Abstract
Chromium is a widespread industrial compound. The soluble hexavalent chromium Cr (VI) is an environmental contaminant widely recognized as carcinogen, mutagen, and teratogen toward humans and animals. The fate of chromium in the environment is dependent on its oxidation state. The reduction of Cr (VI) to Cr (III) results in the formation of reactive intermediates leading to oxidative tissue damage and cellular injury. In the present investigation, Potassium dichromate was given intraperitoneally to Sprague-Dawley rats for 5 days with the doses of 2.5, 5.0, 7.5, and 10 mg/kg body weight per day. Oxidative stress including the level of reactive oxygen species (ROS), the extent of lipid peroxidation and the activity of antioxidant enzymes in both liver and kidney was determined. DNA damage in peripheral blood lymphocytes was determined by single-cell gel electrophoresis (comet assay). The results indicated that administration of Cr (VI) had caused a significant increase of ROS level in both liver and kidney after 5 days of exposure, accompanied with a dose-dependent increase in superoxide dismutase and catalase activities. The malondialdehyde content in liver and kidney was elevated as compared with the control animals. Dose- and time-dependent effects were observed on DNA damage after 24, 48, 72, and 96 h posttreatment. The results obtained from the present study showed that Cr (VI) could induce dose- and time-dependent effects on DNA damage, both liver and kidney show defense against chromium-induced oxidative stress by enhancing their antioxidant enzyme activity. However, liver was found to exhibit more antioxidant defense than the kidney.
Keywords: chromium, malondialdehyde, antioxidant enzymes, superoxide dismutase, catalase, Sprague-Dawley rats, liver, kidneys
INTRODUCTION
Chromium (Cr) is a naturally occurring heavy metal found commonly in the environment in two valence states: trivalent Cr (III) and hexavalent Cr (VI). It is widely used in numerous industrial processes and as a result, is a contaminant of many environmental systems (Cohen et al., 1993). Commercially chromium compounds are used in industrial welding, metal finishes, leather tanning, and wood preservation and it has been a nonnegligible pollutant in the world (Norseth, 1981; Wang et al., 2006). Studies by animal model also found many harmful effects of Cr (VI) on mammals. Subcutaneous administration of Cr (VI) to rats caused severe progressive proteinuria, urea nitrogen, and creatinine, as well as elevation in serum alanine aminotransferase activity and hepatic lipid peroxide formation (Kim and Na, 1991). Similar studies reported by Gumbleton and Nicholls (1988) found that Cr (VI) induced renal damage in rats when administered by single s.c. injections. Bagchi et al. demonstrated that rats received Cr (VI) orally in water induced hepatic mitochondrial and microsomal lipid peroxidation (LPO), as well as enhanced excretion of urinary lipid metabolites including malondialdehyde (MDA) (Bagchi et al., 1995). Moreover, some adverse health effects induced by Cr (VI) have been reported in human. Reports of epidemiological investigations have shown that respiratory cancers had been found in workers occupationally exposed to Cr (VI) compounds (Costa, 1997; Dayan and Paine, 2001). DNA strand breaks in peripheral lymphocytes and LPO products in urine observed in chromium exposed workers in many researches also showed evidence of the Cr (VI)-induced toxicity to humans (Gambelunghe et al., 2003; Goulart et al., 2005).
The carcinogenicity of specific chromium compounds is influenced by both the valence and the solubility of the chromium species. Chromium (VI) compounds have been reported to be more toxic and carcinogenic than chromium (III) compounds (Connett et al., 1983; De Flora et al., 1990) because the former can pass through cell membranes more easily than the latter (De Flora and Wetterhahn, 1989). Once inside the cell, Cr (VI) is reduced to its lower oxidation states [Cr (V)] and [Cr (IV)] and then Cr (III) by low molecular weight molecules, enzymatic, and nonenzymatic reductants (Shi et al., 1999). These reactive chromium intermediates are capable of generating a whole spectrum of reactive oxygen species (ROS), which is an important characteristic of Cr (VI) metabolism (O’Brien et al., 2003). Excessive quantity of ROS generated by these reactions can cause injury to cellular proteins, lipids, and DNA leading to a state known as oxidative stress (Stohs and Bagchi, 1995; Nordberg and Arner, 2001). Therefore, one of the most important damage arosed by extraneous Cr (VI) is massive production of ROS during the reduction of Cr (VI) in the cell.
