Abstract
Background
Adherence to the medical regimen after pediatric organ transplantation is important for maximizing good clinical outcomes. However, the literature provides inconsistent evidence regarding prevalence and risk factors for nonadherence posttransplant.
Methods
A total of 61 studies (30 kidney, 18 liver, 8 heart, 2 lung/heart-lung, and 3 with mixed recipient samples) were included in a meta-analysis. Average rates of nonadherence to 6 areas of the regimen, and correlations of potential risk factors with nonadherence, were calculated.
Results
Across all types of transplantation, nonadherence to clinic appointments and tests was most prevalent, at 12.9 cases per 100 patients per year (PPY). The immunosuppression nonadherence rate was 6 cases per 100 PPY. Nonadherence to substance use restrictions, diet, exercise and other healthcare requirements ranged from 0.6 to 8 cases per 100 PPY. Only the rate of nonadherence to clinic appointments and tests varied by transplant type: heart recipients had the lowest rate (4.6 cases per 100 PPY vs. 12.7–18.8 cases per 100 PPY in other recipients). Older age of the child, family functioning (greater parental distress, lower family cohesion), and the child’s psychological status (poorer behavioral functioning, greater distress) were among the psychosocial characteristics significantly correlated with poorer adherence. These correlations were small to modest in size (r =.12–.18).
Conclusions
These nonadherence rates provide benchmarks for clinicians to use to estimate patient risk. The identified psychosocial correlates of nonadherence are potential targets for intervention. Future studies should focus on improving the prediction of nonadherence risk and on testing interventions to reduce risk.
Keywords: adherence, transplantation, pediatric, kidney, heart, liver, lung
INTRODUCTION
Organ transplantation offers children with end-stage organ disease an opportunity for a significant extension of life as well as major improvements in quality of life.(1–8) Although these benefits are similar to those accrued by adult organ recipients, the value of transplantation for children is even more profound, including the potential for more normalized trajectories of growth and development, the chance to move into adulthood, and the attainment of roles and responsibilities that come with maturation.(3–5,9–13) Poor adherence to the posttransplant regimen —i.e., the degree to which patients’ behavior fails to coincide with medical recommendations(14,15) —undercuts the likelihood of achieving these benefits: poor adherence, especially to medication and clinical follow-up requirements, has repeatedly been linked to morbidity and mortality in pediatric recipients.(16–25) Moreover, adherence is potentially more important in children than adults because of altered pharmacokinetics of immunosuppressive agents in children and the consequent need for meticulous dosing, monitoring, and clinical follow-up care.(26–28).
Recognition of these issues has led to remarkable growth in the literature on adherence in pediatric organ recipients. However, there is little consensus on the prevalence of nonadherence to each of the multiple components of the medical regimen among these children. For example, literature reviews have reported crude rates of immunosuppression nonadherence (unadjusted for duration of follow-up) ranging from 5% to 71%.(7,8,28–33) A recent small meta-analysis of 12 studies primarily in pediatric renal recipients in the United States reported an average rate of nonadherence (without distinguishing the components of the regimen involved) of 28%.(34) This analysis reported substantial between-study variability in nonadherence rates (range, 4% to 50%), but it was unable to evaluate study differences that might have contributed to this variability. In general, reviews of this literature have faced difficult challenges in estimating average prevalence rates of nonadherence after pediatric transplantation due to (a) differences across studies in the type of transplant children received, (b) duration of study follow-up, (c) areas of nonadherence assessed (e.g., medication taking vs. clinic appointment attendance, etc.), and (d) mode of adherence assessment (e.g., paper and pencil vs. parental/other informant report vs. medication monitoring).(3,28,29,35–37) Because of uncontrolled variability related to these factors, the clinical interpretation of the nonadherence prevalence data summarized in past reviews remains difficult.
An additional concern noted in past reviews is that hypothesized risk factors for nonadherence in pediatric transplant populations are understudied and show inconsistent effects.(4,7,29,31,38) In particular, although adolescents have long been viewed as being at considerably greater risk for nonadherence than younger children,(4,27,35,39–41) some empirical studies support this clinical observation(17,24,42) while others do not.(43–47) In their meta-analysis, Kahana et al. (34) found that older age of the child was moderately associated with greater nonadherence. However, they noted significant variability in the size of the age-nonadherence association across studies. Similar to interpretations of variability in nonadherence prevalence rates, methodologic and other study differences are usually emphasized as likely contributors to the mixed findings on risk factors.(29,36) Even so, there has been no direct evaluation of the impact of these differences in any review.
In the present report, we performed a meta-analysis of the full literature on nonadherence in pediatric organ transplantation, published in any of multiple languages and involving cohorts worldwide. We had two goals. First, we sought to estimate the rate of nonadherence to each component of the medical regimen, both across all types of pediatric organ transplantation and within specific types of transplant. Where there was substantial variation in rates across studies, we examined whether it could be explained by differences in study characteristics (e.g., design). Second, we aimed to determine whether nonadherence was associated with patient risk factors and whether any associations varied by transplant type and other study characteristics.
Our goals, and the use of meta-analysis to achieve them, are consistent with best-practice standards in evidence-based medicine.(48,49) These standards call for meta-analysis in order to advance a research field as well as to provide clinicians with optimal information. With respect to the latter, our analyses were designed to yield essential data for transplant clinicians who must gauge nonadherence risk in their pediatric patient populations in order to provide appropriate care and monitoring. Such data are also critical for the design of intervention research to evaluate promising clinical strategies for nonadherence risk reduction.
METHODS
The methodology for conducting and reporting the meta-analysis followed established guidelines(50,51) and was similar in approach to our meta-analyses of adult posttransplant adherence.(52,53)
Search strategy and study selection
Figure 1 summarizes our study retrieval and selection strategy. From 1,136 citations meeting initial inclusion/exclusion criteria, 343 were retrieved. Their bibliographies were searched, yielding 27 additional papers. This group of 370 articles received detailed evaluation, as depicted in the figure.
Figure 1.
Identification of independent studies for the meta-analysis.
Data extraction
Nonadherence outcomes
Pairs of us (one of whom was M.A.D.) reviewed all studies. We examined several outcomes: immunosuppression medication nonadherence; nonadherence to clinic appointments and required tests; nonadherence to diet and exercise requirements; alcohol and illicit drug use; and tobacco use. In addition, some studies reported a nonspecific “global” nonadherence outcome (reflecting nonadherence in multiple, often unspecified, areas). For some areas (e.g., diet and exercise), we grouped behaviors together because few studies examined each individually.
Following Cochrane Collaboration standards(54) and other meta-analyses of nonadherence in transplant and other chronic disease populations,(34,52,55,56) we extracted information from each study on the occurrence of nonadherence in each outcome area based on the original study authors’ definition of clinically significant nonadherence (e.g., taking less than a specified percentage of medication; failing to engage in a behavior at an acceptable level; having a blood test value outside an acceptable range). Authors’ definitions reflected their transplant programs’ requirements. (These definitions necessarily varied depending on studies’ nonadherence assessment method; we evaluated the impact of method differences on outcome as described below.)
