005;26:486–90. doi: 10.1016/j.ijantimicag.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindbäck E, Gharizadeh B, Ataker F, Airell Å, Jalal S, Nyrén P, Wretlind B. DNA gyrase gene in Neisseria gonorrhoeae as indicator for resistance to ciprofloxacin and species verification. Int J STD AIDS. 2005;16:142–7. doi: 10.1258/0956462053057675. [DOI] [PubMed] [Google Scholar]
- 23.Sandström E, Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Path Microbiol Scand [B] 1980;88:27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandström E, Lindell P, Härfast F, Blomberg A, Ryden AC, Bygdeman S. Evaluation of a new set of Neisseria gonorrhoeae serogroup W-specific monoclonal antibodies for serovar determination. In: Schoolnik GK, editor. The pathogenic Neisseria Am Soc Microbiology, Washington. 1985. pp. 26–30. [Google Scholar]
- 25.Knapp JS, Tam MR, Nowinski RC, Holmes KK, Sandström EG. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984;150:44–8. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Tenover FC, Arbeit RD, Goering RV. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: review for healthcare epidemiologists. Infect Control Hosp Epidemiol. 1997;18:426–39. doi: 10.1086/647644. [DOI] [PubMed] [Google Scholar]
- 27.Unemo M, Olcén P, Jonasson J, Fredlund H. Molecular typing of Neisseria gonorrhoeae by pyrosequencing of highly polymorphic segments of the porB gene. J Clin Microbiol. 2004;42:2926–34. doi: 10.1128/JCM.42.7.2926-2934.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004;189:1497–505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]
- 29.Unemo M, Norlén O, Fredlund H. The porA pseudogene of Neisseria gonorrhoeae – low level of genetic polymorphism and a few, mainly identical, inactivating mutations. APMIS. 2005;113:410–9. doi: 10.1111/j.1600-0463.2005.apm_206.x. [DOI] [PubMed] [Google Scholar]
- 30.Ronaghi M, Uhlén M, Nyrén P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:651–5. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 31.van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–70. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 32.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. Changing epidemiologic profile of quinolone-resistant Neisseria gonorrhoeae in London. J Infect Dis. 2005;192:1191–5. doi: 10.1086/444429. [DOI] [PubMed] [Google Scholar]
- 33.Choudhury B, Risley CL, Ghani AC, Bishop CJ, Ward H, Fenton KA, et al. Identification of individuals with gonorrhoea within sexual networks: a population-based study. Lancet. 2006;368:139–46. doi: 10.1016/S0140-6736(06)69003-X. [DOI] [PubMed] [Google Scholar]
- 34.Palmer HM, Young H, Martin IM, Ison CA, Spratt BG. The epidemiology of ciprofloxacin resistant isolates of Neisseria gonorrhoeae in Scotland 2002: a comparison of phenotypic and genotypic analysis. Sex Transm Infect. 2005;81:403–7. doi: 10.1136/sti.2004.013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke SJ, de la Paz H, Poh CL, Ison CA, Heckels JE. Variation within serovars of Neisseria gonorrhoeae detected by structural analysis of outer-membrane protein PIB and by pulsed-field gel electrophoresis. Microbiology. 1997;143:1415–22. doi: 10.1099/00221287-143-4-1415. [DOI] [PubMed] [Google Scholar]
- 36.Fjeldsøe-Nielsen H, Unemo M, Fredlund H, Hjorth SV, Berthelsen LM, Palmer HM, et al. Phenotypic and genotypic characterization of prolyliminopeptidase-negative Neisseria gonorrhoeae isolates in Denmark. Eur J Clin Microbiol Infect Dis. 2005;24:280–3. doi: 10.1007/s10096-005-1319-5. [DOI] [PubMed] [Google Scholar]
- 37.Poh CL, Lau QC, Chow VTK. Differentiation of Neisseria gonorrhoeae IB-3 and IB-7 serovars by direct sequencing of protein IB gene and pulsed-field gel electrophoresis. J Med Microbiol. 1995;43:201–7. doi: 10.1099/00222615-43-3-201. [DOI] [PubMed] [Google Scholar]
- 38.van Looveren M, Ison CA, Ieven M, Vandamme P, Martin IM, Vermeulen K, et al. Evaluation of the discriminatory power of typing methods for Neisseria gonorrhoeae. J Clin Microbiol. 1999;37:2183–8. doi: 10.1128/jcm.37.7.2183-2188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Rourke M, Ison CA, Renton AM, Spratt BG. Opa-typing: a high-resolution tool for studying the epidemiology of gonorrhoea. Mol Microbiol. 1995;17:865–75. doi: 10.1111/j.1365-2958.1995.mmi_17050865.x. [DOI] [PubMed] [Google Scholar]
- 40.Dewi BE, Akira S, Hayashi H, Ba-Thein W. High occurrence of simultaneous mutations in target enzymes and MtrRCDE efflux system in quinolone-resistant Neisseria gonorrhoeae. Sex Transm Dis. 2004;31:353–9. doi: 10.1097/00007435-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Trees DL, Sandul AL, Neal SW, Higa H, Knapp J. Molecular epidemiology of Neisseria gonorrhoeae exhibiting decreased susceptibility and resistance to ciprofloxacin in Hawaii, 1991–1999. Sex Transm Dis. 2001;28:309–14. doi: 10.1097/00007435-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Vereshchagin VA, Ilina EN, Malakhova MV, Zubkov MM, Sidorenko SV, Kubanova AA, Govorun VM. Fluoroquinolone-resistant Neisseria gonorrhoeae isolates from Russia: molecular mechanisms implicated. J Antimicrob Chemother. 2004;53:653–6. doi: 10.1093/jac/dkh145. [DOI] [PubMed] [Google Scholar]
- 43.Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. Gonococcal resistance to β-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother. 1998;42:2799–803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka M, Nakayama H, Haraoka M, Saika T. Antimicrobial resistance of Neisseria gonorrhoeae and high prevalence of ciprofloxacin-resistant isolates in Japan, 1993 to 1998. J Clin Microbiol. 2000;38:521–5. doi: 10.1128/jcm.38.2.521-525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindbäck E, Islam S, Unemo M, Lang C, Wretlind B. Transformation of ciprofloxacin-resistant Neisseria gonorrhoeae gyrA, parE and porB1b genes. Int J Antimicrob Agents. 2006;28:206–11. doi: 10.1016/j.ijantimicag.2006.04.003. [DOI] [PubMed] [Google Scholar]