LPO, the oxidative catabolism of polyunsaturated fatty acids, is widely accepted as a general mechanism for cellular injury and death (Gutteridge and Quinlan, 1983; Halliwell, 1984). LPO and free radical generation are complex and deleterious processes that are closely related to toxicity (Murray et al., 1988). LOP has been implicated in diverse pathological conditions, including atherosclerosis (Holvoet and Collen, 1998), aging (Spiteller, 2001), rheumatoid arthritis (Henrotin et al., 1992), and cancer (Marnett, 2000). It is also involved in the toxicity of pesticides (Bismuth et al., 1990), solvents (Brattin et al., 1985), and metals (Kasprzak, 1995). The extension of the oxidative catabolism of lipid membranes can be evaluated by several endpoints, but the most widely used method is the quantification of MDA, one of the stable aldehydic products of lipoperoxidation, present in biological samples (Gutteridge, 1995; De Zwart et al., 1999).
Antioxidants enzymes are frequently used as markers of oxidative stress (Gutteridge, 1995). Among these biomarkers, superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) are important in the preservation of homeostasis for normal cell function. Enzymes control the rate of metabolic reactions by lowering the amount of activation energy needed to start a reaction. Enzymes exhibit specificity by binding to particular substrates. CAT is found in the peroxisomes of the liver and kidney and acts to metabolize hydrogen peroxide, a toxic by-product of metabolic reactions, to water and free oxygen (Knight, 1997). SOD scavenges the highly toxic superoxide and converts it to hydrogen peroxide (Fridovich, 1989). The hydrogen peroxide that is produced by SOD is maintained at a safe concentration by the glutathione system. GPX protects the membrane lipids from oxidative damage (Kantola et al., 1988).
Although chromium and chromium-containing compounds have been the subject of important toxicology research, there exists a lack of appropriate animal model for oxidative stress assessment, DNA damage, as well as scarcity of scientific data describing the antioxidant defense relationship with respect to their toxicity in in vivo systems. Therefore, the present work was undertaken to study the role of oxidative stress and DNA damage in hexavalent chromium-induced toxicity and the modulation of intracellular antioxidant defense mechanisms in Sprague-Dawley rats.
MATERIALS AND METHODS
Chemicals
Potassium dichromate, sodium chloride, sucrose, Triton X-100, hydrochloric acid, histopaque-1077, NaOH, ethanol, trypan-blue, and EDTA were obtained from Sigma-Aldrich (St. Louis, MO). They were of analytical grade or highest grade available. Lipid peroxidation (LPO) and SOD, assay kits were purchased from Calbiochem (San Diego, CA). Phosphate buffer (pH 7.4) was obtained from GIBCO (New York, NY). Comet assay kit was purchased from Trevigen (Gaithersburg, MD).
Animal Maintenance
Healthy adult male Sprague-Dawley rats [5–7 weeks of age, with average body weight (BW) of 60 ± 2 g] were used in this study. They were obtained from Harlan-Sprague-Dawley Breeding laboratories (Indianapolis, IN). The animals were randomly selected and housed in polycarbonate cages (three rats per cage) with steel wire tops and corn-cob bedding. They were maintained in a controlled atmosphere with a 12 h:12 h dark/light cycle, a temperature of (22 ± 2)°C and 50–70% relative humidity with free access to pelleted feed and fresh tap water. The animals were supplied with dry food pellets commercially available from PMI Feeds (St. Louis, MO). They were allowed to acclimate for 10 days before treatment. Whole blood samples were collected from the tail vein at 24, 48, 72, and 96 h posttreatment in heparinized tubes for the single-cell gel electrophoresis [comet assay].