The only exceptions to our reliance on study authors’ own definitions of nonadherence were for tobacco and other substance use, for which we simply recorded information on any vs. no use. In most studies reporting on substance use, it was not explicitly stated that any use constituted nonadherent behavior. However, since most samples consisted exclusively of minors and substance use is not recommended for any pediatric transplant population, we defined nonadherence as any substance use.
Assessment-related and other study characteristics
We categorized the method used to assess nonadherence outcomes into five broad groupings(52,55): (a) self-report (e.g., patient interview, paper-and-pencil survey); (b) collateral report by family or healthcare professional (e.g., through interview or survey); (c) biologic or other “indirect”(57,58) measure (e.g., blood level from laboratory reports, electronic medication monitoring, pill count, claims data); (d) clinical data retrieved from medical records (i.e., summaries of clinic visits and other encounters with patients and families), and (e) combinations of these methods (e.g., self report+clinical records data). We recorded descriptive information about each investigation (e.g., design, sample age composition). Following current meta-analysis standards,(50) pairs of us rated each study on five components of methodologic quality using a validated scoring system for each.(52,59) We employed a consensus approach where any disagreements were resolved before assigning a final rating. The five components (each rated as 1=yes, 0=no) were whether: (a) the sample was clearly described (e.g., including demographic information, transplant dates), (b) the patients approached for enrollment were shown to be representative of the study site’s transplant population, (c) the sample enrolled was shown to be representative of those approached, (d) the definition of nonadherence, source of nonadherence data and time period covered by the nonadherence measure were clearly described, and (e) analyses of nonadherence rates were appropriate (i.e., if patients varied in follow-up duration, rates were calculated via survival analysis techniques). A composite quality score was created for each study which was the count of the number of the five areas rated as “yes” (total score range, 0–5). (A scoring manual, with criteria for each rated component, is available from M.A.D.)
Risk factors for nonadherence
We extracted information from each study on the size of the association of each nonadherence outcome with a series of potential risk factors. We aimed to examine as many as possible of the characteristics hypothesized in the pediatric transplant literature to affect risk.(4,7,8,11–13,23,26–36,38–43) However, there were only sufficient numbers of studies (i.e., ≥4) to examine the following: (a) 6 patient sociodemographic characteristics (gender, race/ethnicity, age, whether the parents’ marriage was intact, socioeconomic status, receipt of public health insurance), (b) 2 background medical characteristics (receipt of a deceased donor kidney transplant, receipt of dialysis before kidney transplant) and (c) 6 posttransplant characteristics (time since transplant, parental distress and/or feelings of burden related to the child’s health, level of family cohesiveness and support, level of child behavioral functioning, level of child psychological distress, and child perceptions of medical regimen side effects). Because of the small numbers of studies examining most of these variables, we could not distinguish in our analyses whether the variables were based on patient or parent/other informant report.
Statistical analysis
Examination of nonadherence rates across outcome areas
Patients in most studies had unequal follow-up time, typically because they entered a given study at different time points (e.g., different transplant dates) or were lost to follow-up at varying time points (e.g., because of death). As in other clinical epidemiologic contexts when the goal is to compare rates in the face of differences in duration of observation,(52,60–63) we examined event rates—cases of nonadherence—per 100 persons per year (i.e., per 100 person-years of observation). If total person-years of observation was not reported directly, we calculated it from either reported cumulative probabilities or descriptive information about the distribution of follow-up duration in the sample.(61)
We calculated the pooled, or average, estimate of the nonadherence rate in each outcome area (rate=cases per 100 persons per year [PPY]) across all contributing studies, as well as within each organ transplant type. The pooled estimate is a weighted average that takes within-study variance into account. It was generated under a random effects model, in order to allow generalizability beyond the retrieved studies.(64) For each statistically significant pooled estimate, we evaluated the impact of publication bias (i.e., that studies finding nonadherence rates significantly different from zero may have been more likely to have been published) by calculating the “fail-safe N.” This is the number of missing studies obtaining null findings that would need to be added to the analysis so that the pooled estimate would no longer be statistically significant.(65,66)
When there was substantial variability across studies in nonadherence rates in a given outcome area (based on the Q test for heterogeneity), we used random effects meta-regression to determine whether the variability could be explained by five study characteristics: geographic location, design, quality, nonadherence assessment methodology, and (for the subset of studies examining specific age groups) whether the cohort under study consisted exclusively of adolescents or younger children.
Examination of potential risk factors for nonadherence
The association of nonadherence with each factor was examined by extracting or calculating r, the Pearson correlation coefficient. This effect size (ES) indicates the strength and direction of association between pairs of variables. It was chosen for its applicability across measurement scenarios (i.e., with continuous, dichotomous, or ranked variables).(67) For each statistically significant average ES, we calculated the fail-safe N to estimate the impact of publication bias. We calculated Q to determine whether there was significant variability in ESs across studies. When there was significant variability, we examined whether study characteristics accounted for it.
RESULTS
Retrieved studies
A total of 61 studies met inclusion criteria; descriptive information is provided in Table 1. (Appendix A lists the studies and nonadherence outcomes examined; a bibliography is available from M.A.D.) Almost half of the studies focused on kidney recipients (49%), followed by liver recipients (30%) and heart recipients (13%). Over 3,800 pediatric patients were included across all studies, contributing almost 11,600 person-years of observation.
Table 1.
Descriptive information for 61 independent studies of nonadherence after pediatric organ transplantation
| Type of organ transplant |
||||||
|---|---|---|---|---|---|---|
| Characteristic | Total | Kidney | Liver | Heart | Lung/Heart-Lung | Mixed Samplea |
| Number of studies | 61 | 30 | 18 | 8 | 2 | 3 |
| Year of earliest publication of any study examining nonadherence outcomes | 1989 | 1989 | 1990 | 1991 | 1993 | 2006 |
| Study location, continent, % | ||||||
| North America | 68.9 | 60.0 | 77.8 | 87.5 | 50.0 | 67.7 |
| Asia | 14.8 | 26.7 | 5.6 | 0.0 | 0.0 | 0.0 |
| Europe | 11.5 | 3.3 | 16.7 | 12.5 | 50.0 | 33.3 |
| Africa | 3.3 | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| South America | 1.6 | 3.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total no. of countries represented | 13 | 11 | 5 | 3 | 2 | 2 |
| Total no. of patients studied | 3,834 | 1,930 | 1,313 | 355 | 43 | 193 |
| Sample size: mean (SD) | 63 (78) | 64 (103) | 73 (56) | 44 (13) | 21 (3) | 64 (21) |
| median | 47 | 33 | 59 | 49 | 21 | 75 |
| range | 11–591 | 11–591 | 14–234 | 27–61 | 19–24 | 40–78 |
| Total person years of observation | 11,573 | 5,110 | 4,557 | 1,410 | 67 | 429 |
| Duration of observation or follow-up, years: mean (SD) | 3.5 (2.3) | 3.5 (3.3) | 3.6 (4.2) | 4.1 (3.50) | 1.6 (0.9) | 3.2 (4.7) |
| median | 3.4 | 2.6 | 2.0 | 2.7 | 1.6 | 0.8 |
| range | 0.04–15.1 | 0.04–13.6 | 1.0–15.1 | 1.0–10.0 | 1.0–2.3 | 0.2–8.6 |
| Study sample age distribution,b % | ||||||
| children only | 6.6 | 10.0 | 5.6 | 0.0 | 0.0 | 0.0 |
| adolescents only | 27.9 | 23.3 | 33.3 | 12.5 | 50.0 | 66.7 |
| children & adolescents | 65.6 | 66.7 | 61.1 | 87.5 | 50.0 | 33.3 |
| absolute age rangec | 0 – 24.9 | 0 – 23 | 0 – 21 | 0 – 19 | 4 – 19 | 7 – 24.9 |
| Study design, % cross-sectional/retrospective (vs. prospective) | 85.2 | 86.7 | 88.9 | 87.5 | 50.0 | 66.7 |
| Study quality evaluation, % | ||||||
| Lower (score 0–2) | 65.6 | 66.7 | 66.7 | 75.0 | 0.0 | 66.7 |
| Higher (3–5) | 34.4 | 33.3 | 33.3 | 25.0 | 100.0 | 33.3 |
| Outcomes examined, no. of studies | ||||||
| Immunosuppression nonadherenced | 41 | 18 | 13 | 7 | 1 | 2 |
| Nonadherence to clinic appts. and tests | 10 | 3 | 3 | 4 | 0 | 0 |
| Diet and exercise nonadherence | 3 | 0 | 0 | 2 | 0 | 1 |
| Alcohol and illicit drug use | 6 | 1 | 1 | 1 | 1 | 2 |
| Tobacco use | 4 | 1 | 0 | 1 | 1 | 1 |
| Global nonadherence | 11 | 4 | 2 | 4 | 1 | 0 |
Samples included recipients of either a kidney, liver, heart, lung, or heart-lung transplant; studies did not provide data separately by transplant type.
