Abstract
Fabry disease (α-galactosidase A (α-Gal A, GLA) deficiency) is a panethnic inborn error of glycosphingolipid metabolism. Since optimal therapeutic outcomes depend on early intervention, a pilot program was designed to assess newborn screening for this disease in 171,977 consecutive Taiwanese newborns by measuring their dry blood spot (DBS) α-Gal A activities and β-galactosidase/α-Gal A ratios. Of the 90,288 male screenees, 638 (0.7%) had DBS α-Gal A activity <30% of normal mean and/or activity ratios >10. A second DBS assay reduced these to 91 (0.1%). Of these, 11 (including twins) had <5% (Group-A), 64 had 5–30% (Group-B), and 11 had >30% (Group-C) of mean normal leukocyte α-Gal A activity. All 11 Group-A, 61 Group-B, and 1 Group-C males had GLA gene mutations. Surprisingly, 86% had the later-onset cryptic splice mutation c.936+919G>A (also called IVS4+919G>A). In contrast, screening 81,689 females detected two heterozygotes. The novel mutations were expressed in vitro, predicting their classical or later-onset phenotypes. Newborn screening identified a surprisingly high frequency of Taiwanese males with Fabry disease (~1 in 1,250), 86% having the IVS4+919G>A mutation previously found in later-onset cardiac phenotype patients. Further studies of the IVS4 later-onset phenotype will determine its natural history and optimal timing for therapeutic intervention.
Keywords: Fabry disease, α-galactosidase A deficiency, newborn screening, GLA
Introduction
Fabry disease (MIM# 301500) is an X-linked lysosomal storage disorder resulting from the deficient activity of the lysosomal glycohydrolase α-galactosidase A (α-Gal A) [Desnick et al., 2001]. The enzymatic defect leads to progressive accumulation of globotriaosylceramide (GL-3) and related glycosphingolipids primarily in the vascular endothelium of the skin, kidney, heart, and brain. Affected males who have little or no α-Gal A activity exhibit the classic phenotype with onset of angiokeratomas, acroparesthesias, and hypohidrosis in childhood [Desnick et al., 2004]. With advancing age, the occurrence of renal failure, cardiac disease and stroke lead to premature death. The clinical manifestations in heterozygous women with mutations causing the classic phenotype range from asymptomatic to as severe as those in affected males. To date, over 500 disease-causing mutations in the α-Gal A gene (GLA; MIM# 300644) have been identified [Stenson et al., 2003]. The mutations include missense, nonsense, various frame-shift lesions (small deletions, insertions, and rearrangements), and splicing mutations [Desnick et al., 2001]. Most mutations are “private,” occurring in one or a few affected families.
The incidence of classic Fabry disease was initially estimated to be 1 in 40,000 to 60,000 males [Meikle et al., 1999; Desnick et al., 2001]. However, during the past decade the clinical spectrum of Fabry disease expanded from the classic phenotype to include the later-onset phenotype detected in males with renal, cardiac, and cerebrovascular disease [von Scheidt et al., 1991; Desnick et al., 2001; Nakao et al., 1995; Nakao et al., 2003; Rolfs et al., 2005]. Screening by plasma α-Gal A activity identified previously undiagnosed Fabry patients in 0.25% to 1% of males undergoing hemodialysis [Nakao et al., 2003; Kotanko et al., 2004; Tanaka et al., 2005;], 3% to 4% of males with left ventricular hypertrophy or hypertrophic cardiomyopathy [Nakao et al., 1995; Sachdev et al., 2002; Monserrat et al., 2007], and up to 4.9% of males with acute cryptogenic strokes [Rolfs et al., 2005]. In a recent study, newborn screening of over 37,000 consecutive males in Italy identified 12 infants with α-Gal A mutations including 11 who had lesions that expressed residual activity consistent with the later-onset phenotype [Spada et al., 2006]. In the Italian study, the frequencies of newborns predicted to have the classic and later-onset phenotypes were ~1 in 37,000 and 1 in 3,373 males, respectively, while the overall frequency of Fabry disease was ~1 in 3,100 Caucasian males.
Recently, recombinant α-Gal A replacement therapy has been shown to clear microvascular endothelial deposits of GL-3 from the kidneys, heart, and skin in patients with Fabry disease, reversing the primary pathogenesis of the clinical manifestations of this disease [Eng et al., 2001; Wilcox et al., 2004; Banikazemi et al., 2007; Germain, 2007]. These studies revealed that early treatment, before significant renal impairment (glomerular filtration rate <60 ml/min) [Wilcox et al., 2004; Banikazemi et al., 2007; Germain, 2007] or cardiac disease [evidence of late enhancement on magnetic resonance imaging (MRI)] [Moon et al., 2003; Weidemann et al., 2003], is important for optimal therapeutic outcomes. Although early treatment is critical in Fabry disease to preserve organ function, many patients are diagnosed late after significant disease progression, due to the non-specific nature of the clinical manifestations early in the disease [Desnick et al., 2003; Eng et al., 2006]. Common Fabry symptoms in childhood, such as gastrointestinal problems, acroparesthesias, and hypohidrosis, can easily be attributed to other etiologies or even be regarded as psychological problems [Desnick et al., 2003]. Additionally, the early symptoms of the classic phenotype are usually absent in patients with the later-onset phenotype [von Scheidt et al., 1991; Desnick et al., 2001; Nakao et al., 1995; Nakao et al., 2003; Rolfs et al., 2005]. Even when diagnosed later in life, enzyme replacement therapy has been shown to slow the disease progression [Banikazemi et al., 2007].
To determinate the frequency of the disease in an Asian population, newborn screening was undertaken in over 170,000 consecutive male and female Taiwanese babies. We identified newborns carrying both reported and novel α-Gal A gene mutations. By evaluation of at-risk consenting family members, previously undiagnosed individuals affected by Fabry disease were identified. Of note, a high frequency (~1 in 1,410) of the g.9331G>A (c.936+919G>A; often called IVS4+919G>A) cryptic splice mutation, previously reported in Japanese cardiac variants [Ishii et al., 2002], was identified in the Taiwanese newborn males.
