Abstract
A large proportion of perinatally HIV-infected (PHIV) children are becoming adolescents and exploring their sexuality. This study explored the prevalence of sexual behaviors (kissing, touching, engaging in oral sex, or having vaginal/anal intercourse) in a sample of predominantly ethnic minority youths (N = 339; 54.1% Black and 30.4% Latino; 51% female; ages 9–16) perinatally exposed to HIV (61% HIV+). Using logistic regression, we tested the association between sexual behavior and HIV status, demographic characteristics, and peer influences regarding sexual behavior. PHIV youth were less likely to be sexually active. Among sexually active youth, PHIV youth were more likely to engage in touching behavior than HIV-negative youth and were less likely to engage in penetrative sex. Youths reporting that a greater number of their peers believed that sexually active boys were “cool” or “popular” were more likely to report sexual behavior. The association between sexual behavior and peers believing sexually active girls were “cool” or “popular” varied by age, gender, and HIV status. Furthermore, friends’ sexual activity was associated with sexual intercourse. Prevention programs should strengthen messages addressing peer norms regarding sexuality, as well as address specific issues related to adolescent HIV.
Keywords: Perinatal HIV-infection, Seroreverters, Adolescents, Sexual Behavior, Peer Norms
With the advent of antiretroviral treatment (ART), many HIV-infected children are reaching adolescence and young adulthood (Gortmaker, Hughes, Cervia, Brady, Johnson, Seage et al., 2001). For example, in New York City, where this study takes place, over 67% of perinatally, HIV-infected (PHIV) children are 13 years and older (New York City Department of Health and Mental Hygiene [NYCDOHM], 2007). Sexual exploration typically begins during adolescence and most often includes sexual activities like kissing, touching, and sexual intercourse (Miller, Christopherson, & King, 1993). Adolescent sexual activity in the United States commonly begins because of a series of biological, psychological, and social transitions (Pedlow & Carey, 2004). These transitions include the onset of puberty, the exploration of intimate romantic relationships, and participation with peers in settings unsupervised by adults. In uninfected populations, adolescence has been considered a period of heightened risk for transmission of sexually transmitted diseases (STDs), including HIV/AIDS, as adolescents who initiate sex early have been shown to have multiple partners and to engage in inconsistent condom use (Alan Guttmacher Institute, 2006).
In the general population, health education programs have aimed to decrease adolescents’ sexual risk-taking through delaying sexual initiation or teaching successful condom use; yet, the efficacy of these efforts has been inconsistent or insufficient (DiClemente, Salazar, & Crosby, 2007; Malow, Kershaw, Sipsma, Rosenberg, & Dévieux, 2007), as evidenced by the approximately 40,000 new HIV infections, mostly acquired via sexual behavior prior to age 25 (UNAIDS, 2008). Furthermore, there is a growing racial/ethnic disparity in HIV/AIDS incidence. Estimates from the Center for Disease Control and Prevention (CDC, 2008) indicate that, compared to White youth, African Americans (9:1 incidence ratio) and Latinos (4:1 incidence ratio) are more likely to account for most new HIV/AIDS infections, respectively.
Mirroring this racial/ethnic disparity, PHIV youths are largely represented by ethnic minorities residing in poor urban neighborhoods, and may be particularly vulnerable to health risk behaviors including earlier onset and higher rates of sexual risk behavior, substance abuse, and non-adherence to ART due to environmental and familial risk factors compounded by the biopsychosocial sequelae of HIV (Battles & Weiner, 2002). For PHIV youth, sexual risk behaviors may not only compromise their well-being, but also present a public health concern for increased HIV transmission, including transmission of ART resistant strains, to partners and offspring (CDC, 2003). To date, we know surprisingly little about the sexual development and sexual risk behavior of PHIV youth; this is partly because they are just now reaching adolescence in large enough numbers to be studied (NYCDOHM, 2007). Consequently, it is vital to study PHIV youths’ sexual risk-taking behaviors to inform HIV prevention programs for this adolescent population.
Sexual risk behaviors among HIV+ youths
There is a great deal of literature describing the sexual risk behaviors of HIV+ youths who were behaviorally infected, with these youths engaging in a higher prevalence of sexual risk behaviors than the general population (Kadivar, Garvie, Sinnock, Heston, & Flynn, 2006; Koenig, Espinoza, Hodge, & Ruffo, 2007; Lightfoot, Swendeman, Rotheram-Borus, Comulada, & Weiss, 2005; Murphy, Durako, Moscicki, Vermund, Ma, Schwartz et al, 2001; Naar-King, Wright, Parsons, Frey, Templin, & Ondersma, 2006). Extrapolating data from samples of adolescents with behaviorally acquired HIV, however, may be inadequate or insufficient to tailor harm reduction strategies for PHIV youth. PHIV youth have grown up with a highly stigmatized and transmittable illness. Many of these youths have been shown to be delayed developmentally or have cognitive deficits, and may have experienced multiple hospitalizations, losses and other stressors that may complicate decisions related to HIV disclosure and affect friendships, sexuality, and further the risk of HIV transmission (Havens & Mellins, in press). Taken together, these distinctive developmental factors may influence PHIV youths’ sexual risk-taking behaviors and underscore the need to explore how to maximize opportunities for HIV prevention among PHIV.
Clinical reports suggest that PHIV youth may not engage in high-risk sexual activity as often as HIV-negative youth because of fear of disclosing their HIV status to a partner or as a harm reduction strategy to avoid infecting their partners (Kang, Mellins, Ng, Robinson, & Abrams, 2008). Conversely, perinatally HIV-infected youths may also rush into sexual risk behavior at an early age thinking they might not have a full life span (Kang et al., 2008). Ezeanolue and colleagues (2006) are one of the few groups to study the sexual behaviors of a cohort of N=77 perinatally HIV-infected adolescents and young adults (13–24 years). Results from their cross sectional survey revealed that 79% of participants reported ever having a boyfriend or girlfriend and 33% had ever had vaginal intercourse. Among those who were sexually active, 26% reported their first sexual encounter before 15 years of age. In addition, five out of the ten girls who reported being sexually active had been pregnant. There are several additional studies reporting pregnancy rates among PHIV youth. For example, within a cohort of 638 girls (13 years and older) from 75 pediatric infectious disease clinics in 24 states in the US, 17% of girls had experienced a first pregnancy by 19 years of age (Brogly et al., 2007). These studies, however, provide limited data to inform interventions for this unique cohort as actual rates of sexual behaviors and their determinants are not described in detail. Understanding the risk and protective factors associated with sexual behaviors have been critical to informing interventions for other vulnerable populations.
In this study, we expand on the aforementioned studies of PHIV youths by identifying the prevalence of sexual behaviors, comparing them to those of a group of perinatally-exposed HIV-negative youth (seroreverters), and exploring the role of peer norms in youths’ sexual decision-making across early and middle adolescence. Seroreverters are an adequate comparison group for perinatally HIV-infected youths (Mellins, Smith, O’Driscoll, Magder, Brouwers, Chase et al., 2003). Most sociodemographic and family characteristics, including perinatal exposure to maternal HIV disease, are similar (Agostini, Riva, Esposito Ferraris, Principi & Zuccotti, 2000; Knight, Mellins, Levenson, Arpadi & Kairam, 2000). Although there may be some maternal pregnancy characteristics that are different between these two groups, seroreverters provide an opportunity to examine the contribution of HIV infection to youths’ sexual risk correlates.