Studies that focused on specific age groups, or that conducted analyses comparing adolescents to younger children, varied in the cut point used to define adolescence. The largest group (n=11) of the 23 studies using a cut point defined children as aged 10 and adolescents as aged >10. Five studies used age >11 as the cut point and 7 studies used age >12.
See Appendix A for age range of individual studies included in meta-analysis.
Includes 2 studies that did not clearly exclude nonimmunosuppression medications when examining this outcome. These 2 studies were indistinguishable from the remaining studies in their rates of nonadherence in this outcome area. See Appendix A for outcome areas examined in each study included in the meta-analysis.
Appendix A.
Studies included in meta-analysis of rates and psychosocial risk factors for nonadherence to the medical regimen after pediatric organ transplantation.a
| Study first author, year (and first author, year of related publications) | Country | Age rangeb | Areas of nonadherence examined | Examined psychosocial variables that were included in the meta-analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immuno. meds. | Clinic appts. | Tests | Diet | Exercise | Alcohol use | Illicit drug usec | Tobacco use | Global nonadher. | ||||
| Kidney transplant studies | ||||||||||||
| Rovelli, 1989 (Schweizer, 1990; Swanson, 1991) | USA | < 20 | X | |||||||||
| Ettinger, 1991, pre-adolescent cohort (Ettinger, 1991a, 1991b) | USA | < 13 | X | X | ||||||||
| Ettinger, 1991, adolescent cohort (Ettinger, 1991a, 1991b) | USA | 13 – 21 | X | X | ||||||||
| McBride, 1991 | USA | Not provided | X | |||||||||
| Foulkes, 1993 (Fennell, 1993; Foulkes, 1995) | USA | 6 – 21 | X | X | ||||||||
| Morgenstern, 1994 | USA | 2.5 – 19 | X | |||||||||
| Sharma, 1994 | USA | 0.75 – 14 | X | |||||||||
| Meyers, 1995 | South Africa | 6.5 – 15 | X | X | X | |||||||
| Blowey, 1996 (Blowey, 1997) | Canada | 12.5 – 17.9 | X | X | ||||||||
| Conley, 1996 | USA | ≤ 18 | X | X | ||||||||
| Davis, 1996 | USA | 5 – 18 | X | |||||||||
| Iitaka, 1996 | Japan | 5 – 15 | X | |||||||||
| Meyers, 1996 | South Africa | 2.5 – 20.8 | X | |||||||||
| Fennell, 2001 (Tucker, 2001, Tucker, 2002) | USA | 6 – 20 | X | |||||||||
| Fernandez de Preliasco, 2002 | Argentina | 9 – 22 | X | |||||||||
| Fukunishi, 2002 | Japan | 0 – 19 | X | |||||||||
| Rizvi, 2002 | Pakistan | 6 – 17 | X | |||||||||
| Penkower, 2003 | USA | 13 – 18 | X | X | X | X | ||||||
| Shaw et al., 2003, pre-adolescent cohort (Sarwal, 2003) | USA | < 11 | X | X | ||||||||
| Shaw et al., 2003, adolescent cohort | USA | ≥ 11 | X | X | ||||||||
| Gerson, 2004 | USA | 2.4 – 20.9 | X | |||||||||
| Feinstein, 2005 | Israel | 1.7 – 23.0 | X | X | ||||||||
| Berber, 2006 | Turkey | 11 – 17 | X | |||||||||
| Remorino, 2006 | UK | 17 – 20 | X | X | ||||||||
| Abeyeskera, 2007 | Sri Lanka | 8 – 16 | X | X | ||||||||
| Chisholm, 2007 | USA | < 5 | X | |||||||||
| Shellmer, 2007 | USA | 8 – 19 | X | |||||||||
| Takemoto, 2007 | USA | 0 – 18 | X | |||||||||
| Wu, 2008 | China | 12 – 17 | X | X | ||||||||
| Vasudevan, 2008 | India | < 18 | X | |||||||||
| Liver Transplant Studies | ||||||||||||
| Kennard, 1990 | USA | 0.25 – 12.5 | X | |||||||||
| Lurie, 2000 (Shemesh, 2000) | USA | 13 – 18 | X | |||||||||
| Fukunishi, 2002 | Japan | 0 – 19 | X | |||||||||
| Kelly, 2002, pre-adolescent cohort (Kelly, 2005, 2006) | UK | ≤ 10 | X | X | ||||||||
| Kelly, 2002, adolescent cohort (Kelly, 2005, 2006) | UK | > 10 | X | X | ||||||||
| Avitzur, 2004 | Canada | 0.5 – 17.3 | X | |||||||||
| Falkenstein, 2004 | USA | Not provided | X | |||||||||
| Rumbo, 2004 | USA | 3 – 20 | X | |||||||||
| Shemesh, 2004 (Shemesh, 2007, Emre, 2006) | USA | 2 – 21 | X | X | ||||||||
| Berquist, 2006 | USA | 12 – 21 | X | X | ||||||||
| Annunziato, 2007 | USA | ≥ 12 | X | |||||||||
| Bueno, 2007 | Spain | 0.42 – 15 | X | |||||||||
| Fredericks, 2007 | USA | 2.33 – 16 | X | X | X | |||||||
| Berquist, 2008 | USA | 12 – 21 | X | X | X | X | X | |||||
| Fredericks, 2008 | USA | 12 – 17.9 | X | X | X | |||||||
| Shemesh, 2008 | USA | 8 – 21 | X | |||||||||
| Stuber, 2008 | USA | < 21 | X | |||||||||
| Venkat, 2008 | USA | Not provided | X | |||||||||
| Heart Transplant Studies | ||||||||||||
| Baum, 1991 | USA | 0.25 – 18.9 | X | X | ||||||||
| Douglas, 1993 (Addonizio, 1993) | USA | ≤ 18 | X | X | X | |||||||
| Serrano-Ikkes, 1998 | UK | Not provided | X | X | X | X | ||||||
| Flippen, 2000 | USA | 0 – 18 | X | |||||||||
| Chartrand, 2001 | Canada | 0.83 – 19 | X | |||||||||
| Ringewald, 2001 | USA | Not provided | X | X | ||||||||
| Farley, 2005 (Farley, 2007) | USA | 1 – 18 | X | X | X | X | ||||||
| Stilley, 2005 (Stilley, 2006) | USA | < 21 | X | X | X | X | X | X | X | |||
| Lung/Heart-Lung Transplant Studies | ||||||||||||
| Whitehead, 1993 (Serrano-Ikkes, 1998) | UK | 3.6 – 18.6 | X | X | X | |||||||
| Durst, 2001 | USA | 11 – 17 | X | X | ||||||||
| Mixed Samples of Transplant Recipients | ||||||||||||
| Maikranz, 2006 (Dreyer, 2006; Maikranz, 2007) | USA | 7 – 18 | ||||||||||
| Wray, 2006 | UK | 12.3 – 24.9 | X | X | X | X | X | |||||
| Simons, 2007 (Simons, 2008) | USA | 11 – 21 | X | X | ||||||||
Each row in this table describes an independent study of a given transplant recipient cohort. A few investigations included more than one cohort (e.g., Fukunishi, 2002 studied a kidney recipient cohort and a liver recipient cohort). Each study is listed under each type of transplantation for which it contributed a separate, independent sample.