Materials and methods
Newborn screening
For 24 months (July, 2006 through June, 2008), 171,977 consecutive male and female newborns were screened for α-Gal A deficiency on day 3 of life. This pilot program was conducted by the Newborn Screening Center at the National Taiwan University Hospital (NTUH), which screens approximately 40% of all newborns in Taiwan. The dried blood spots (DBS) used in this study were punched from routine newborn filter cards. This study was approved by the Institutional Review Board of the National Taiwan University Hospital, and informed consent was obtained from the parents of participating newborns.
Screening Assay on DBS
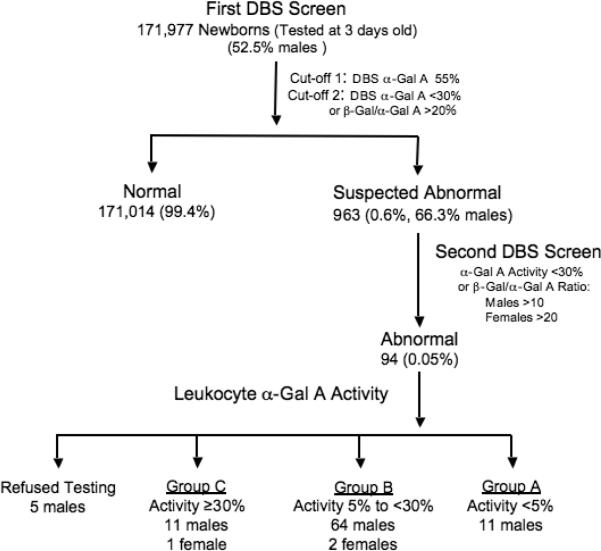
To determine α-Gal A activities in the DBS, assays were carried out essentially as previously described for total α-Gal A activity [Chamoles et al., 2001], and for β-galactosidase (β-Gal) activity [Lukacs et al., 2005] which served as a control enzyme. A two-tiered format was employed (Figure 1): samples which had α-Gal A activities <55% of the normal mean (5.87 μmol/L whole blood/h, SD=3.04, range 0.6 – 50.97 μmol/L/h, n=6947) were analyzed again for both α-Gal A and β-Gal activities and an β-Gal/α-Gal A activity ratio was determined (normal mean ratio = 5.01 ± 1.56, range 1.21–11.30, n=606). For samples with α-Gal A activities <30% and/or a ratio >10, a second newborn filter card was requested. If the second DBS had an α-Gal A activity <30% and/or a ratio >10 in males, or an α-Gal A activity <30% and a ratio >20 in females, the respective newborn's parents would be asked for a blood sample from the infant to determine the leukocyte α-Gal A activity. The cut-offs for females were stricter since it is more difficult to diagnose Fabry disease by enzyme assay in women due to random X-inactivation [Dobrovolny et al., 2005; Maier et al., 2006].
Figure 1.
Scheme used to identify and confirm α-Gal A deficiency in newborns. Note cut-offs for male (M) and female (F) newborns differ. The number and percentage of newborns detected under each category are indicated.
Confirmatory α-Gal A Assay in Isolated Leukocytes
Leukocyte α-Gal A activities were determined with the fluorogenic substrate, 4-methylumbelliferyl-α-D-galactopyranoside (Sigma-Aldrich, MO, USA) in the presence of α-N-acetyl-D-galactosamine (Sigma-Aldrich, MO, USA) to inhibit α-N-acetylgalactosaminidase activity [Mayes et al., 1981]. The diagnosis of Fabry disease was based on a leukocyte α-Gal A activity ≤ 4.76 nmol/hr/mg protein (5% of normal mean; normal mean ± SD = 95.1 ± 30.3 nmol/hr/mg protein; n=40).
For newborns with low leukocyte α-Gal A activities, family pedigrees were obtained, and at-risk family members were contacted, counseled and asked to provide blood samples for diagnostic studies, with informed consent. Genomic DNAs were isolated from whole blood, and each of the seven exons and their flanking intronic sequences of GLA gene (NC_000023.9; GI:89161218) were amplified as previously described [Shabbeer et al., 2006] (protocol available on request). Each amplicon was then sequenced with an ABI Prism 3700 Capillary Array Sequencer, using the ABI Prism BigDye Terminator Ready Reaction Mix (Perkin-Elmer-Cetus). Mutations were confirmed by repeat amplification and sequencing from the opposite strand and by cosegregation of the lesion and disease in other family members. The cDNA (NM_000169.1) was numbered with +1 corresponding to the A of the ATG translation initiation codon (www.hgvs.org/mutnomen). The initiation codon is codon 1. The frequency of the IVS4 cryptic splicing mutation in the Taiwanese population was estimated by amplification of intron 4 and BfaI cleavage of genomic DNAs isolated from 428 normal X-chromosomes from unrelated individuals (130 males and 149 females) [Ishii et al., 2002], but no IVS4+919G>A allele was detected. Urinary GL-3 was measured to determine the relative amount of storage material [Kitagawa et al., 2005].
Mutagenesis and in vitro expression
Site-specific mutagenesis of the wild-type α-Gal A cDNA was performed as previously described [Yasuda et al., 2003]. Each mutated cDNA was subcloned into pcDNA3.1/myc-his vector (Invitrogen, CA, USA), and the expression constructs were confirmed by sequencing. Eight GLA missense mutations and one in-frame three base deletion identified in the newborns, as well as the wild-type GLA cDNA, were expressed in vitro. Plasmid DNA (0.5 μg) was transfected into COS-1 cells grown in 6 cm dishes with Transfectamine-2000 (Invitrogen, CA, USA). Cells were harvested 40 hr after transfection and the α-Gal A activities were determined as described above for leukocytes.