The role of peer norms in adolescent sexual risk behaviors
Adolescents are embedded within social contexts (e.g., school) where peer relationships are prominent, highlighting the importance of being involved and participating in a peer group (Bronfenbrenner, 1979). Youths become involved with groups whose values and norms are perceived as attractive or similar and, in turn, enact these norms into their behaviors (Crosnoe & McNeely, 2008). Accordingly, peers have been identified as an important influence on the sexual behavior of adolescents in a wide range of populations (Buhi & Goodson, 2007; Pedlow & Carey, 2004). The salience of these peer relationships on youths’ behaviors, however, changes across adolescence and assumes greater importance as the youth begin to individuate and achieve independence from caregivers (Hartup, 1993). Perceptions of peer sexual activity and condom use among older youth, for example, may be more prominent in sexual decision-making than among younger youth (Romer, Black, Izabel, Feigelman, Kaljee, Galbraith et al., 1994). These patterns reflect youths’ transition from activity-based to peer-based friendships, particularly across early adolescence into mid-adolescence. As these friendships mature during adolescence, youths’ need to fit in with the values and behaviors of their close-knit group of friends gains prominence as these peer-based friendships provide youths’ with greater stability, support, and intimacy than the childhood years (Brown, Dolcini & Leventhal, 1997). Consequently, we anticipate the role of peer norms on PHIV youths’ sexual behaviors will become more salient from early and middle adolescence.
Theoretically-informed HIV and teenage-pregnancy prevention programs have acknowledged the association of peer norms and adolescents’ sexual decision-making through constructs such as the Theory of Planned Behavior’s subjective norm (Montaño & Kasprzyk, 2002) and the Health Belief Model’s cues to action (Janz, Champion, & Strecher, 2002). The influence of peer norms on adolescent sexual behavior has been reviewed extensively in the scientific literature (Buhi & Goodson, 2007; Pedlow & Carey, 2004). In a recent review of the literature on non-infected adolescents, Buhi and Goodson (2007) identified peer norms regarding acceptability of sexual behavior and the number of peers who are perceived to be sexually active as two peer-related indicators that were associated with sexual onset and sexual risk behaviors among adolescents. Thus, the role of peer norms is vital in developing behavior change interventions targeting adolescents.
From a social influence perspective, peers within adolescents’ social networks may reinforce positive and/or negative attitudes and behaviors regarding safer sex practices (Albarracin, Kumkale, & Johnson, 2004; Lewis, DeVellis, & Sleath, 2002), and may promote condom use and shift group norms among peers (Fang, Stanton, Li, Feigelman, & Baldwin, 1998). In a recent review of HIV/STI prevention interventions, Pedlow and Carey (2004) found that seven interventions measured the effect of peer norms on adolescent sexual behavior. Five of these seven interventions found peer norms supporting abstinence and safer sex delayed sexual initiation and improved condom use. Furthermore, six of the seven interventions reported an increase in safer sex peer norms from baseline to follow-up. Thus, peer norms are an important component that may have lasting effects in adolescent HIV/AIDS prevention programs.
Bearman and Bruckner (1999) found that a youth’s peer network might exert a greater influence on sexual initiation than a single friend (e.g., a best friend). From a social network perspective, youth who believe that most of their peers have had sex are more likely to be more motivated to begin their own sexual activity, to start at an earlier age, and to gain their friends’ respect and acceptance if they too became sexually active (Kinsman, Romer, Furstenberg, & Schwarz, 1998; Sieving, Eisenberg, Pettingell, & Skay, 2006). Nonetheless, a third of the studies reviewed by Buhi and Goodson (2007) found “no statistically significant association between the perception of peers’ sex behaviors and sexual behavior outcomes” (p. 8). Furthermore, research suggests that gender may moderate the relationship between peer norms and youths’ sexual behaviors. Research exploring the association between peer norms and sexual behaviors for boys and girls, respectively, suggests that the effect is stronger for girls (Sheeran & Abraham, 1999). Compared to boys, girls assign greater weight to their social relationships and may be more likely to comply with peer norms to avoid rejection, leading to greater sexual risk-taking behaviors (e.g., inconsistent condom use), decreased self-esteem (Salazar, Crosby, DiClemente, Wingood, Lescano, Brown et al., 2005), and sexual power imbalances in condom use negotiation (DiClemente, Wingood, Harrington, Lang, Davies, Hook et al., 2004). Consequently, the role of gender merits particular attention when exploring the association between peer influences and sexual behaviors.
While evidence suggests that adolescents are highly influenced by peer beliefs and norms, we know very little about peer relationships and their influence on PHIV youths’ sexual behaviors. For PHIV youth, the potential moderating effects of developmental stage and gender on the associations of peer norms on sexual behavior remain unknown. Many PHIV youth have often missed school and social opportunities because of multiple medical appointments, hospitalizations, or home schooling. Furthermore, caregivers may be overprotective of PHIV youth, reducing their social opportunities (Havens & Mellins, in press). Consequently, PHIV youth must contend with managing a stigmatizing illness that may force them to the fringes of social groups and potential sexual isolation (Henry, Schoeny, Deptula, & Slavick, 2007), or may serve to increase or accelerate their sexual behavior as they attempt to normalize their socials status and accrue social capital (Dishion, McCord, & Poulin, 1999). Given that many abstinence-focused and safer sex interventions for adolescents focus on peer-based norms (Kirby, 2002), understanding the role of peer normative beliefs for PHIV youth may be important to inform prevention interventions for this population.
Study Objectives and Hypotheses
This paper aims to describe the sexual behaviors of a sample of perinatally HIV-infected and perinatally HIV-exposed youth (9–16 year old) and to examine the association of these sexual behaviors with peer influences. First, we examined whether sexual behaviors differed between PHIV early and middle adolescent youth and perinatally exposed HIV-negative youth from a multisite clinic-based sample. We hypothesized that PHIV youth would report fewer sexual behaviors than perinatally-exposed HIV-negative youth given clinical reports that PHIV youth are delayed in their psychosocial development (Battles & Weiner, 2002), or because of fear of disclosing their HIV status to a partner or as a harm reduction strategy to avoid infecting their partners (Kang et al., 2008). Second, we explored the associations between sexual behaviors and sexuality-related peer norms. Consistent with previous findings on peer norms on sexual behavior (Buhi & Goodson, 2007), we hypothesized that the likelihood of engaging in sexual behaviors would be higher among youths who perceived that their peers were sexually active and/or would endorse sexual activity would be more likely to report sexual activity. Consistent with previous findings on age differences in peer norms (Brown et al., 1997), we hypothesized that developmental stage, early (ages 9–12) and mid (ages 13–16) adolescence would modify the associations between peer-norms and sexual behaviors, with the effect of peer norms on the onset of sexual behavior being greater for mid-adolescents. Similarly, we hypothesized that the association between peer norms and sexual behaviors would be more salient for girls (DiClemente et al., 2004; Sheeran & Abraham, 1999). Based on our findings, we present implications for peer-based HIV/AIDS interventions for this population.
Method
Participants and Procedures
Data for this paper come from the baseline interview of a longitudinal study on risk and resilience with PHIV youth, Project CASAH. Participants for Project CASAH were recruited from four medical centers in New York City (NYC) that provide family-focused, medical care, and supportive services for HIV-affected families. Inclusion criteria for study participants were: 1) youth ages 9 to 16 years with perinatal exposure to HIV, 2) adequate caregiver and youth cognitive capacity to complete interview, 3) English or Spanish speaking, and 4) caregiver with legal capacity to sign consent for the child’s participation (foster care parents can not provide consent for child participation in research in NYC). We recruited youths ages 9 to 16 in order to obtain a large enough sample of PHIV youth and to examine differences between early and middle adolescence.