The age range describes transplant recipients at the inception of the follow-up period examined in a given investigation. For some studies, this was the age at transplant (e.g., when studies examined adherence during the entire period since transplant); for other studies, it was the age at which the study began to monitor patients’ adherence (e.g., when studies examined adherence during a specific time period at some point during the years post-transplant). In a few studies no age range was provided, although the authors typically provided other descriptive information (e.g., mean or median age), indicating that they were studying pediatric recipients.
Includes studies that do not explicitly note if alcohol use was included or excluded from the substances examined.
The number of reports contributing data on each nonadherence outcome are also shown in Table 1. Studies of immunosuppression medication nonadherence were by far the most common.
Nonadherence rates and rate differences by type of transplant
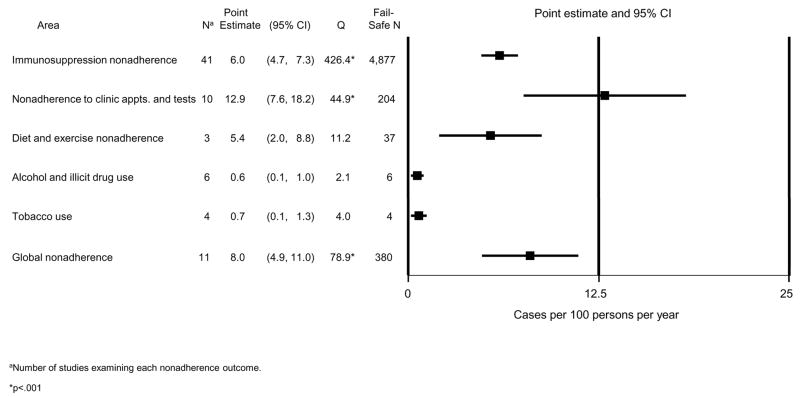
Figure 2 shows the average rate and 95% confidence interval (CI) for each nonadherence outcome. For example, the average rate for immunosuppression nonadherence was 6 cases per 100 PPY. The average rates for all outcomes differed significantly from zero (i.e., CIs do not include zero). The rates of alcohol or drug use, and tobacco use were very low, with narrow CIs. We found higher rates, with wider CIs, for immunosuppression nonadherence, diet and exercise nonadherence, and global nonadherence. The highest rate of nonadherence was for clinic appointments and tests. The generally large fail-safe N’s for the rates in Figure 2, especially for immunosuppression nonadherence, indicate that these average rates are very robust to the discovery of many additional studies reporting null findings.
Figure 2.
Pooled estimates of nonadherence rates after pediatric organ transplantation in 6 outcome areas.
The Q tests in Figure 2 indicate that there was significant variability in the rates across studies for three outcome areas: immunosuppression nonadherence; clinic appointment and test nonadherence; and global nonadherence. While we hypothesized that this variability might be explained by differences between types of transplant, we found no evidence of such differences for immunosuppression or global nonadherence: for these outcomes, a meta-ANOVA comparing rates across kidney, liver, and heart recipient studies was nonsignificant: overall test statistic, Q(2)=1.92, p=.383 and Q(2)=2.67, p=.263, for the two outcomes, respectively (lung recipients could not be included because only one study examined these outcomes). However, there were transplant-related differences in the rate of nonadherence to clinic appointments and tests (Q(2)=8.63, p=.013): it was greatest in liver recipients (rate=18.8, SE=3.6, n=4 studies) and kidney recipients (rate=12.7, SE=4.4, n=4 studies), and smallest in heart recipients (rate=4.6, SE=3.3, n=2 studies).
Study-related characteristics accounting for variability in nonadherence rates
We focused on the three nonadherence areas where there was significant variability across studies and, for each, we used multivariate meta-regression to test the independent effects of study design, quality, and nonadherence assessment method. (An additional characteristic, study geographic location, could not be examined because location was confounded with other methodologic characteristics. For example, all Asian studies used retrospective designs and clinical records reviews; all European studies except two used retrospective designs.) Study sample age group could not be included in the multivariate analyses because only a subset of studies reported rates specifically for adolescents (most often defined in this literature as aged >10; see footnote b, Table 1) or for younger children. However, we performed a univariate test of the impact of this characteristic (i.e., a meta-regression with age group as the single predictor).
For each of the three nonadherence areas, Table 2 shows the estimated nonadherence rate according to each study characteristic. For example, the average immunosuppression nonadherence rate was 5.5 cases per 100 PPY for the 33 cross-sectional/retrospective studies, and 14.7 for the 8 longitudinal/prospective studies. Meta-regression results show that studies with higher quality ratings found higher immunosuppression nonadherence rates than lower quality studies (Z=4.27, p<.001). Concerning nonadherence assessment methods, studies using indirect methods or a multiple-method approach showed significantly higher nonadherence rates than studies relying on clinical records reviews. (An additional comparison of indirect vs. multiple method approaches yielded no statistically significant difference between these two rates, Z=0.27, p=.788). Self-report was also associated with a higher nonadherence rate but could not be tested in the meta-regression because only one study relied exclusively on that approach. The single study using collateral reports showed a very low nonadherence rate.
Table 2.