To determine the stability of the expressed α-Gal A wild-type and mutant enzymes, the COS-1 cells were lysed and the expressed human α-Gal A activities in the cell lysates were incubated at pH 4.6 and 37°C and aliquots were removed at intervals over a 180 minute period for assay as described above. For each expressed enzyme activity, the stability profile was fitted for first order exponential decay kinetics [Desnick et al., 2001], and the half-lives were determined using the data analysis software of the OriginPro Program v.8 (Northampton, MA, USA).
Results
Newborn Screening
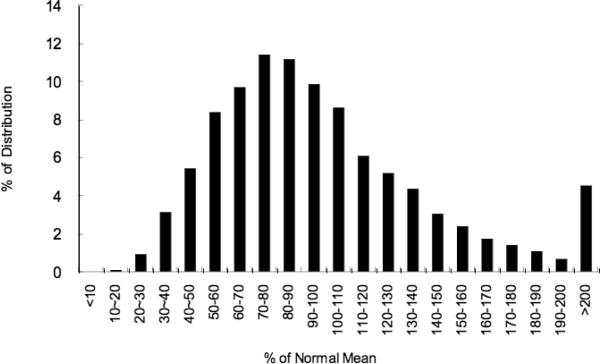
Normal DBS α-Gal A activity was determined in 6,947 consecutive newborns (52.5% males). The mean and median α-Gal A activities were 5.87 and 5.26 μmol/hr/L whole blood. DBS enzyme activities were normally distributed from 10.2% to 560% of the mean (Figure 2). The mean values of DBS α-Gal A activities for normal males and females were not statistically different.
Figure 2.
Distribution of α-Gal A activity in 606 normal newborns. The X- and Y-axes indicate the percent (%) of normal mean and the percent (%) of the distribution for each activity percentage, respectively.
Among the 171,977 consecutive male and female newborns screened, 963 (0.63%), including 325 females, had <30% of the mean normal DBS α-Gal A activity and/or a β-Gal/α-Gal A ratio >10, and were recalled for a second DBS sample. Of these, 94, including three females, (0.055% of the total population) were recalled to determinate their leukocyte α-Gal A activities (Figure 1). As indicated in Table 1, 11 males of the 89 newborns who provided samples had leukocyte α-Gal A activities <5% of the mean normal activity (Group-A), 64 males and two females had activities between 5 and 30% (Group-B), and 11 males and one female had activities >30% of mean normal activities (Group-C).
Table 1.
Baseline characteristics for α-Gal A mutation-confirmed newborns grouped by leukocyte α-Gal A activity2
| Groupa | Proband No |
Sex | 1st spot α-Gal Activity |
1st spot β-Gal/ α-Gal A Ratio |
Leukocyte α-Gal A Activity |
GLA Mutation | Predicted effect | Urine GL-3 (μg/mg creatinine) |
|---|---|---|---|---|---|---|---|---|
| Aa | 1 | M | 5.13% | 169.0 | 1.07% | c.277G>A | p.D93N | 74.0 |
| 2 | M | 11.5% | 40.6 | 3.55% | g.9331G>Ab | c | - | |
| 3 | M | 27.1% | 9.6 | 4.03% | g.9331G>A | - | ||
| 4 | M | 19.3% | 19.2 | 4.67% | g.9331G>A | - | ||
| 5 | M | 1.87% | 284.0 | 3.26% | c.34_42del | p.C12_L14del | 33.7 | |
| 6 | M | 17.4% | 48.4 | 3.05% | c.1078G>T | p.G360C | 11.7 | |
| 7 | M | 5.75% | 62.4 | 3.46% | c.1078G>T | p.G360C | - | |
| 8 | M | 4.20% | 61.4 | 3.63% | c.1078G>T | p.G360C | - | |
| 9 | M | 12.3% | 63.8 | 3.57% | c.137A>C | p.H46P | - | |
| 10 | M | 7.15% | 135.0 | 4.42% | c.358C>G | p.L120V | 11.0 | |
| 11 | M | 45.6% | 99.7 | 4.47% | c.656T>C | p.I219T | 19.2 | |
|
| ||||||||
| Ba | 12 | M | 16.0% | 34.3 | 6.22% | g.9331G>A | - | |
| 13 | M | 12.7% | 10.9 | 7.15% | g.9331G>A | - | ||
| 14 | M | 9.02% | 35.1 | 7.23% | g.9331G>A | - | ||
| 15 | M | 12.9% | 17.0 | 7.44% | g.9331G>A | - | ||
| 16 | M | 23.3% | 32.5 | 7.90% | g.9331G>A | 1.59 | ||
| 17 | M | 25.0% | 20.3 | 8.45% | g.9331G>A | - | ||
| 18 | M | 19.3% | 20.7 | 8.74% | g.9331G>A | - | ||
| 19 | M | 41.7% | 28.9 | 8.75% | g.9331G>A | - | ||
| 20 | M | 10.9% | 34.2 | 9.07% | g.9331G>A | 2.62 | ||
| 21 | F | 11.7% | 35.9 | 9.20% | g.9331G>A heterozygote | - | ||
| 22 | M | 18.8% | 24.9 | 9.40% | g.9331G>A | - | ||
| 23 | M | 22.9% | 26.5 | 9.49% | g.9331G>A | 0.55 | ||
| 24 | M | 18.8% | 46.9 | 9.63% | g.9331G>A | - | ||
| 25 | M | 23.2% | 25.0 | 9.69% | g.9331G>A | 3.33 | ||
| 26 | M | 8.55% | 22.6 | 9.71% | g.9331G>A | 7.38 | ||
| 27 | M | 13.5% | 18.8 | 9.82% | g.9331G>A | - | ||