During the recruitment period (2003–2007), of 443 eligible participants, 11% refused contact with the research team and the providers could not contact 6%. A total of 367 eligible participants (83%) were approached, of whom 92% were enrolled. Data were not collected on patients who were not approached or who refused to participate.
Two sources of data were collected: 1) caregiver and adolescent interviews, and 2) medical chart abstractions. Baseline data were obtained over two interview sessions from each caregiver-child dyad. Both caregivers and children were interviewed simultaneously but separately; interviews lasted 60–90 minutes and were approximately 2–4 weeks apart. Data from this paper comes from 339 caregiver-youth dyads; 206 HIV+ and 133 HIV- youths. Among the 339 caregiver-child dyads enrolled, 339 children and their caregivers completed baseline session 1, and 322 children (195 HIV+, 127 HIV-) and caregivers completed both baseline session 1 and session 2. Thus, 95% of each group (HIV+ and HIV-) completed both sessions. The primary reasons for failure to complete session 2 in both groups were caregiver death and/or relocation. The psychiatric interview with demographic questions was administered in Session 1. All other measures were collected during the second session. Medical chart data were collected on all HIV+ youth. This paper focuses on Session 2 data only (N = 322). This study received Institutional Review Board approval from all of the participating sites. All caregivers provided written informed consent for themselves and youths. Youths provided written assent. Monetary reimbursement for time and transportation was provided.
Measures
Child sexual behavior
Child sexual behavior was assessed with the Adolescent Sexual Behavior Assessment (Dolezal, Mellins, Brackis-Cott, & Meyer-Bahlburg, 2006), developed in preparation for this project. The Sexual Risk Behavior Assessment Schedule for Youths (SERBAS-Y; Meyer-Bahlburg, Ehrhardt, Exner, Gruen, & Dugan, 1995) was an important reference throughout the development process. In brief, the goal was to have a relatively brief assessment of sexual behavior that was explicit enough to distinguish among various specific sexual practices, but yet would be appropriate for the younger children in the study (via gateway questions). The assessment was randomly administered via ACASI (audio computer assisted self-interview) or face-to-face interview. There were no differences in youths’ sexual behavior reporting across these two data collection procedures. The following sexual behaviors (yes/no) were examined: lifetime kissing, touching a partner’s genitals, engaging in oral sex, and penetrative (vaginal or anal) sex. We aggregated reports of vaginal and anal sex behavior into one variable (“penetrative sex”) given the low frequency of vaginal and anal sex, respectively, and the high-risk of transmission associated with both behaviors. We were unable to distinguish consensual from non-consensual sexual behaviors.
Peer norms regarding sexuality
Based on our clinical experience with PHIV youth, we measured peer norms by adapting an existing measure (Silver & Bauman, 2006) that ascertained how many peers within participants’ social networks perceived being-sexually active makes someone “cool” or “popular”. The popularity of each of statement was constructed to be gender-specific (five items measuring how many peers would think a boy or a girl, respectively, would be popular or cool for each statement). The 5 items read: “Having sexual intercourse at my age is a cool thing for a [boy/girl] to do,” “Having sexual intercourse with someone besides his/her steady partner makes a [boy/girl] cool”, “Having sex with as many people as possible makes a [boy/girl] cool,” “Having sex with 2 different people on the same day makes a [boy/girl] cool,” and “It’s cool [for girls to get pregnant/for guys to get a girl pregnant] while in high school.” Adolescents answered each statement using a 4-point ordinal scale: 0=None, 1=Some, 2=Most, and 3=All. We created a mean composite score for each gender-specific set of items. We found strong reliability for both scales (male gender norms Cronbach’s α = .87); female gender norms Cronbach’s α = .81). High scores indicate beliefs that participants had more friends in their social network who perceived sexual practices made them “cool” or “popular.”
Perceived peer sexual behavior
Participants were asked to indicate their perceptions regarding how many of their friends were sexually active (Bauman & Silver, 2002). Participants answered four items that presented the possibility of their peers’ engaging in risky sexual behavior. These items read: “How many of your friends think condoms are too much trouble to use?,” “How many of your friends do you think have had sexual intercourse without a condom because they were high from drinking alcohol?”, “How many of your friends do you think have had sexual intercourse without a condom because they were high from drugs?,” and “How many of your friends do you think are or have been pregnant or have gotten someone pregnant?”. Adolescents answered each item using a 4-point ordinal scale: 0=None, 1=Some, 2=Most, and 3=All. We created a mean composite score from these 4 items (Cronbach’s α = .78). High scores indicated participants’ perception that more friends were engaging in risky sexual behavior.
Demographic variables
We collected data on children’s age, gender, and race/ethnicity as well as their caregiver’s employment status, years of education, household income, and number of people living in the household. Household income was measured by aggregating the total earnings from work, unemployment insurance, disability, public assistance, spouse or family member support, or other sources, and creating several income categories (1 = $5,000 or less, 2 = $5,001 to $10,000, 3 = $10,001 to $15,000, 4 = $15,001 to $20,000, 5 = $20,001 to $25,000, 6 = $25,001 to $30,000, 7 = $30,001 to $40,000, 8 = $40,001 to $50,000, 9 = $50,001 to $75,000, 10 = $75,001 to $100,000, and 11 = $100,001 to $150,000). Using household income and the number of people living per household, we created a dichotomous variable that indicated whether a family o met the New York City poverty criteria for a family of four ($20,650; New York City Center for Economic Opportunity, 2008).
Analytic Approach
We used the subsample of participants who completed the second baseline assessment where sexual behavior measures were asked for all analyses (N = 316). First, we verified whether our study variables were normally distributed. We found the peer-norm indicators were skewed, yet transformations (e.g., log-10) did not alleviate the skewed distribution. Given logistic regression’s robustness to deviations from normality in the explanatory variables (Kay & Little, 1987), we used the variables in their original metric to maximize the interpretability of these variables. Second, we compared the study variables by HIV status using t-tests for continuous variables and χ2 tests for categorical variables. Third, we created a logistic regression model to test the association between HIV-status and youths’ sexual behaviors. Given that the sexual behaviors measured are not mutually exclusive, we tested the association between HIV-status and all four sexual behaviors (consequently, being sexually inactive was the referent category) in a logistic regression model. This approach allowed us to identify sexual risk taking patterns by comparing the likelihood of engaging in one type of behavior across HIV-status, after adjusting for the probability of having engaged in other sexual behaviors (Hypothesis 1). Finally, we created four logistic regression models to test the association between each sexual behavior and participants’ HIV status, their demographic characteristics, and their perceived peer norms and perceived peer sexual behaviors (Hypothesis 2). While we wished to test for age, HIV status and gender interactions with the peer-related indicators (Hypothesis 3), we were concerned about diminishing our statistical power due to the low rates of sexual behaviors in our sample. Consequently, as an exploratory step, we used forward stepwise logistic regression models with an inclusion criterion of p ≤ .05 for the gender, age and HIV status, and peer-related indicators interactions, respectively.
Results
Sample Description
On average, participants in our sample were early adolescents (M = 12.16 years, SD = 2.26), evenly split by gender, and predominantly Black (54.1%) or Latino (30.4%). As shown in Table 1, caregivers reported having less than a high school education (M = 11.59 years, SD = 3.21). The average household income for the sample ranged between $20,000 and $30,000; however, households with HIV+ youth reported slightly higher income and were less likely to meet poverty criteria than HIV- youths’ households.
Table 1.