Study characteristics potentially accounting for variability in outcome rates (per 100 persons per year) across independent studies of pediatric organ transplant recipients.
| Outcome Domain |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Immunosuppression Nonadherence | Clinic appointment and test nonadherence | Global nonadherence | |||||||
| Study Characteristica | rate (SE) | Nb | Z | rate (SE) | Nb | Z | rate (SE) | Nb | Z |
| Study design | |||||||||
| Cross-sectional/retrospective | 5.5 (0.6) | 33 | 1.14 | 11.5 (2.7) | 8 | 1.01 | 7.6 (1.6) | 9 | 1.01 |
| Prospective | 14.7 (2.9) | 8 | 24.0 (8.6) | 2 | 16.1 (7.5) | 2 | |||
| Study quality | |||||||||
| Lower (0–2) | 4.9 (0.8) | 28 | 4.27*** | 12.5 (3.5) | 6 | 0.04 | 7.3 (2.3) | 5 | 1.00 |
| Higher (3–5) | 8.4 (1.1) | 13 | 12.7 (3.9) | 4 | 9.5 (2.6) | 6 | |||
| Method of assessment | |||||||||
| Clinical records review | 4.2 (0.8) | 19 | (ref) | 16.3 (4.0) | 6 | 0.50 | 5.2 (1.4) | 7 | 2.29** |
| Self-report | 13.9 (8.5) | 1c | --- | 24.1 (9.1) | 2 | --- | |||
| Collateral | 2.0 (2.7) | 1c | --- | 2.7 (7.6) | 1c | --- | --- | ||
| Indirect | 13.8 (1.6) | 14 | 5.90*** | --- | --- | ||||
| Multiple methods | 8.0 (2.1) | 6 | 3.56*** | 6.6 (7.6) | 1c | --- | 18.7 (3.2) | 4 | |
| Study sample age groupd | |||||||||
| Children only | 2.4 (1.4) | 4 | 4.96*** | --- | --- | --- | --- | ||
| Adolescents only | 7.1 (1.1) | 12 | 16.1 (3.5) | 7 | 14.9 (4.3) | 8 | |||
For each nonadherence outcome domain, multivariate meta-regression tested the independent effects of study design, quality and method of assessment on nonadherence rate. Because fewer studies reported rates for specific age group, this variable could not be included in the multivariate analyses. A univariate test of this variable is provided (i.e., meta-regression with a single predictor variable).
Number of studies included in each category of a given study characteristic, e.g., there were 32 studies that used a cross-sectional/retrospective design to examine immunosuppression nonadherence.
There were too few studies in this category to be examined in the analyses.
Studies focused on specific age groups most commonly defined children as subjects aged ≤ 10 and adolescents as subjects aged > 10. See also footnote b, Table 1.
p < .05
p < .01
p < .001
The lowermost section of Table 2 also shows that cohorts for 16 of the 41 studies of immunosuppression nonadherence consisted solely of adolescents or solely of younger children (remaining studies included children in both age groups and did not present separate rates for each). The nonadherence rate among the 12 studies of adolescent cohorts was significantly higher than that for younger cohorts (Z=4.96, p<.001).
Finally, Table 2 shows that none of the study characteristics accounted for significant variability in rate of clinic appointment and test nonadherence. For global nonadherence, the use of a multimethod assessment strategy was associated with a nonadherence rate three times greater than that of a clinical records approach.
Associations of nonadherence rates with potential risk factors
The numbers of studies examining each individual nonadherence outcome in relation to potential risk factors were small. For the outcomes of diet and exercise, alcohol and drug use, and tobacco use, only one to two studies of each outcome considered any possible correlates, and thus we could not examine them further. We collapsed the remaining three outcomes (immunosuppression nonadherence, clinic appointment and test nonadherence, and global nonadherence) and first analyzed the association of the risk factor variables with any type of nonadherence across all available studies. Then, if we detected significant ES heterogeneity across studies, we examined whether it could be explained by differences in the specific type of nonadherence outcome considered in the studies, as well as other study characteristics.
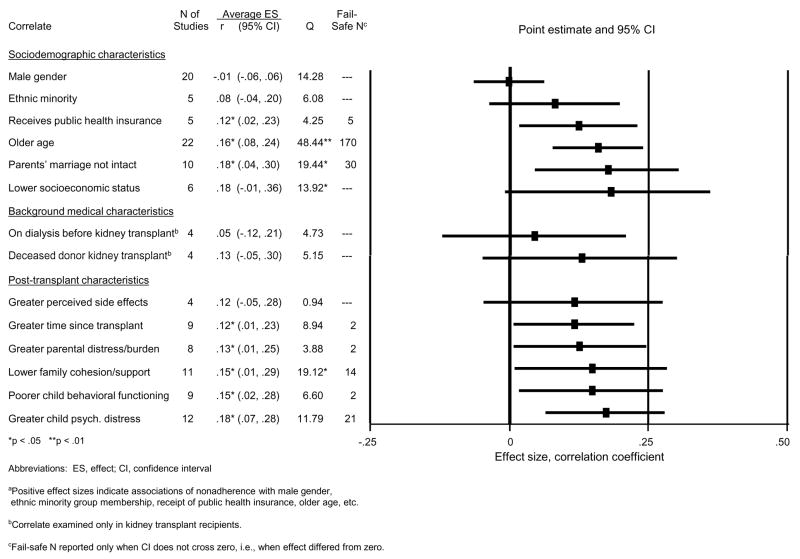
Figure 3 displays the potential risk factors’ correlations with nonadherence. For example, across the 20 studies contributing data on the gender-nonadherence correlation, the average ES was r= −.01 (CI, −.06, .06). Within each of the three broad categories of risk factors, the correlations are shown ordered by size.
Figure 3.
Associations between pediatric post-transplant nonadherence and potential risk factors.a
A number of significant correlations were found (i.e., p’s<.05, CIs not overlapping with zero). The average ESs were generally small to modest (r’s of .12 to .18). Among sociodemographic characteristics, receipt of public health insurance, older age of the child, and a non-intact parental marriage were associated with greater rates of nonadherence (average ESs of .12, .16, and .18, respectively). The fail-safe N of 5 for receipt of public health insurance is small, but the fail-safe Ns of 30 to 170 for parental marriage and older child age suggests that these latter associations are robust to the discovery of additional reports of null effects. Neither of the background medical factors was reliably associated with nonadherence. All posttransplant psychosocial variables but one (the child’s perception of more medical regimen side effects) were significantly associated with nonadherence. However, the fail-safe Ns for some effects were very small. The most robust associations (with larger fail-safe Ns) were found for lower family cohesion/support and greater child psychological distress.
Characteristics accounting for variability in the size of associations between potential risk factors and nonadherence outcomes
Q was significant for several ESs in Figure 3. However, the between-study variability in the sizes of these associations was largely unexplained by differences in specific type of nonadherence, type of transplant, or study characteristics (e.g., design) (p’s >.05). There was one exception: studies with higher quality ratings found older age of the child to be more strongly associated with nonadherence (ES, r=.31, SE=.06) than did studies with lower quality ratings (ES, r=.09, SE=.04; Z from meta-regression =2.28, p=.023).
DISCUSSION
Although the volume of research on adherence in pediatric organ transplantation has lagged behind that for adult transplantation,(4,22,34,36,38) we found a surprising number of studies conducted since the advent of modern immunosuppression that have considered pediatric adherence outcomes. This literature has focused on medication adherence, and we identified almost as many empirical studies documenting immunosuppression nonadherence in pediatric samples (n=41) as we found in our recent meta-analysis of adult organ recipients (n=46)(52).