| 28 | M | 11.7% | 19.8 | 10.3% | g.9331G>A | 2.12 | ||
| 29 | M | 16.8% | 36.7 | 10.4% | g.9331G>A | 3.85 | ||
| 30 | M | 35.0% | 35.7 | 10.5% | g.9331G>A | - | ||
| 31 | M | 6.38% | 39.5 | 10.9% | g.9331G>A | 1.32 | ||
| 32 | M | 43.7% | 34.9 | 11.3% | g.9331G>A | 9.25 | ||
| 33 | M | 45.7% | 30.9 | 11.4% | g.9331G>A | - | ||
| 34 | M | 16.3% | 23.4 | 11.5% | g.9331G>A | - | ||
| 35 | M | 27.7% | 12.8 | 11.6% | g.9331G>A | 1.77 | ||
| 36 | M | 11.7% | 14.0 | 11.7% | g.9331G>A | - | ||
| 37 | M | 16.9% | 10.0 | 11.9% | g.9331G>A | - | ||
| 38 | M | 25.0% | 27.0 | 12.1% | g.9331G>A | 3.69 | ||
| 39 | M | 37.9% | 31.2 | 12.2% | g.9331G>A | 1.45 | ||
| 40 | M | 14.9% | 37.2 | 12.3% | g.9331G>A | - | ||
| 41 | M | 37.3% | 6.14 | 12.3% | g.9331G>A | - | ||
| 42 | M | 24.7% | 39.6 | 13.0% | g.9331G>A | - | ||
| 43 | M | 21.5% | 40.7 | 13.0% | g.9331G>A | 3.79 | ||
| 44 | M | 19.6% | 42.6 | 13.0% | g.9331G>A | - | ||
| 45 | M | 49.0% | 21.7 | 13.2% | g.9331G>A | 3.17 | ||
| 46 | M | 48.4% | 39.3 | 13.4% | g.9331G>A | - | ||
| 47 | M | 34.4% | 23.9 | 13.5% | g.9331G>A | - | ||
| 48 | M | 30.5% | 27.2 | 13.7% | g.9331G>A | - | ||
| 49 | M | 16.3% | 22.6 | 13.9% | g.9331G>A | - | ||
| 50 | M | 19.4% | 25.8 | 14.1% | g.9331G>A | - | ||
| 51 | M | 19.1% | 26.5 | 14.3% | g.9331G>A | - | ||
| 52 | M | 4.51% | 76.4 | 14.6% | g.9331G>A | - | ||
| 53 | M | 21.8% | 15.0 | 14.7% | g.9331G>A | - | ||
| 54 | M | 20.8% | 42.5 | 14.8% | g.9331G>A | 0.99 | ||
| 55 | M | 15.9% | 38.3 | 14.9% | g.9331G>A | - | ||
| 56 | M | 20.5% | 27.2 | 15.8% | g.9331G>A | 5.35 | ||
| 57 | M | 35.3% | 15.7 | 16.1% | g.9331G>A | 5.42 | ||
| 58 | M | 19.0% | 32.5 | 17.7% | g.9331G>A | 3.75 | ||
| 59 | M | 23.2% | 17.5 | 18.2% | g.9331G>A | - | ||
| 60 | M | 17.4% | 37.8 | 18.6% | g.9331G>A | - | ||
| 61 | M | 34.5% | 30.9 | 18.9% | g.9331G>A | - | ||
| 62 | M | 23.5% | 13.9 | 19.1% | g.9331G>A | - | ||
| 63 | M | 41.2% | 21.8 | 19.8% | g.9331G>A | 4.56 | ||
| 64 | M | 16.9% | 38.0 | 20.4% | g.9331G>A | - | ||
| 65 | M | 14.0% | 35.5 | 21.0% | g.9331G>A | - | ||
| 66 | M | 49.5% | 29.7 | 21.8% | g.9331G>A | 3.02 | ||
| 67 | M | 33.3% | 50.5 | 22.3% | g.9331G>A | 3.1 | ||
| 68 | M | 21.5% | 41.5 | 22.3% | g.9331G>A | - | ||
| 69 | M | 23.6% | 50.5 | 22.4% | g.9331G>A | - | ||
| 70 | M | 14.0% | 21.2 | 24.5% | g.9331G>A | - | ||
| 71 | M | 22.7% | 33.4 | 28.5% | g.9331G>A | 0.92 | ||
| 72 | M | 17.1% | 51.5 | 12.5% | c.1067G>A | p.R356Q | - | |
| 73 | M | 18.2% | 43.8 | 19.1% | c.1067G>A | p.R356Q | - | |
| 74 | F | 21.8% | 20.7 | 11.9% | c.1078G>T heterozygote | p.G360C heterozygote | - | |
| M | 35.9% | 31.0 | 11.5% | No mutation | - | |||
| M | 12.1% | 30.4 | 14.7% | No mutation | - | |||
| M | 23.5% | 13.9 | 16.2% | No mutation | - | |||
|
| ||||||||
| Ca | 75 | M | 43.5% | 10.6 | 56.9% | c.196G>C | p.E66Q | - |
|
| ||||||||
| F | 35.8% | 13.1 | 76.6% | No mutation | ||||
|
| ||||||||
| Normal Range | 5.87±3.04 (mmol/L/h) | 5.01±1.56 | 95.13±30.3 (nmol/mg/h) | 0.17±0.13 (μg/mg Creatinine) | ||||
Group-A and -B newborns had <5 and 5 to 30% of normal mean leukocyte α-Gal A activity, respectively. Group-C newborns had >30% of normal mean leukocyte α-Gal A activity, and/or β-Gal A/α-Gal A ratio >10 for males, >20 for females.
g.9331G>A = c.639+919G>A = IVS4+919G>A; a previously reported cryptic splice variant [Ishii et al., 2002].
Alternative splicing due to the IVS4+919G>A mutation results in a markedly reduced level of the normal α-Gal A glycoprotein.
Genomic mutation nomenclature is based on GenBank NC_000023.9.
cDNA mutation nomenclature is based on GenBank NM_000169.1 with +1 corresponding to the A of the ATG translation initiation codon (www.hgvs.org/mutnomen). The initiation codon is codon 1.