Descriptive characteristics of a sample of perinatally HIV-infected (N = 191) and perinatally HIV-exposed (N = 125) youth.
| Variable | HIV− | HIV+ | Total Sample | t/χ2 |
|---|---|---|---|---|
| Age (in years) | 11.95(2.35) | 12.30 (2.18) | 12.16 (2.25) | −1.36 |
| Gender | 0.12 | |||
| Male | 61 (48.8%) | 97 (50.8%) | 158 (50.0%) | |
| Female | 64 (51.2%) | 94 (49.2%) | 158 (50.0%) | |
| Ethnicity | 3.47 | |||
| Black | 62 (49.6%) | 109 (57.1%) | 171 (54.1%) | |
| Latino | 38 (30.4%) | 58 (30.4%) | 96 (30.4%) | |
| Other/Mixed | 25 (20.0%) | 24 (12.5%) | 49 (15.5%) | |
| Household income | 5.03(2.51) | 5.80 (2.91) | 5.50 (2.78) | −2.46** |
| Caregiver’s years of education |
11.43(2.96) | 11.70 (3.37) | 11.59 (3.21) | −0.74 |
| Caregiver’s currently working |
26 (20.8%) | 57 (29.8%) | 83 (26.3%) | 3.19 |
| Family meets poverty criteria |
68 (57.1%) | 67 (37.2%) | 135 (45.2%) | 11.48*** |
| Sexual Behavior | ||||
| Kissing | 42 (33.6%) | 75 (39.3%) | 117 (37.0%) | 1.04 |
| Touching | 19 (15.2%) | 39 (20.4%) | 58 (18.4%) | 1.37 |
| Oral Sex | 10 (8.0%) | 12 (6.3%) | 22 (7.0%) | 0.34 |
| Vaginal Sex | 17 (13.6%) | 18 (9.5%) | 35 (11.1%) | 1.30 |
| Anal Sex | 4 (3.2%) | 6 (3.1%) | 10 (3.2%) | 0.001 |
| Peer Norms | ||||
| Number of peers assigning popularity to sexually active boys |
0.56 (.80) | 0.65 (.80) | 0.62 (.80) | −1.03 |
| Number of peers assigning popularity to sexually active girls |
0.38 (.59) | 0.45 (.60) | 0.42 (.59) | −1.09 |
| Perceived friends’ sexual activity |
0.37 (.55) | 0.41 (.55) | 0.21 (.48) | −0.68 |
p ≤ .05
p ≤ .01
p ≤ .001
Youths’ sexual behaviors varied: 58% reported no sexual behavior, 35% reported kissing, 17% reported touching, 7% reported oral sex, and 11% reported vaginal (11.1%) or anal (2.9%) penetrative sex. HIV+ youth reported an earlier age of sexual onset (N = 19; M = 11.95 years, SD = 2.25) than HIV-negative youth (N = 18; M = 13.39 years, SD = 1.79), t (35) = 2.15, p < .05. Although a large range was observed, on average, youth reported that none to a few of their friends thought being sexually active was cool or popular for boys and girls, respectively. Participants also reported that none or a few of their friends were engaging in risky sexual behavior.
Differences across Sexual Behaviors by HIV-Status
When we assessed prevalence of each sexual behavior independently (see Table 1) across HIV+ and HIV- youth, we found no statistically significant differences. However, when we explored the association between HIV-status and prevalence of youths’ experience across all sexual behaviors simultaneously (see Table 2), we found HIV-positive youth were less likely to be sexually active (as defined by not reporting any kissing, touching, or oral or penetrative sex) than HIV-negative youth (OR = 1.36, p ≤ .05). Furthermore, HIV-positive youth were four times less likely to engage in penetrative sex (OR = .22, p ≤ .05), but were three times more likely to engage in touching behavior (OR = 3.68, p ≤ .05) than HIV-negative youth, after accounting for the likelihood of engaging in all other sexual behaviors (see Table 2). We found no differences by HIV-status in the prevalence of kissing behavior or oral sex.
Table 2.
Multivariable logistic regression associating HIV-status to multiple sexual behaviors in a sample of perinatally HIV-infected (N = 191) and perinatally HIV-exposed (N = 125) youth.
| Sexual Behavior | Odds Ratio (95% C.I.) b |
|---|---|
| Kissing | 1.29(0.73, 2.27) |
| Touching | 3.68(1.17, 11.54)* |
| Oral Sex | 0.76(0.21, 2.73) |
| Penetrative Sex | 0.22(0.06, 0.83)* |
| Constant a | 1.36* |
p ≤ .05
p ≤ .01
p ≤ .001
Constant reflects no sexual behavior
Each coefficient represents the likelihood of HIV-positive youth engaging in the behavior, compared to HIV-negative youth, after accounting for the likelihood of reporting other types of sexual behavior.
Demographic Characteristics, Peer Norms, and Sexual Behaviors
Seventy percent of PHIV youth knew their status. Given that participants’ awareness of their HIV status was highly correlated with age (r = .59, p ≤ .01), we did not include HIV status awareness in the logistic regression models to avoid multicollinearity.
Kissing
On average, kissing was associated with being in mid (ages 13–16) versus early (9–12) adolescence (OR = 12.72, p ≤ .001), having a greater number of peers who felt sexually active boys were popular (OR = 2.23; p ≤ .01), and having a greater number of peers who felt sexually active girls were popular (OR = 2.93; p ≤ .05). As shown in Table 3, we found age moderated the relationship between having a greater number of peers who felt sexually active girls were popular and kissing behavior (OR = .31; p ≤ .01). The odds of reporting kissing behavior increased if a greater number of peers perceived sexually active girls were popular, yet this association was stronger for early adolescents (ages 9–12) than for mid adolescent (ages 13–16) participants. We found no other statistically significant interactions between the peer-related indicators and gender or HIV status, respectively.
Table 3.
Logistic regressions for multiple sexual behaviors by demographic characteristics, perceived peer norms, and sexual behavior by age, HIV status, and gender interactions in a sample of perinatally-infected and perinatally-exposed HIV youth (N = 296).
| Kissing OR (95% CI) |
Touching OR (95% CI) |
Oral Sex OR (95% CI) |
Penetrative Sex OR (95% CI) |
|
|---|---|---|---|---|
| Age a | 12.72(5.70,31.90)*** | 1.42(1.15,1.76)** | 2.11(1.38,3.22)*** | 2.10(1.40,3.14)*** |
| Gender b | 0.68(.35,1.32) | 0.69(.32,1.49) | 7.00(.72, 68.27) | 1.21(.26,5.60) |
| HIV Status | 1.00(.52,1.92) | 1.33(.62,2.83) | 0.80(.27,2.34) | 0.15(.03,.68)* |
| Ethnicity c | ||||
| Latino | 1.02(.51,2.08) | 0.50(.21,1.15) | 0.54(.16,1.83) | 0.26(.08,.84)* |
| Other/Mixed | 0.98(.40,2.41) | 0.38(.12,1.22) | 0.59(.12,2.85) | 0.35(.08,1.60) |
| Family meets poverty criteria | 1.19(.62,2.26) | 0.74(.35,1.56) | 1.00(.32,3.07) | 0.36(.12,1.09) |
|
Perceived Peer Norms and Sexual Behavior | ||||
| Popularity of sexually active boys | 2.23(1.29,3.84)** | 2.97(1.68,5.23)*** | 3.43(1.01,11.66)* | 3.35(1.56,7.17)** |
| Popularity of sexually active girls | 2.93(1.06,8.05)* | 0.78(.40,1.55) | 0.83(.30,2.33) | 0.05(.01,.79)* |
| Friends’ sexual activity | 1.35(.67,2.73) | 1.44(.68,3.04) | 2.86(.95,8.65) | 5.83(2.01,16.89)*** |
| Peer Interactions | ||||
| Popularity of sexually active boys x Gender |
--- | --- | 0.13(.03,.57)** | --- |
| Popularity of sexually active girls x Age |
0.31(.11,.89)* | --- | --- | --- |
| Popularity of sexually active girls x Gender |
--- | --- | --- | 0.12(.02,.74)** |
| Popularity of sexually active girls x HIV status |
--- | --- | --- | 5.46(1.13,26.39)* |
p ≤ .05
p ≤ .01
p ≤ .001
For analyses where an age interaction is present, the age variable is a dichotomous variable (0 = Early Adolescence: Ages 9–12; 1 = Mid Adolescence: Ages 13–16) instead of a continuous variable.