Our results indicate that most pediatric recipients are adherent to the multiple components of their medical regimen. Nevertheless, significant proportions of recipients are nonadherent; the rates shown in Figure 2 provide important benchmarks for clinicians to use in estimating risk for nonadherence in their pediatric cohorts. These rates represent annualized risk (cases per year); over time posttransplant the rates would cumulate and, in fact, we found a significant correlation between time since transplantation and nonadherence risk (Figure 3).
The observed nonadherence rates are uniformly lower than those obtained in our meta-analysis of adult recipients.(52) For example, while transplant healthcare providers can expect to see, on average, about 23 adult recipients per 100 patients per year who are nonadherent to immunosuppression medications, the present findings indicate that only 6 such pediatric recipients per 100 per year would be expected. However, unlike adults—for whom taking immunosuppression and following diet and exercise requirements were among the most problematic areas of the regimen(52)—we found that clinic appointment and test nonadherence was the most common difficulty in pediatric samples, with an average rate of almost 13 cases per 100 PPY. This was also the only area of the regimen in which there were differences across types of transplantation. Nonadherence to clinic appointments and tests was more than twice as prevalent in pediatric liver recipients and kidney recipients (~13 to 19 cases per 100 PPY) than in heart recipients (5 cases per 100 PPY). This may relate to the fact that heart recipients require more intensive clinical surveillance to detect graft rejection and related complications.(68) The critical importance of these follow-up activities may lead to more attention on the part of transplant professionals and patients’ families to ensuring that appointments and tests are not missed.
No other study-related characteristics accounted for significant variability in nonadherence rates for clinic appointments and tests. However, studies of higher methodologic quality yielded higher immunosuppression nonadherence rates than did lower quality studies. In addition, studies employing methods other than clinical record reviews (either indirect methods, self-report, or multi-method approaches) found rates of immunosuppression nonadherence and global nonadherence that were two to over three times higher than rates in studies relying exclusively on clinical record reviews. Clinical record reviews alone, then, appear markedly inadequate for the assessment of nonadherence in pediatric transplant populations.
One of our key aims was to examine correlates of nonadherence. We found converging evidence that children’s age mattered: first, our between-study analyses comparing studies of adolescent cohorts to studies of younger cohorts found the rate of immunosuppression nonadherence to be over twice as high in adolescents. Second, our analyses examining within-study age-nonadherence associations found a significant correlation between these variables. Moreover, this correlation was stronger in higher quality studies (r=.31, indicating a moderate sized effect[69]), than in lower quality studies (r=.09). A variety of other psychosocial variables also emerged as significant correlates of nonadherence. Consistent with clinical observations regarding the critical role of the family system for pediatric patients’ adherence,(27,38,40,41,45,70) we observed significant correlations between nonadherence and both lower family cohesion and greater parental feelings of distress. Nonadherence was also correlated with poorer child behavioral functioning and greater psychological distress. Unfortunately, the predictive direction of these effects cannot be specified, given the cross-sectional nature of most studies.
The sizes of most of the correlations between psychosocial variables and nonadherence—while slightly larger than the ESs of .05–.12 for most psychosocial variables in our meta-analysis of adult recipients(52)— were nevertheless modest at best. We and others have suggested that other classes of variables, namely provider-related and healthcare systems-level factors, may ultimately prove to be stronger risk factors for nonadherence in both adult and pediatric patients.(29,38,52,71,72) Many such factors—e.g., patient-provider communication styles, costs of medications, accessibility of care, the structure of pediatric to adult care transition programs—have been found to influence adherence behaviors in other chronic disease groups,(15,29,73,74) but have rarely been examined in pediatric transplant samples.(39,58,75)
The present meta-analysis also has other limitations that reflect the body of research that we synthesized. Few studies considered areas of the regimen beyond immunosuppression adherence. Therefore, we had to group some areas of nonadherence crudely (e.g., diet and exercise) rather than conduct more fine-grained analyses. Studies varied in the age cut point used to define adolescence; this may have affected the strength of the association between adolescence and nonadherence. There were few to no studies of adherence in some types of pediatric transplant recipients (e.g., lung recipients, intestine recipients). We could not examine geographic variations in studies’ nonadherence rates because study site location was confounded with methodologic factors. There were too few studies to perform finer-grained analyses of variations in nonadherence rates according to specific strategies for adherence assessment (e.g., within the category of “indirect” assessments, comparing studies using electronic monitoring to those using another approach). Each of these limitations suggests areas for future work.
In sum, our findings have direct clinical care and research implications, as detailed in Table 3. A particularly important avenue of work is the development and testing of interventions to improve adherence. Although some nonadherence risk factors are not modifiable (e.g., children’s age), such factors identify groups most in need of immediate intervention research attention. Other potentially modifiable psychosocial correlates of nonadherence (e.g., parental and child psychological distress) might reasonably become focal points for prospective research to establish their predictive effects, followed by intervention effectiveness studies. However, intervention testing need not await definitive information on risk factors if a model of “universal prevention”(76) is adopted, in which preventive interventions are offered to all pediatric transplant recipients. While this might seem to be less efficient and more costly than targeting interventions on the basis of risk, universal prevention in the form of education and counseling is offered routinely to patients and their families pre- and posttransplant. Formally evaluating the effectiveness of the educational and other novel clinical follow-up strategies already in use in pediatric programs(36,77,78) may be the critical next step in reducing nonadherence after pediatric organ transplantation.
Table 3.
Recommendations based on meta-analytic findings on nonadherence in pediatric transplantation.
Recommendations for clinicians caring for pediatric recipients
|
Recommendations for researchers in pediatric transplantation
|
Acknowledgments
This article was supported in part by a grant from the International Transplant Nurses Society, a grant from Astellas Pharma Global Development, Inc., and grant MH072718 from the National Institute of Mental Health, Rockville, MD, USA. None of the funding agencies reviewed the contents of the article or provided comments on it.
Footnotes
*Authors’ specific contributions to the work: Dew (research conceptualization, design, data collection, data analysis, interpretation, report preparation, obtained funding for research); DeVito Dabbs, Myaskovsky, Shellmer, DiMartini, Steel, Unruh, Switzer (research conceptualization, data collection, interpretation, report preparation); Shyu (data collection, interpretation, report preparation); Shapiro (interpretation, report preparation); Greenhouse (design, data analysis, interpretation, report preparation).