Mutation analysis and in vitro expression
Mutation analysis was performed to identify which newborns with decreased α-Gal A activities had gene mutations. All 11 Group-A newborns, who had <5% of normal mean leukocyte α-Gal A activity, were males who had α-Gal A mutations (Table 1). Proband 1 had the previously reported c.277G>A (p.D93N) mutation found in patients with the classical phenotype [Ishii et al., 2002; Lukacs et al., 2005]. Probands 2, 3, and 4 had the cryptic splicing mutation IVS4+919G>A previously described in Japanese patients with the later-onset cardiac phenotype [Ishii et al., 2002]. The other seven male newborns had novel mutations including an in-frame 9 bp deletion [c.34_42del (p.C12_L14del)] in the enzyme's leader sequence (Proband 5), and novel missense mutations c.1078G>T (p.G360C) in Proband 6 and twin Probands 7 and 8, c.137A>C (p.H46P) in Proband 9, c.358C>G (p.L120V) in Proband 10, and c.656T>C (p.I219T) in Proband 11.
In the 66 Group-B newborns (64 males, 2 females) who had α-Gal A activities between 5 and 30% of mean normal leukocyte activity, 59 males and one female had the IVS4+919G>A mutation (Probands 12–71), two males (Probands 72 and 73) had the novel c.1067G>A (p.R356Q) mutation, and one female (Proband 74) was heterozygous for the c.1078G>T (p.G360C) lesion. No mutation was detected in three males who had α-Gal A leukocyte activities of 11.5 to 16.2% of normal mean leukocyte α-Gal A activity.
Among the 12 Group-C newborns (11 males, 1 female) with leukocyte α-Gal A activities higher than 30% of normal mean, and/or a β-Gal/α-Gal A ratio >10 for males and >20 for females, there was only one male (Proband 75) who had a leukocyte α-Gal A activity of 56.9%, but had a β-Gal/ α-Gal A ratio of 10.6. He was found to have a previously reported mutation, c.196G>C (p.E66Q), which was identified in patients with later-onset renal disease [Ishii et al., 1992].
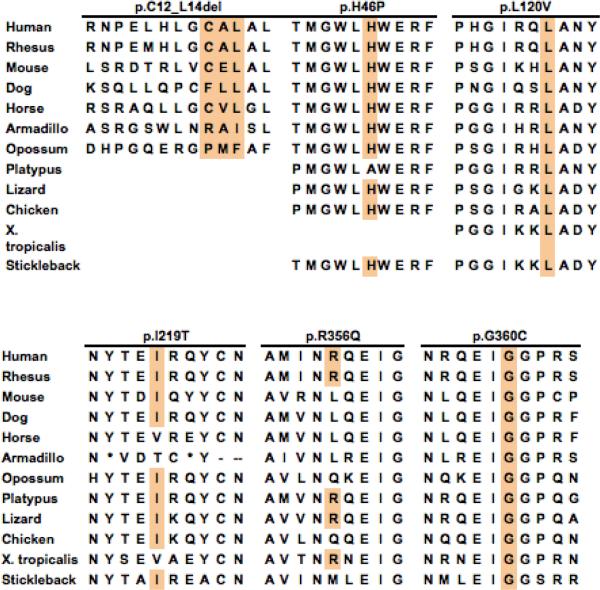
All five novel missense mutations predicted amino acid substitutions (p.H46P, p.L120V, p.I219T, p.R356Q, and p.G360C) that occurred at highly conserved residues in the α-Gal A protein (Figure 3), and none was detected in 400 X-chromosomes from normal Taiwanese individuals. The c.34_42del (p.C12_L14del) mutant enzyme was an in-frame deletion of three amino acid residues that occurred in the leader sequence, but had markedly reduced α-Gal A activity. In vitro expression in COS-1 cells revealed that mutant enzymes p.C12_L14del and p.G360C had very low α-Gal A activities (4% and 6% of mean wild-type expressed activity) (Table 2) predicting that these mutations cause the classic phenotype. In contrast, mutant enzymes p.H46P, pE66Q, p.L120V, p.I219T, and p.R356Q had significant residual activities in the COS-1 cells, 36%, 52%, 42%, 46%, and 15% of expressed mean wild-type activity, respectively. Compared to the half-life (4.3 hr) of the expressed wild-type activity at 37°C and pH 4.6, mutant enzymes p.H46P, p.E66Q, p.L120V, p.I219T, and p.R356Q had half-lives of 1.5, 0.7, 1.9, 8.0, and 1.5 hr, respectively (Table 2).
Figure 3.
Phylogenetic conservation of wild-type human α-Gal A amino acid residues, highlighting the amino acids substituted in mutant enzymes p.C12_L14del, p.H46P, p.L120V, p.I219T, p.R356Q, and p.G360C due to the novel missense mutations identified in the newborns.
Table 2.
In vitro expression and stability of wild-type and mutant α-Gal A constructs
| Expressed Mutant Enzymes | Activity % of WT | Stability at pH 4.6b | |
|---|---|---|---|
| 1hr | 3hr | ||
| WT | 100% | 90% | 61% |
| p.H46P | 36% | 65% | 23% |
| p.E66Q | 52% | 48% | 13% |
| p.L120V | 42% | 73% | 34% |
| p.I219T | 46% | 94% | 77% |
| p.R356Q | 15% | 65% | 23% |
| p.F383La | 35% | 98% | 78% |
| p.G360C | 6% | 66% | 13% |
| p.D93Na | 0% | - | - |
| p. C12_L14del | 4% | - | - |
Novel mutations in bold type
p.D93N and p.F383L mutant proteins were previously reported [Stenson et al., 2003]
Percent of initial activity
In sum, 72 male (not counting an affected twin) and two female newborns had low α-Gal A enzymatic activities and α-Gal A mutations, an overall frequency of ~1 in 1,250 males and ~1 in 40,840 females. Four males (counting twin Probands 7 and 8 as 1), who had the predicted mutant proteins, p.D93N, p.G360C, and p.C12_L14del, that expressed very low α-Gal A activities, were predicted to have the classic phenotype, a frequency of about 1 in 22,570 newborn males. The six males with later-onset missense mutations and the 59 male newborns with the IVS4 cryptic splicing mutation gave an estimated frequency of the later-onset phenotype of ~1 in 1,390 male newborns. The IVS4 cryptic splicing mutation was detected in ~1 in 1,460 male newborns.