Males serve as the comparison group.
African American youths serve as the comparison group.
Genital Touching
On average, genital touching was associated with being older (OR = 1.42, p ≤ .01) and having a greater number of peers who felt sexually active boys were popular (OR = 2.97; p ≤ .05). We found no statistically significant association between genital touching and any other covariate in our model (See Table 3), nor did we find any statistically significant interactions between the peer-related indicators and gender, HIV status, or age group, respectively.
Oral Sex
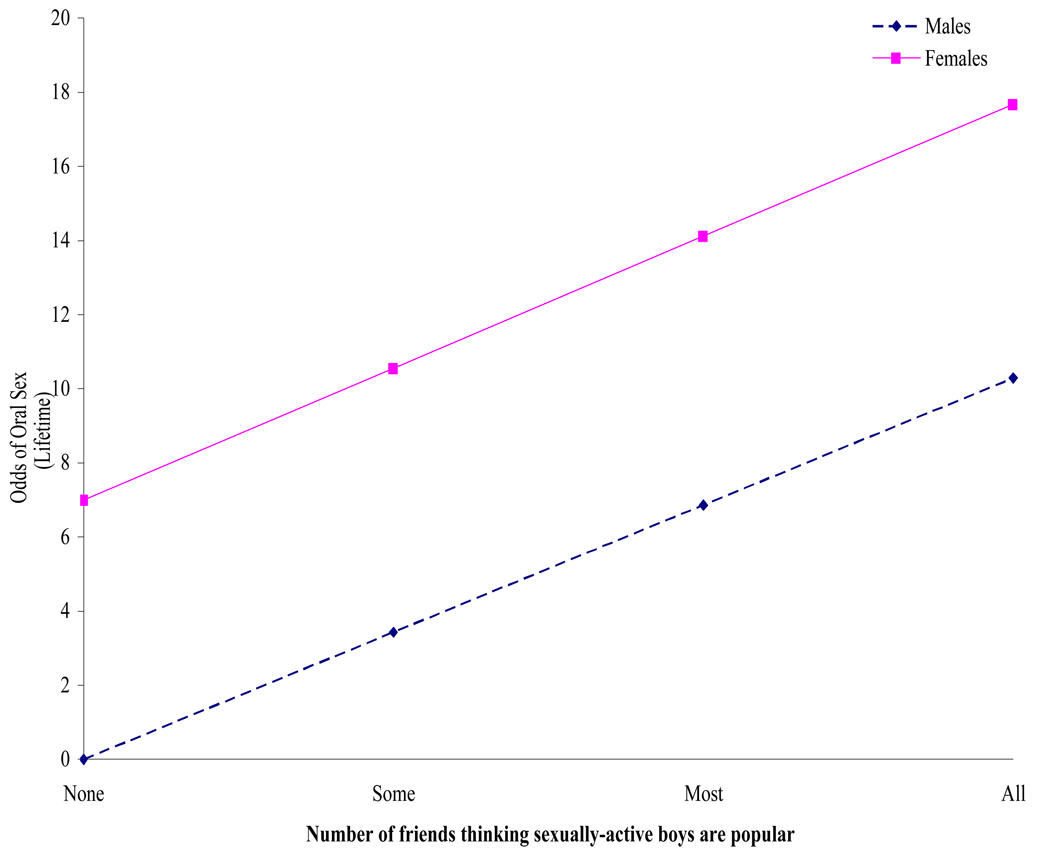
On average, oral sex was associated with being older (OR = 2.11, p ≤ .001) and having a greater number of peers who felt sexually active boys were popular (OR = 3.43; p ≤ .05). We found, however, that gender moderated the association between oral sex and the number of peers considering sexually active boys to be popular (OR = 0.13; p ≤ .05). As shown in Figure 1, adolescent girls were more likely to report oral sex if a greater number of peers considered sexually active boys to be popular. We found no other statistically significant associations between oral sex and the other covariates in our model (See Table 3). We did find a trend suggesting that the association between oral sex and the number of peers considering sexually active boys to be popular was greater for HIV-positive participants yet, while compelling, this interaction did not meet our specified criteria to achieve statistical significance (data not shown).
Figure 1.
Interaction Effects of Gender by Number of Peers who Think that Sexually Active Girls are Popular on Likelihood of Engaging in Oral Sex.
Penetrative Sex
On average, youth who reported engaging in anal or vaginal sex were more likely to be older (OR = 2.10, p ≤ .001). Youth were less likely to engage in penetrative sex if they were HIV-positive (OR = .11, p ≤ .01) or Latino (OR = 0.26; p < .05). Furthermore, as shown in Table 3, youth were more likely to report engaging in penetrative sex if a greater number of peers considered sexually active boys to be popular (OR = 3.35; p ≤ .01) or if a greater number of their peers were engaging in risky sexual behavior (OR = 5.83; p ≤ .001).
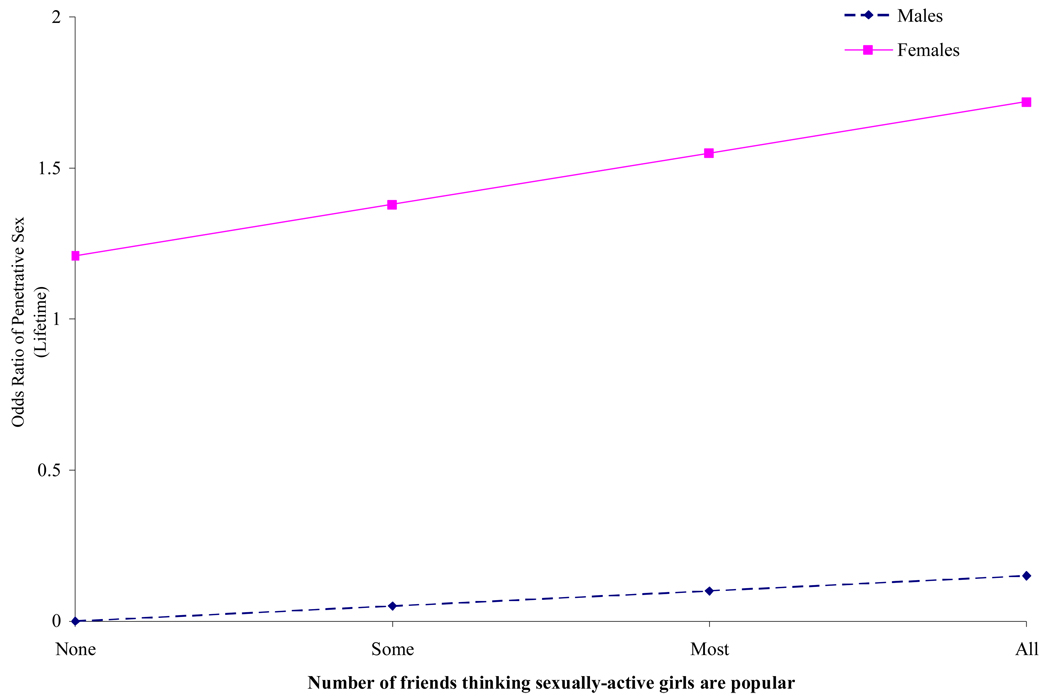
We found that gender and HIV status moderated the association between penetrative sex and the number of peers considering sexually active girls to be popular, respectively. As shown in Figure 2, adolescent girls were more likely than boys to report penetrative sex if a greater number of peers considered sexually-active girls to be popular (OR = .12; p < .01). We also found HIV status moderated the association between penetrative sex and the number of peers considering sexually active girls to be popular (OR = 5.46, p < .01). The likelihood of engaging in penetrative sex increased among HIV-positive youth if a greater number of peers considered sexually active girls to be popular.