References
- 1.U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients, 2007. Annual Report, Transplant Data 1997–2006. Dept. of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association; Ann Arbor, MI: 2007. [Last accessed April 23, 2009]. ( http://www.ustransplant.org/annual_reports/current/default.htm, or http://optn.transplant.hrsa.gov/data/annualReport.asp) [Google Scholar]
- 2.Magee JC, Krishnan SM, Benfield MR, et al. Pediatric transplantation in the United States, 1997–2006. Am J Transplant. 2008;8:935. doi: 10.1111/j.1600-6143.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- 3.Bucuvalas JC, Alonso E. Long-term outcomes after liver transplantation in children. Curr Opin Organ Transplant. 2008;13:247. doi: 10.1097/MOT.0b013e3282f94aab. [DOI] [PubMed] [Google Scholar]
- 4.Bucuvalas JC, Alonso E, Magee JC, et al. Improving long-term outcomes after liver transplantation in children. Am J Transplant. 2008;8:2506. doi: 10.1111/j.1600-6143.2008.02432.x. [DOI] [PubMed] [Google Scholar]
- 5.DeBolt AJ, Stewart SM, Kennard BD, et al. A survey of psychosocial adaptation in long-term survivors of pediatric liver transplants. Children’s Health Care. 1995;24:79. doi: 10.1207/s15326888chc2402_1. [DOI] [PubMed] [Google Scholar]
- 6.Gillen DL, Stehman-Breen CO, Smith JM, et al. Survival advantage of pediatric recipients of a first kidney transplant among children awaiting kidney transplantation. Am J Transplant. 2008;8:2600. doi: 10.1111/j.1600-6143.2008.02410.x. [DOI] [PubMed] [Google Scholar]
- 7.Laine J, Jalanko H, Ronnholm K, et al. Paediatric kidney transplantation. Annals Medicine. 1998;30:45. doi: 10.3109/07853899808999384. [DOI] [PubMed] [Google Scholar]
- 8.Rianthavorn P, Al-Akash SI, Ettenger RB. Kidney transplantation in children. In: Weir MR, editor. Medical management of kidney transplantation. Philadelphia: Lippincott; 2005. p. 198. [Google Scholar]
- 9.Ettenger RB, Rosenthal JT, Marik J, et al. Cadaver renal transplantation in children: Results with long-term cyclosporine immunosuppression. Clin Transplant. 1990;4:329. [Google Scholar]
- 10.Fine RN, Alonso EM, Fischel JE, et al. Pediatric transplantation of the kidney, liver and heart: summary report. Pediatr Transplant. 2004;8:75. doi: 10.1111/j.1399-3046.2004.2s050.x. [DOI] [PubMed] [Google Scholar]
- 11.Kelly DA. Current issues in pediatric transplantation. Pediatr Transplant. 2006;10:712. doi: 10.1111/j.1399-3046.2006.00567.x. [DOI] [PubMed] [Google Scholar]
- 12.Neu AM. Special issues in pediatric kidney transplantation. Advances Chronic Kidney Dis. 2006;13:62. doi: 10.1053/j.ackd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Waite E, Laraque D. Pediatric organ transplant patients and long-term care: A review. Mount Sinai J Medicine. 2006;73:1148. [PubMed] [Google Scholar]
- 14.Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Baltimore: Johns Hopkins University Press; 1979. [Google Scholar]
- 15.Sabate E World Health Organization. Adherence to long-term therapies: Evidence for action. Geneva: WHO; 2003. [Last accessed April 23, 2009]. ( http://whqlibdoc.who.int/publications/2003/9241545992.pdf) [Google Scholar]
- 16.Broyer M, Charbit M, Lebihan M, et al. Loss of graft by chronic rejection in a series of pediatric kidney transplantation: Predictive value of biopsy. Transplant Proc. 1998;30:2815. doi: 10.1016/s0041-1345(98)00822-7. [DOI] [PubMed] [Google Scholar]
- 17.Fridell JA, Jain A, Reyes J, et al. Causes of mortality beyond 1 year after primary pediatric liver transplant under tacrolimus. Transplantation. 2002;74:1721. doi: 10.1097/00007890-200212270-00014. [DOI] [PubMed] [Google Scholar]
- 18.Jarzembowski T, John E, Panaro F, et al. Impact of non-compliance on outcome after pediatric kidney transplantation: an analysis in racial subgroups. Pediatr Transplant. 2004;8:367. doi: 10.1111/j.1399-3046.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 19.Molmenti E, Mazariegos G, Bueno J, et al. Noncompliance after pediatric liver transplantation. Transplant Proc. 1999;31:408. doi: 10.1016/s0041-1345(98)01682-0. [DOI] [PubMed] [Google Scholar]
- 20.Ringewald JM, Gidding SS, Crawford SE, et al. Nonadherence is associated with late rejection in pediatric heart transplant recipients. J Pediatrics. 2001;139:75. doi: 10.1067/mpd.2001.115067. [DOI] [PubMed] [Google Scholar]
- 21.Sellers M, Singer A, Maller E, et al. Incidence of late acute rejection and progression to chronic rejection in pediatric liver recipients. Pediatr Transplant. 1997;29:428. doi: 10.1016/s0041-1345(96)00165-0. [DOI] [PubMed] [Google Scholar]
- 22.Shaw RJ, Palmer L, Blasey C, Sarwal M. A typology of non-adherence in pediatric renal transplant recipients. Pediatr Transplant. 2003;7:489. doi: 10.1046/j.1397-3142.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 23.Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113:825. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 24.Sigfusson G, Fricker FJ, Bernstein D, et al. Long-term survivors of pediatric heart transplantation: A multicenter report of sixty-eight children who have survived longer than five years. J Pediatrics. 1997;130:862. doi: 10.1016/s0022-3476(97)70270-1. [DOI] [PubMed] [Google Scholar]
- 25.Sudan DL, Shaw BW, Langnas AN. Causes of late mortality in pediatric liver transplant recipients. Annals Surg. 1998;227:289. doi: 10.1097/00000658-199802000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deierhoi MH, Haug M., 3rd Review of select transplant subpopulations at high risk of failure from standard immunosuppressive therapy. Clin Transplant. 2000;14:439. doi: 10.1034/j.1399-0012.2000.140501.x. [DOI] [PubMed] [Google Scholar]
- 27.Dhanireddy KK, Maniscalco J, Kirk AD. Is tolerance induction the answer to adolescent non-adherence? Pediatr Transplant. 2005;9:357. doi: 10.1111/j.1399-3046.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 28.Hsu DT. Biological and psychological differences in the child and adolescent transplant recipient. Pediatr Transplant. 2005;9:416. doi: 10.1111/j.1399-3046.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 29.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: Non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant. 2005;9:381. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 30.Kelly DA. Strategies for optimizing immunosuppression in adolescent transplant recipients: A focus on liver transplantation. Paediatr Drugs. 2003;5:177. doi: 10.2165/00128072-200305030-00004. [DOI] [PubMed] [Google Scholar]
- 31.Loghman-Adham M. Medication noncompliance in patients with chronic disease: Issues in dialysis and renal transplantation. Am J Managed Care. 2003;9:155. [PubMed] [Google Scholar]
- 32.Mekechuk J. A review of compliance studies in kidney transplanted adolescents. International J Health Care Qual Assurance Incorporating Leadership in Health Serv. 2004;17(4–5):i. doi: 10.1108/13660750410550548. [DOI] [PubMed] [Google Scholar]
- 33.Rianthavorn P, Ettenger RB, Malekzadeh M, et al. Noncompliance with immunosuppressive medications in pediatric and adolescent patients receiving solid-organ transplants. Transplantation. 2004;77:778. doi: 10.1097/01.tp.0000110410.11524.7b. [DOI] [PubMed] [Google Scholar]
- 34.Kahana SY, Frazier TW, Drotar D. Preliminary quantitative investigation of predictors of treatment non-adherence in pediatric transplantation: A brief report. Pediatr Transplant. 2008;12:656. doi: 10.1111/j.1399-3046.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 35.Nevins TE. Non-compliance and its management in teenagers. Pediatr Transplant. 2002;6:475. doi: 10.1034/j.1399-3046.149.ptr1s077.1.x. [DOI] [PubMed] [Google Scholar]