Family studies
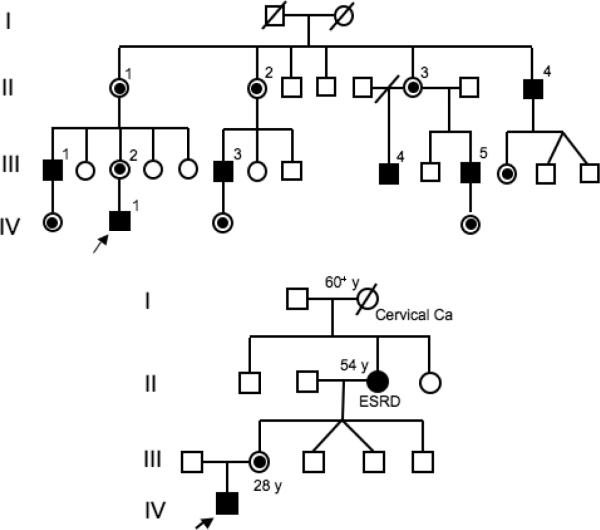
Although privacy is highly regarded in Taiwanese culture, medical privacy is even more respected. When asked for information about family members, only three families would provide information for publication, two with classic mutations and one with a later-onset lesion. All three families had previously undiagnosed symptomatic family members. Proband 1, who had the c.227G>A (p.D93N) classic phenotype mutation, had a large number of maternal relatives, including five males who had histories of acroparesthesias and hypohidrosis, but had not been previously diagnosed as having Fabry disease. As shown in Figure 4 (upper panel), III-1, a 39 year old male, had a history of severe acroparesthesias since early childhood which lead to school problems due to frequent long absences for unexplained pain episodes. He had a stroke at age 35 and was hemiparetic for two years. Brain MRI revealed old infarctions. He had difficulty ambulating, had bilateral ventricular hypertrophy on echocardiography, and renal involvement with a decreased creatinine clearance (79 ml/min/1.73m2) and proteinuria (1.3 g/day). A renal biopsy revealed the typical Fabry pathology. III-3, a 39 year old uncle, had a similar history of acroparesthesias, hypohidrosis and heat intolerance during childhood. Angiokeratoma were present in the typical distribution. He had microalbuminuria (microalbumin/creatinine = 0.05) and a renal biopsy revealed the typical pathology.
Figure 4.
Pedigrees of two Fabry families detected by newborn screening: a) Proband 1, affected males and obligate female heterozygotes are indicated, and b) Proband 11, note that the maternal grandmother had end stage renal disease (ESRD).
The grandmother of Proband 11, who had the c.656T>C (p.I219T) mutation, was a 54 year old heterozygote who had been on hemodialysis for end-stage renal disease for two years (Figure 4, lower panel). Proband 74, a newborn female with 11.9% of normal mean leukocyte α-Gal A activity, inherited the mutation from her 33 year old father who had markedly decreased (2.7% of normal mean) leukocyte α-Gal A activity, a normal EKG, and no proteinuria.
Biochemical identification of Classic and Later-Onset Phenotypes
The elevated β-Gal A/ α-Gal A ratios in newborn males with mutations predicting the classic phenotype ranged from 48.4 to 284, whereas the missense mutations and IVS4 splice mutation predicting the later-onset phenotype had ratios ranging from 10.6 to 135 and 6.14 to 76.4, respectively [data not shown]. Thus, the ratios overlapped between the two phenotypes. Urinary GL-3 levels were measured in five Group-A patients who provided specimens (Table 1, 3 classic and 2 later-onset mutations). Compared to the mean and range from 30 normal newborns (0.17 ± 0.13 μg/mg creatinine), all five newborns had elevated GL-3 levels. The three newborns predicted to have the classic phenotype had GL-3 levels of 11, 33.7 and 74 μg/mg creatinine, which could not be discriminated from those of the two infants predicted to have the later-onset phenotype who had levels of 11 and 19 μg/mg creatinine.
Discussion
Newborn screening provides the opportunity to determine the incidence of an inherited disease, to prospectively document the early disease manifestations, and to initiate therapy prior to irreversible organ damage. In 2006, Spada and co-workers reported the screening of 37,104 consecutive Italian newborn males for Fabry disease by determining their DBS α-Gal A activities and found that 12 neonates, or 1 in 3,100 males, had deficient α-Gal A enzyme activities and confirmed α-Gal A gene mutations [Spada et al., 2006]. Mutation analysis predicted that one of the newborns had the classic phenotype (1 in ~37,000), whereas 11 of the newborns were predicted to have the later-onset phenotype (1 in ~3,400).
Screening of 1 7 1,977 consecutive Taiwanese newborns for Fabry disease by determination of their DBS α-Gal A activities detected a total of 75 newborns, including two females, who had low leukocyte α-Gal A activities and α-Gal A mutations (Table 1). Of the 90,288 male newborns screened, 72 (considering the affected twins as 1) had α-Gal A mutations for a frequency of ~1 in 1,250, about 2.5 times more frequent than that found in the Italian newborns [Spada et al., 2006]. Six novel α-Gal A mutations were detected among these newborns. The five novel missense mutations and the in-frame deletion of three amino acids in the leader sequence occurred in conserved amino acid residues (Figure 3) and were absent in over 400 normal control alleles. In vitro expression and enzyme stability studies (Table 2) revealed that the previously reported mutant enzyme p.D93N and novel mutant enzymes p.G360C and p.C12_L14del had very low α-Gal A activities, predicting the classic phenotype. The other two previously reported mutations, c.196G>C (p.E66Q) and IVS4+919G>A, and four novel mutations, c.137A>C (p.H46P), c.358C>G (p.L120V), c.656T>C (p.I219T) and c.1067G>A (p.R356Q), had residual activities and stabilities that predicted the later-onset phenotype.