Figure 2.
Interaction Effects of Gender by Number of Peers who Think that Sexually Active Boys are Popular on Likelihood of Engaging in Penetrative Sex.
Discussion
Peer norms have been shown to influence adolescent sexual behaviors in a wide range of populations (Brown et al., 2000; Buhi & Goodson, 2007). Similar to other adolescent populations, our findings suggest that peer norms exert an important influence on perinatally infected and perinatally exposed youths’ sexual behaviors. Furthermore, consistent with previous research, we found the role of peer norms was more salient for girls than boys (Sheeran & Abraham, 1999). To our knowledge, this is one of the first studies to explore the association between sexual behaviors and peer influences in a sample of perinatally exposed youths, 61% of whom are HIV-positive. We found low rates of sexual behavior in our sample (i.e., 7% engaged in oral sex, 11% vaginal sex, 3% anal sex). This is not surprising given our age range of 9–16 years, making comparisons to the general adolescent population difficult given the low prevalence of sexual behaviors (CDC, 2006). Among the sexually active, the mean age of sexual onset (e.g., reporting oral, vaginal or anal sex) was earlier for PHIV youth (M = 12 years) than perinatally-exposed HIV negative youth (M = 13.4 years), yet older youths in our sample were more likely to report engaging all sexual behaviors regardless of their HIV status. Our rates appear to be lower than the 33% of sexually active PHIV youths in Ezeanolue et al.’s (2006) study; however, the youths in that study ranged in age from 13–24 years, making direct comparisons impossible.
Contrary to our expectations, we found no bivariate differences by HIV status in the prevalence of sexual behaviors (i.e., kissing, touching, oral sex, and anal/vaginal penetration) between our two groups. Nevertheless, when we compared youths’ engagement in a particular sexual behavior after accounting for the likelihood of having participated in any other sexual behavior, we found PHIV youth were less likely to be sexually active than HIV-negative youth. However, if sexually active, PHIV youth seemed to delay penetrative sex through genital touching behavior. Taken together, these findings suggest that our sample of PHIV youth may avoid anal/vaginal penetration through delaying sexual onset or engaging in more touching behavior as harm reduction strategies. From a methodological standpoint, it is interesting to note that the different analytical approaches (i.e., independent t-tests versus multivariate logistic regression) used to compare youths’ sexual behaviors by HIV status reveal different patterns of sexual risk taking. Consequently, when possible, future research should assess youths’ sexual behaviors concurrently rather than assuming that one sexual behavior precludes another as the findings may lead to different implications to inform HIV prevention programs. Furthermore, qualitative and quantitative research is needed to understand these risk-taking patterns in detail.
We explored two interpersonal influences previously associated with adolescent sexual behavior, gender-specific peer norms regarding sexuality and the number of peers perceived to be sexually-active in youths’ social networks (Buhi & Goodson, 2007). Overall, our findings suggest that youths who reported that a greater number of their peers believed that sexually-active boys were “cool” or “popular” were more likely to report engaging in any sexual behavior, even after accounting for HIV status and sociodemographic characteristics. These findings are consistent across all sexual behaviors and underscore the importance of addressing masculinity and popularity when discussing sexuality among adolescents (Tolman, Striepe, & Harmson, 2003). We also found gender moderated this relationship. Girls were more likely to report engaging in oral sex as the number of their peers who perceived that sexually active boys were “cool” or “popular” increased. This finding offers support for the role of gender as moderating the relationship between sexual behavior and peer influences, and underscores the importance of gender norms in HIV prevention programs.
The association between peers considering sexually active girls as “cool” or “popular” and sexual behavior was conditioned by age, HIV status, and gender. The number of peers believing that sexually-active girls were cool and popular was associated with kissing, yet youths in mid-adolescence (ages 13–16) were more likely than early adolescents (ages 9–12) to report kissing if they had a greater number of peers who believed that sexually-active girls were popular. Furthermore, we found that the likelihood of engaging in penetrative sex increased as the number of peers regarding sexually active girls as cool or popular increased, particularly among HIV-positive adolescents. Similarly, girls were more likely to report engaging in penetrative sex if a greater number of peers perceived sexually active girls were cool or popular. A possible interpretation for these interactions is twofold. First, girls may feel more peer pressure to carry out sexual behaviors requiring greater intimacy (e.g., oral or penetrative sex) if they perceive that the social rewards of carrying-out the behavior are greater (Halpern-Felsher, Cornell, Kropp, & Tschann, 2005). Second, the relationship between girls engaging in oral sex and perceptions of sexually-active boys as being “cool” and “popular” may be the indirect effect of male pressure on girls’ sexual behavior (O'Donnell, Myint-U, O'Donnell, & Stueve, 2003). Taken together, these findings are consistent with previous research on gender-based differences regarding the influence of peers on youths’ sexual behavior and highlight the need for sexual health programs that promote skills where youth, particularly girls, can learn to resist peer pressure and shift or change gender and social norms and expectations (DiClemente et al., 2004).
Our study has several other limitations. First, the study’s cross-sectional data do not establish causality. Future research should explore the causal relationship between the study variables as youth transition from early/mid to late adolescence. The ability to detect significant associations will be easier as the prevalence of sexual behaviors increase as youth age. Second, we were unable to identify whether any of the sexual behaviors reported were unwanted. Third, our study findings may not be generalizable to all PHIV youth. Participants in this study come from a New York City clinic-recruited sample that may have access to an array of social and medical services that include health education messages; therefore, it is plausible that PHIV youth with less comprehensive access to care in other parts of the United States or other countries may be different. Finally, while we have collected individual (i.e., egocentric) data on youths’ peer influences, our understanding of peer influences on sexual behavior may be strengthened by conducting network-level studies (Bearman, Moody, & Stovel, 2002). Network-level analyses may elucidate increasingly prominent, structural-level approaches to HIV prevention (Amirkhanian, Kelly, Kabakchieva, Kirsanova, Vassileva, Takacs et al., 2005; Woods, Samples, Melchiono, & Harris, 2003).
These limitations not withstanding, our study builds on the relationship between peer influences and sexual behavior among youth in several ways. First, this study explores the relationship between sexual behavior and peer influences in a sample of perinatally HIV-infected and HIV-exposed sample of adolescents. Second, our study accounted for the role of gender, age, and HIV status as moderating the relationship between sexual behaviors and peer influences. A deeper understanding of peer influences on adolescents boys and girls’ sexual behaviors, respectively, may help elucidate the similarities and differences that exist between HIV-positive and HIV-negative youth, and assist in developing appropriate HIV prevention interventions.
Increasingly, public health efforts in the United States are developing prevention programs for HIV-positive youth. While prevention programs should strengthen messages addressing peer norms regarding sexuality in the context of adolescent development, attention on how to best deliver HIV-focused gender-specific, multilevel prevention education to PHIV youth is required. This approach will provide PHIV youth with harm reduction strategies such as resisting peer pressure to engage in risky sexual behaviors, using condoms adequately and consistently, and/or having the skills to disclose their HIV status to potential partners. Future research and interventions focused on PHIV youth are needed and should expand this study’s focus to include other interpersonal influences that may shape youths’ social networks (e.g., parental involvement and participation in school and extracurricular activities) and exert an influence in youths’ sexual decision-making.