- 36.Rianthavorn P, Ettenger RB. Medication non-adherence in the adolescent renal transplant recipient: A clinician’s viewpoint. Pediatr Transplant. 2005;9:398. doi: 10.1111/j.1399-3046.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 37.Zelikovsky N, Schast AP. Eliciting accurate reports of adherence in a clinical interview: Development of the Medical Adherence Measure. Pediatr Nurs. 2008;34:141. [PubMed] [Google Scholar]
- 38.Griffin KJ, Elkin TD. Non-adherence in pediatric transplantation: A review of the existing literature. Pediatr Transplant. 2001;5:246. doi: 10.1034/j.1399-3046.2001.005004246.x. [DOI] [PubMed] [Google Scholar]
- 39.Bunchman TE. Compliance in pediatric transplant. Pediatr Transplant. 2000;4:165. doi: 10.1034/j.1399-3046.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 40.Cole BR. The psychosocial implications of pre-emptive transplantation. Pediatr Nephrol. 1991;5:158. doi: 10.1007/BF00852875. [DOI] [PubMed] [Google Scholar]
- 41.Stuber ML. Psychiatric aspects of organ transplantation in children and adolescents. Psychosomatics. 1993;34:379. doi: 10.1016/S0033-3182(93)71840-X. [DOI] [PubMed] [Google Scholar]
- 42.Feinstein S, Keich R, Becker-Cohen R, et al. Is noncompliance among adolescent renal transplant recipients inevitable? Pediatrics. 2005;115:969. doi: 10.1542/peds.2004-0211. [DOI] [PubMed] [Google Scholar]
- 43.Conley SB, Salvatierra O., Jr Noncompliance among adolescents: Does it impact the success of transplantation? Nephrol News Issues. 1996;10:18. [PubMed] [Google Scholar]
- 44.Fennell RS, Tucker C, Pedersen T. Demographic and medical predictors of medication compliance among ethnically different pediatric renal transplant patients. Pediatr Transplant. 2001;5:343. doi: 10.1034/j.1399-3046.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 45.Foulkes LM, Boggs SR, Fennell RS, Skibinski K. Social support, family variables, and compliance in renal transplant children. Pediatr Nephrol. 1993;7:185. doi: 10.1007/BF00864393. [DOI] [PubMed] [Google Scholar]
- 46.Meyers KE, Weiland H, Thomson PD. Paediatric renal transplantation non-compliance. Pediatr Nephrol. 1995;9:189. doi: 10.1007/BF00860742. [DOI] [PubMed] [Google Scholar]
- 47.Meyers KE, Thomson PD, Weiland H. Noncompliance in children and adolescents after renal transplantation. Transplantation. 1996;62:186. doi: 10.1097/00007890-199607270-00007. [DOI] [PubMed] [Google Scholar]
- 48.Center for Evidence Based Medicine. [Last accessed April 23, 2009];Levels of evidence and grades of recommendation. 2009 March; www.cebm.net/levels_of_evidence.asp.
- 49.Roberts AR, Yeager KR. Evidence-based practice manual: Research and outcome measures in health and human services. NY: Oxford University Press; 2004. [Google Scholar]
- 50.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology (MOOSE): A proposal for reporting. JAMA. 2000;283:2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 51.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Lancet. 1999;354:1896. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 52.Dew MA, DiMartini AF, DeVito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 53.Dew MA, DiMartini AF, Steel J, et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transplant. 2008;14:159. doi: 10.1002/lt.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: A systematic review. Arch Internal Med. 2007;167:540. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 55.DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med Care. 2004;42:200. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 56.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: A meta-analysis. Med Care. 2007;45:521. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 57.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Therapeutics. 1999;21:1074. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 58.Fine RN, Becker Y, De Geest S, et al. Nonadherence Consensus Conference summary report. Am J Transplant. 2009;9:35. doi: 10.1111/j.1600-6143.2008.02495.x. [DOI] [PubMed] [Google Scholar]
- 59.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lilienfeld DE, Stolley PD. Foundations of epidemiology. 3. NY: Oxford University Press; 1994. [Google Scholar]
- 61.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 62.Alsheikh-Ali AA, Trikalinos TA, Kent DM, Karas RH. Statins, low-density lipoprotein cholesterol, and risk of cancer. J Am Coll Cardiol. 2008;52:1141. doi: 10.1016/j.jacc.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 63.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influence on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 64.Fleiss JL. The statistical basis for meta-analysis. Stat Methods Med Res. 1993;2:121. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 65.Begg CB. Publication bias. In: Cooper H, Hedges LV, editors. Handbook of Research Synthesis. NY: Russell Sage; 1994. p. 399. [Google Scholar]
- 66.Rosenthal R. The “file-drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638. [Google Scholar]
- 67.Rosenthal R, DiMatteo MR. Meta-analysis: Recent developments in quantitative methods for literature reviews. Ann Rev Psychology. 2001;52:59. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- 68.Webber SA. The current state of, and future prospects for, cardiac transplantation in children. Cardiol in the Young. 2003;13:64. doi: 10.1017/s104795110300012x. [DOI] [PubMed] [Google Scholar]
- 69.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 70.Fredericks EM, Lopez MJ, Magee JC, Shieck V, Opipari-Arrigan L. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant. 2007;7:1974. doi: 10.1111/j.1600-6143.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 71.De Geest S, Dobbels F, Fluri C, Paris W, Troosters T. Adherence to the therapeutic regimen in heart, lung, and heart- lung transplant recipients. J Cardiovasc Nurs. 2005;20(5 Suppl):S88. doi: 10.1097/00005082-200509001-00010. [DOI] [PubMed] [Google Scholar]
- 72.Benfield M. Insurance, non-adherence--a call to action. Pediatr Transplant. 2007;11:236. doi: 10.1111/j.1399-3046.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 73.Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Therapeutics Clin Risk Management. 2005;1:189. [PMC free article] [PubMed] [Google Scholar]
- 74.Kennedy A, Sawyer S. Transition from pediatric to adult services: Are we getting it right? Curr Opin Pediatr. 2008;20:403. doi: 10.1097/MOP.0b013e328305e128. [DOI] [PubMed] [Google Scholar]
- 75.Bell LE, Bartosh SM, Davis CL, et al. Adolescent transition to adult care in solid organ transplantation: A consensus conference. Am J Transplant. 2008;8:2230. doi: 10.1111/j.1600-6143.2008.02415.x. [DOI] [PubMed] [Google Scholar]
- 76.Kellam SG, Langevin DJ. A framework for understanding “evidence” in prevention research and programs. Preven Sci. 2003;4:137. doi: 10.1023/a:1024693321963. [DOI] [PubMed] [Google Scholar]
- 77.Emre S. Posttraumatic stress disorder in posttransplant children: Creating a clinical program to address their needs. CNS Spectr. 2006;11:118. doi: 10.1017/s1092852900010658. [DOI] [PubMed] [Google Scholar]
- 78.Shemesh E, Annunziato RA, Shneider BL, et al. Improving adherence to medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12:316. doi: 10.1111/j.1399-3046.2007.00791.x. [DOI] [PubMed] [Google Scholar]