Of particular note, was the surprisingly high prevalence (~1 in 1,460 males) of the IVS4+919G>A splicing mutation in the Taiwanese newborns. This mutation was first discovered in Japanese patients with the later-onset cardiac phenotype who had ~10% residual α-Gal A activity in lymphoblasts [Ishii et al., 2002]. The G to A transversion enhanced the percent expression of an alternatively spliced α-Gal A variant that was normally expressed at low levels, and included a 57-nucleotide intronic sequence that caused a frameshift mutation, resulting in a truncated enzyme polypeptide of 222 amino acid residues that had no detectable enzymatic activity. The amount of the wild-type transcript was markedly reduced to <10% of the wild-type expression. In the study by Ishii and colleagues, 1,603 consecutive male patients with non-specific cardiac symptoms were screened and the 230 who had left ventricular hypertrophy had their plasma α-Gal A activities determined. Seven (3%) had markedly decreased plasma α-Gal A activities and five of the seven had the IVS4+919G>A mutation [Ishii et al., 2002]. Since the entire intronic regions are not routinely evaluated when sequencing α-Gal A, the occurrence of the IVS4+919G>A and other intronic disease-causing lesions may be underestimated. The relatives in the newborn's families and other patients with the IVS4 mutation should be clinically followed in order to document the severity and variability of this later-onset genotype. A recent study suggested that the intronic lesion g.9273C>T also caused a similar splicing defect which resulted in clinical manifestations of Fabry disease [Filoni et al., 2008].
Thus, in Taiwan, the overall detection of mutation-confirmed males with Fabry disease was ~1 in 1,250 with frequencies of the classic and later-onset phenotypes of ~1 in 22,570 males and ~1 in 1,390 males. The disease was more frequent than in the Italian newborn study which detected an overall incidence of 1 in 3,080 males with predicted classic and later-onset frequencies of ~1 in 37,800 and ~1 in 3,400 males, respectively [Spada et al., 2006].
Notably, screening over 80,000 newborn females resulted in the detection of only two heterozygotes, one with a classic and the other with the IVS4 later-onset mutation. Based on the prevalence of the IVS4 mutation in newborn males, the sensitivity for detecting heterozygotes for this cryptic splicing lesion was unexpectedly low. These findings indicate that newborn screening for Fabry disease is markedly more effective and cost beneficial when screening is limited to male newborns. Although the natural history of the later-onset phenotype has not been systematically documented in female heterozygotes, most appear to have minimal life-threatening manifestations.
Recognizing the ethical and psychosocial issues raised by the identification of the later-onset variants among the newborn males, efforts were directed to predict the disease phenotype in the first DBS screening by determining the β-Gal/α-Gal A ratios and plotting them against the α-Gal A activity. In addition, the urinary GL-3 levels were determined in a few newborn males who had low enzyme activities (<5% of the normal leukocyte mean). Neither individually nor together did these determinations reliably predict newborns to have the classic or later-onset phenotype. To date, the most reliable predictor is the specific α-Gal A mutation. Clearly, expert laboratories and clinicians should establish a genotype/phenotype registry to document the clinical manifestations of each genotype. If modifier genes are identified, these too should be part of the genotype/phenotype predictions. In this way, newborn screening for Fabry disease could focus on males with the classic phenotype who would benefit from early therapeutic intervention.
In summary, newborn screening for Fabry disease in Taiwan identified a high frequency of Fabry disease (~1 in 1,250 males) of which the majority (86%) had the later-onset phenotype (~1 in 1,390 males). These studies highlight the fact that newborn screening can identify large numbers of patients who will remain clinically asymptomatic until later in life. Although investigation of family members may identify older affected relatives with this X-linked disease who may benefit from therapeutic intervention, the newborn diagnosis of infants presents predicted to have the later-onset phenotype is controversial. This concern will become even more problematic for common later-onset disorders when multi-disease chip analyses or whole genome sequencing become commonplace for prospective parents and newborns. Clearly, well-documented genotype/phenotype registries will become essential for the future selection of patients for early therapeutic intervention.
Acknowledgments
The authors thank Ms. Nicole Kelly for her expert editorial assistance, Ms Yi-Li Liu and I-Ching Huang for their assistance with DNA sequencing, and all the doctors and nurses who helped collect the samples for newborn screening. This study was supported by an unrestricted research grant of Genzyme Corporation, Cambridge, MA, USA, a grant from the Taiwanese National Science Council (NSC96-2314-B-002-044-MY3), and grants from the National Institutes of Health including an NIH Merit Award for research (5 R37-DK34045) to RJD, and a grant (5 MO1 RR00071) for the Mount Sinai General Clinical Research Center Program from the National Center of Research Resources.
Footnotes
Conflict of interest: Wuh-Liang Hwu received a research grant from the Genzyme Corporation to support newborn screening for Fabry disease. Robert J. Desnick is a consultant and receives grants, and is an inventor of patents licensed to the Genzyme Corporation, and has founder stock and is a consultant to Amicus Therapeutics, Inc.