Acknowledgments
Drs. Bauermeister and Elkington are supported by a NIMH training grant (Behavioral Research in HIV Infection; T32 MH19139 Behavioral Sciences Research in HIV Infection; PI: Anke A Ehrhardt, Ph.D.). This research was supported by a grant from the National Institute of Mental Health (R01-MH63636; PI: Claude Ann Mellins, Ph.D.), and a center grant from the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30 MH43520; Center PI: Anke A. Ehrhardt, Ph.D.).
Abbreviations
- HIV
Human immunodeficiency virus
- HIV+
HIV infected
- HIV−
HIV negative
Contributor Information
Jose A. Bauermeister, Email: jbauerme@umich.edu.
Katherine Elkington, Email: ke2143@columbia.edu.
Elizabeth Brackis-Cott, Email: eb372@columbia.edu.
Curtis Dolezal, Email: dolezalc@childpsych.columbia.edu.
Claude Mellins, Email: cam14@columbia.edu.
References
- Agostoni C, Riva E, Esposito S, Ferraris G, Principi N, Zuccotti GV. Fatty acid composition of plasma lipids in HIV-infected children: Comparison with seroreverters. Acta Paediatrica. 2000;89:172–176. doi: 10.1080/080352500750028780. [DOI] [PubMed] [Google Scholar]
- Alan Guttmacher Institute. [Accessed on May 7, 2008];Sex education: Needs, Programs and Policies. 2006 Available at www.agi-usa.org/presentations/ed_slides.html.
- Albarracin D, Kumkale GT, Johnson BT. Influences of social power and normative support on condom use decisions: a research synthesis. AIDS Care. 2004;16(6):700–723. doi: 10.1080/09540120412331269558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirkhanian YA, Kelly JA, Kabakchieva E, Kirsanova AV, Vassileva S, Takacs J, et al. A randomized social network HIV prevention trial with young men who have sex with men in Russia and Bulgaria. AIDS. 2005;19(16):1897–1905. doi: 10.1097/01.aids.0000189867.74806.fb. [DOI] [PubMed] [Google Scholar]
- Battles H, Weiner LS. From adolescence through young adulthood: Psychosocial adjustment associated with long-term survival of HIV. Journal of Adolescent Health. 2002;30:161–168. doi: 10.1016/s1054-139x(01)00341-x. [DOI] [PubMed] [Google Scholar]
- Bauman LJ, Silver EJ. Gender Beliefs and HIV/STD Risk. Pediatric Academic Societies Annual Meeting, Baltimore, MD. May. 2002 [Google Scholar]
- Bearman PS, Bruckner H. Peer effects on adolescent girls' sexual debut and pregnancy risk. Pregnancy Prevention for Youth Network. 1999;2(3):3–4. [Google Scholar]
- Bearman PS, Moody J, Stovel K. Institute for Social and Economic Research and Policy - Columbia Univeristy Working Papers. New York: Columbia University; 2002. Chains of affection: The structure of adolescent romantic and sexual networks; p. 36. [Google Scholar]
- Brogly SB, Watts H, Ylitalo N, Franco EL, Seage GR, Oleske J, Eagle M, Van Dyke R. Reproductive health of adolescent girls perinatally infected with HIV. American Journal of Public Health. 2007;97(6):1047–1052. doi: 10.2105/AJPH.2005.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Cambridge, Massachusetts: Harvard University Press; 1979. [Google Scholar]
- Brown BB, Dolcini MM, Leventhal A. Transformations in peer relations at adolescence: Implications for health-related behavior. In: Schulenberg J, Maggs JL, Hurrelmann K, editors. Health risks and developmental transitions during adolescence. New York: Cambridge University Press; 1997. pp. 161–189. [Google Scholar]
- Brown LK, Schultz JR, Parsons JT, Butler RB, Forsberg AD, Kocik SM, King G, Manco-Johnson M, Aledort L. Sexual behavior change among human immunodeficiency virus-infected adolescents with hemophilia. Pediatrics. 2000;106(2):e22. doi: 10.1542/peds.106.2.e22. [DOI] [PubMed] [Google Scholar]
- Buhi ER, Goodson P. Predictors of adolescent sexual behavior and intention: A theory-guided systematic review. Journal of Adolescent Health. 2007;1:4–27. doi: 10.1016/j.jadohealth.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance- United States, 2005. MMWR. 2006;55:SS5, 78. [Google Scholar]
- Centers for Disease Control and Prevention. Pregnancy in Perinatally HIV-infected adolescents and young adults- Puerto Rico, 2002. MMWR. 2003;52:149–151. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Estimated numbers of cases and rates (per 100,000 population) of AIDS, by race/ethnicity, age category, and sex, 2006—50 states and the District of Columbia. 2008 Accessed via the World Wide Web at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2006report/pdf/table5a-b.pdf.
- Corby NH. Priority-population assessment for HIV prevention program development. In: Corby NH, Wolitski RJ, editors. Community HIV prevention: The Long Beach AIDS Community Demonstration Project. California: University Press; 1997. pp. 125–134. [Google Scholar]
- DiClemente R, Salazar LF, Crosby RA. A review of STD/HIV preventive interventions for adolescents: Sustaining effects using an ecological approach. Journal of Pediatric Psychology. 2007;32(8):888–906. doi: 10.1093/jpepsy/jsm056. [DOI] [PubMed] [Google Scholar]
- DiClemente R, Wingood G, Harrington KF, Lang DL, Davies SL, Hook EW, et al. Efficacy of an HIV prevention intervention for African American adolescent girls. JAMA. 2004;292(2):171–179. doi: 10.1001/jama.292.2.171. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, McCord J, Poulin F. When interventions harm: Peer groups and problem behavior. American Psychologist. 1999;54:755–764. doi: 10.1037//0003-066x.54.9.755. [DOI] [PubMed] [Google Scholar]
- Dolezal C, Mellins C, Brackis-Cott E, Meyer-Bahlburg H. Adolescent sexual Behavior Assessment. 2006 Unpublished Manuscript. Copy Written, 2006. [Google Scholar]
- Ezeanolue EE, Wodi AP, Patel R, Dieudonne A, Oleske J. Sexual behaviors and procreational intentions of adolescents and young adults with perinatally acquired human immunodeficiency virus infection: Experience of an urban tertiary care center. Journal of Adolescent Health. 2006;38:719–725. doi: 10.1016/j.jadohealth.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Fang X, Stanton B, Li X, Feigelman S, Baldwin R. Similarities in sexual activity and condom use among friends within groups before and after a risk-reduction intervention. Youth & Society. 1998;29(4):431–450. doi: 10.1177/0044118x98029004002. [DOI] [PubMed] [Google Scholar]
- Gortmaker SL, Hughes M, Cervia J, Brady M, Johnson GM, Seage GR, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. New England Journal of Medicine. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- Halpern-Felsher BL, Cornell JL, Kropp RY, Tschann JM. Oral versus vaginal sex among adolescents: Perceptions, attitudes, and behavior. Pediatrics. 2005;115(4):845–851. doi: 10.1542/peds.2004-2108. [DOI] [PubMed] [Google Scholar]
- Havens JF, Mellins CA. Psychiatric Aspects of HIV/AIDS in childhood and adolescence. In: Rutter M, Taylor E, editors. Child and Adolescent Psychiatry: Modern Approaches. Fifth Edition. Oxford, UK: Blackwell; (In press). [Google Scholar]
- Hartup WW. Peer relations. In: Hetherington EM, editor. Handbook of Child Psychology. Vol. 4. New York: Wiley; 1993. pp. 103–196. [Google Scholar]
- Henry DB, Schoeny ME, Deptula DP, Slavick JT. Peer selection and socialization effects on adolescent intercourse without a condom and attitudes about the costs of sex. Child Development. 2007;78(3):825–838. doi: 10.1111/j.1467-8624.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Janz NK, Champion V, Strecher VJ. The Health Belief Model. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco: Jossey-Bass; 2002. pp. 45–66. [Google Scholar]
- Kadivar H, Garvie PA, Sinnock C, Heston JD, Flynn PM. Psychosocial profile of HIV-infected adolescents in a southern US urban cohort. AIDS Care. 2006;18(6):544–549. doi: 10.1080/13548500500228763. [DOI] [PubMed] [Google Scholar]
- Kang E, Mellins CA, Ng WYK, Ronbison L, Abrams EJ. Standing between two worlds in Harlem: A developmental psychopathology perspective of perinatally acquired human immunodeficiency virus and adolescence. Journal of Applied Developmental Psychology. 2008;29:277–237. [Google Scholar]
- Kay R, Little S. Transformations of the explanatory variables in the logistic regression model for binary data. Biometrika. 1987;74(3):495–501. [Google Scholar]
- Kinsman SB, Romer D, Furstenberg FF, Schwarz DF. Early sexual initiation: The role of peer norms. Pediatrics. 1998;102(5):1185–1192. doi: 10.1542/peds.102.5.1185. [DOI] [PubMed] [Google Scholar]
- Kirby D. Effective approaches to reducing adolescent unprotected sex, pregnancy, and childbearing. The Journal of Sex Research. 2002;39(1):51–58. doi: 10.1080/00224490209552120. [DOI] [PubMed] [Google Scholar]
- Knight WG, Mellins CA, Levenson RL, Arpadi SM, Kairam R. Brief report: Effects of pediatric HIV infection on mental and psychomotor development. Journal of Pediatric Psychology. 2000;25(8):583–587. doi: 10.1093/jpepsy/25.8.583. [DOI] [PubMed] [Google Scholar]
- Koenig LJ, Espinoza L, Hodge K, Ruffo N. Young, seropositive, and pregnant: Epidemiologic and psychosocial perspectives on pregnant adolescents with human immunodeficiency virus infection. American Journal of Obstetrics & Gynecology, Suppliment. 2007:S123–S131. doi: 10.1016/j.ajog.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lewis MA, DeVellis BM, Sleath B. Social Influence and Interpersonal Communication in Health Behavior. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco: Jossey-Bass; 2002. pp. 240–264. [Google Scholar]
- Lightfoot M, Swendeman D, Rotherum-Borus MJ, Comulada WS, Weiss R. Risk behaviors of youth living with HIV: Pre-and Post-HAART. American Journal of Health Behavior. 2005;29(2):162–171. doi: 10.5993/ajhb.29.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow RM, Kershaw T, Sipsma H, Rosenberg R, Dévieux JG. HIV preventive interventions for adolescents: A look back and ahead. Current HIV/AIDS Reports. 2007;4:173–180. doi: 10.1007/s11904-007-0025-6. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Smith R, O'Driscoll P, Magder LS, Brouwers P, Chase C, et al. High rates of behavioral problems in perinatally HIV-infected children are not linked to HIV disease. Pediatrics. 2003;111(2):384–393. doi: 10.1542/peds.111.2.384. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg, HFL., Ehrhardt, AA., Exner, TM., Gruen, RS., Dugan, T.: Sexual Risk Behavior Assessment Schedule - Youth, Depressed Females, Baseline (SERBAS-Y-DEPR-F-1), 1995 edition.
- Miller B, Christopherson C, King P. Sexual behavior in adolescence. In: Gullotta TP, Adams GR, Montemayor R, editors. Adolescent sexuality. Newbury Park, CA: Sage Publications; 1993. pp. 57–76. [Google Scholar]
- Montaño DE, Kasprzyk D. The Theory of Reasoned Action and the Theory of Planned Behavior. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco: Jossey-Bass; 2002. pp. 67–98. [Google Scholar]
- Murphy DA, Durako SJ, Moscicki AB, Vermund SH, Ma Y, Schwartz DF, et al. No change in high-risk behavior over time among HIV-infected adolescents in care: Role of psychological distress. Journal of Adolescent Health. 2001;29:57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Wright K, Parsons JT, Frey M, Templin T, Ondersma S. Transtheoretical model and condom use in HIV-positive youths. Health Psychology. 2006;25(5):648–652. doi: 10.1037/0278-6133.25.5.648. [DOI] [PubMed] [Google Scholar]
- New York City Center for Economic Opportunity. Poverty in New York City. 2008 Accessed via the World Wide Web at: http://home2.nyc.gov/html/ceo/html/poverty/poverty_facts.html.
- New York City Department of Health and Mental Hygiene. Pediatric and Adolescent HIV/AIDS Surveillance Update New York City: Semiannual Report, December 2007. New York City: Department of Health and Mental Hygiene; 2007. [Google Scholar]
- O'Donnell L, Myint-U A, O'Donnell C, Stueve A. Long-term influence of sexual norms and attitudes on timing of sexual initiation among urban minority youth. The Journal of School Health. 2003;73(2):68–75. doi: 10.1111/j.1746-1561.2003.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Pedlow CT, Carey MP. Developmentally appropriate sexual risk reduction interventions for adolescents: Rationale, review of interventions, and recommendations for research and practice. Annals of Behavioral Medicine. 2004;27(3):172–184. doi: 10.1207/s15324796abm2703_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Black M, Izabel R, Feigelman S, Kaljee L, Galbraith J, et al. Social influences on the sexual behavior of youth at risk for HIV exposure. American Journal of Public Health. 1994;84(6):977–985. doi: 10.2105/ajph.84.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar LF, Crosby RA, DiClemente RJ, Wingood GM, Lescano CM, Brown LK, Harrington J, Davies S. Self-esteem and theoretical mediators of safer sex among African American female adolescents: Implications for sexual risk reduction interventions. Health Education & Behavior. 2005;32(3):413–427. doi: 10.1177/1090198104272335. [DOI] [PubMed] [Google Scholar]
- Sieving RE, Eisenberg ME, Pettingell S, Skay C. Friends' influence on adolescents' first sexual intercourse. Perspectives of Sexual and Reproductive Health. 2006;38(1):13–19. doi: 10.1363/psrh.38.013.06. [DOI] [PubMed] [Google Scholar]
- Silver EJ, Bauman LJ. The association of sexual experience with attitudes, beliefs, and risk behaviors in inner-city adolescents. Journal of Research on Adolescence. 2006;16(1):29–45. [Google Scholar]
- Tolman DL, Striepe MI, Harmon T. Gender matters: Constructing a model of adolescent sexual health. Journal of Sex Research. 2003;40(1):4–12. doi: 10.1080/00224490309552162. [DOI] [PubMed] [Google Scholar]
- UNAIDS. AIDS Epidemic Update – December 2007. Geneva: UNAIDS/WHO; 2008. [Google Scholar]
- Woods ER, Samples CL, Melchiono MW, Harris SK. Boston HAPPENS Program: HIV-positive, homeless, and at-risk youth can access care through youth-oriented HIV services. Semin Pediatr Infect Dis. 2003;14(1):43–53. doi: 10.1053/spid.2003.127217. [DOI] [PubMed] [Google Scholar]