References
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146:77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308:195–196. doi: 10.1016/s0009-8981(01)00478-8. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Kinzler KE, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York; McGraw-Hill: 2001. pp. 3733–3774. [Google Scholar]
- Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Brady RO. Fabry disease in childhood. J Pediatr. 2004;144:S20–26. doi: 10.1016/j.jpeds.2004.01.051. [DOI] [PubMed] [Google Scholar]
- Dobrovolny R, Dvorakova L, Ledvinova J, Magage S, Bultas J, Lubanda JC, Elleder M, Karetova D, Pavlikova M, Hrebicek M. Relationship between X-inactivation and clinical involvement in Fabry heterozygotes. Eleven novel mutations in the alpha-galactosidase A gene in the Czech and Slovak population. Mol. Med. 2005;83:647–654. doi: 10.1007/s00109-005-0656-2. [DOI] [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ. Safety and efficacy of recombinant human alpha-galactosidase A--replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, Hopkin RJ, Bultas J, Lee P, Sims K, Brodie SE, Pastores GM, Strotmann JM, Wilcox WR. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8:539–548. doi: 10.1097/01.gim.0000237866.70357.c6. [DOI] [PubMed] [Google Scholar]
- Filoni C, Caciotti A, Carraresi L, Donati M, Mignani R, Parini R, Filocamo M, Soliani F, Simi L, Guerrini R, Zammarchi E, Morrone A. Unbalanced GLA mRNAs ratio quantified by real-time PCR in Fabry patients' fibroblasts results in Fabry disease. Eur J Hum Genet. 2008;16:1311–1317. doi: 10.1038/ejhg.2008.109. [DOI] [PubMed] [Google Scholar]
- Germain DP. Fabry disease: the need to stratify patient populations to better understand the outcome of enzyme replacement therapy. Clin Ther. 2007;29(Suppl A):S17–18. doi: 10.1016/s0149-2918(07)80122-6. [DOI] [PubMed] [Google Scholar]
- Ishii S, Sakuraba H, Suzuki Y. Point mutations in the upstream region of the alpha-galactosidase A gene exon 6 in an atypical variant of Fabry disease. Hum Genet. 1992;89:29–32. doi: 10.1007/BF00207037. [DOI] [PubMed] [Google Scholar]
- Ishii S, Nakao S, Minamikawa-Tachino R, Desnick RJ, Fan JQ. Alternative splicing in the alpha-galactosidase A gene: increased exon inclusion results in the Fabry cardiac phenotype. Am J Hum Genet. 2002;70:994–1002. doi: 10.1086/339431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotanko P, Kramar R, Devrnja D, Paschke E, Voigtlander T, Auinger M, Pagliardini S, Spada M, Demmelbauer K, Lorenz M, Hauser AC, Kofler HJ, Lhotta K, Neyer U, Pronai W, Wallner M, Wieser C, Wiesholzer M, Zodl H, Fodinger M, Sunder-Plassmann G. Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol. 2004;15:1323–1329. doi: 10.1097/01.asn.0000124671.61963.1e. [DOI] [PubMed] [Google Scholar]
- Kitagawa T, Ishige N, Suzuki K, Owada M, Ohashi T, Kobayashi M, Eto Y, Tanaka A, Mills K, Winchester B, Keutzer J. Non-invasive screening method for Fabry disease by measuring globotriaosylceramide in whole urine samples using tandem mass spectrometry. Molec Genet Metabol. 2005;85:196–202. doi: 10.1016/j.ymgme.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Lukacs Z, Keil A, Kohlschutter A, Beck M, Mengel E. The ratio of alpha-galactosidase to beta-glucuronidase activities in dried blood for the identification of female Fabry disease patients. J Inherit Metab Dis. 2005;28:803–805. doi: 10.1007/s10545-005-0039-4. [DOI] [PubMed] [Google Scholar]
- Maier EM, Osterrieder S, Whybra C, Ries M, Gal A, Beck M, Roscher AA, Muntau AC. Disease manifestations and X inactivation in heterozygous females with Fabry disease. Acta Paediatr Suppl. 2006;95:30–38. doi: 10.1111/j.1651-2227.2006.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Mayes JS, Scheerer JB, Sifers RN, Donaldson ML. Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry's disease. Clin Chim Acta. 1981;112:247–251. doi: 10.1016/0009-8981(81)90384-3. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- Monserrat L, Gimeno-Blanes JR, Marin F, Hermida-Prieto M, Garcia-Honrubia A, Perez I, Fernandez X, de Nicolas R, de la Morena G, Paya E, Yague J, Egido J. J Am Coll Cardiol. 2007;50:2399–2403. doi: 10.1016/j.jacc.2007.06.062. [DOI] [PubMed] [Google Scholar]
- Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. European heart journal. 2003;24:2151–2155. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, Tei C, Desnick RJ. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant”; phenotype. Kidney Int. 2003;64:801–807. doi: 10.1046/j.1523-1755.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Rolfs A, Bottcher T, Zschiesche M, Morris P, Winchester B, Bauer P, Walter U, Mix E, Lohr M, Harzer K, Strauss U, Pahnke J, Grossmann A, Benecke R. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366:1794–1796. doi: 10.1016/S0140-6736(05)67635-0. [DOI] [PubMed] [Google Scholar]
- Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–1411. doi: 10.1161/01.cir.0000012626.81324.38. [DOI] [PubMed] [Google Scholar]
- Shabbeer J, Yasuda M, Benson SD, Desnick RJ. Fabry disease: identification of 50 novel alpha-galactosidase A mutations causing the classic phenotype and three-dimensional structural analysis of 29 missense mutations. Hum Genomics. 2006;2:297–309. doi: 10.1186/1479-7364-2-5-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abevsinghe S Krawczak M, Cooper DN. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. See www.hgmd.cf.ac.uk.webloc. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ohashi T, Kobayashi M, Eto Y, Miyamura N, Nishida K, Araki E, Itoh K, Matsushita K, Hara M, Kuwahara K, Nakano T, Yasumoto N, Nonoguchi H, Tomita K. Identification of Fabry's disease by the screening of alpha-galactosidase A activity in male and female hemodialysis patients. Clin. Nephrol. 2005;64:281–287. doi: 10.5414/cnp64281. [DOI] [PubMed] [Google Scholar]
- von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hubner G, Olsen EG, Christomanou H, Kandolf R, Bishop DF, Desnick RJ. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991;324:395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]
- Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM. Improvements of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–1301. doi: 10.1161/01.CIR.0000091253.71282.04. [DOI] [PubMed] [Google Scholar]
- Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2004;75:65–74. doi: 10.1086/422366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Shabbeer J, Benson SD, Maire I, Burnett RM, Desnick RJ. Fabry disease: characterization of alpha-galactosidase A double mutations and the D313Y plasma enzyme pseudodeficiency allele. Hum Mutat. 2003;22:486–492. doi: 10.1002/humu.10275. [DOI] [PubMed] [Google Scholar]