Experimental Design
Groups of five rats each were treated with four different potassium dichromate dose levels. Potassium dichromate was diluted with distilled water (as required) and intraperitoneally administered to animals at the doses of 2.5, 5, 7.5, and 10 mg/kg BW, one dose per 24 h given for 5 days. We selected intraperitoneal injection because it is the most commonly used method that is simple and also, for many agents such as chromium compounds, it will tend to maximize chemical exposure to the target organs. Each rat received a total of five doses at 24 h intervals. The cumulative dose of potassium dichromate given to each group of rats was thus 12.5, 25, 37.5, or 50 mg/kg BW. Distilled water was administered to the 5 animals of control group in the same manner as in the treatment groups. At the end of the exposure, after overnight starvation, the liver and both kidneys were removed under anesthesia. The organs were washed thoroughly in ice-cold physiological saline and weighed. The biological material not used immediately was stored frozen at −80°C until further analysis.
The local Ethics committee for animal experiments [Institutional Animal Care and Use Committee] at Jackson State University, Jackson, MS, approved this study. Procedures involving the animals and their care conformed to the institutional guidelines, in compliance with national and international laws and guidelines for the use of animals in biomedical research (Giles, 1987).
Preparation of Homogenates
At the end of the 5 days exposure to potassium dichromate, liver and both kidneys were excised under anesthesia. The organs were washed thoroughly in ice-cold physiological saline and weighed. A 10% homogenate of each tissue was prepared separately in 0.05 M phosphate buffer (pH 7.4) containing 0.1 mM EDTA using a motor driven Teflon-pestle homogenizer (Fischer), followed by sonication (Branson sonifer), and centrifugation at 4000 rpm for 10 min at 4°C. The supernatant was decanted and centrifuged at 16 000 rpm for 60 min at 4°C. The supernatant fraction obtained was called “homogenate” and used for the assays.
ROS Detection
Reactive oxygen species (ROS) production was quantified by the DCFH-DA method (Lawler et al., 2003) based on the ROS-dependent oxidation of DCFH-DA to DCF. An aliquot of homogenates was centrifuged at 1000 × g for 10 min (4°C). The supernatants were re-centrifuged at 20 000 × g for 20 min at 4°C, and then the pellet was resuspended. The DCFH-DA solution with the final concentration of 50 µM and resuspension were incubated for 30 min at 37°C. Fluorescence of the samples was monitored at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
Comet Assay
Rat lymphocytes were isolated and resuspended in PBS. Following isolation, the cells were mixed with 0.4% Trypan blue solution, after 15–20 min cells were counted and checked for viability. The remaining cells were immediately used for single-cell gel electrophoresis. The assay was performed according to Singh et al. (1988) with slight modifications. All the steps were conducted under yellow lamp in the dark to prevent additional DNA damage. Stained slides are viewed under automated robotic epiflorescent microscope.
A total of 150 individual cells were screened/sample [duplicate, each with 75 cells}.
Slides were read using DNA damage analysis software [Loats Associates, Westminster, MD].
Malondialdehyde Determination
Malondialdehyde (MDA) concentration was measured in 10% homogenates of liver and kidney using LPO assay kit from Calbiochem (San Diego, CA). Briefly, 0.65 mL of 10.3 mM N-methyl-2-phenyl-indole in acetonitrile was added to 0.2 mL of sample (10% homogenate of liver or kidney). After vortexing for 3–4 s and adding 0.15 mL of 37% HCl, samples were mixed well and closed with a tight stopper and incubated at 45°C for 60 min. The samples were then cooled on ice, centrifuged and the absorbance was measured spectrophotometrically at 586 nm. A calibration curve of an accurately prepared standard MDA solution (from 2 to 20 nmol/mL) was also run for quantitation. Measurements of each group were performed in triplicate. The standard deviations were less than ± 10%.
Antioxidant Enzymes Assay
The activity of SOD was determined in 10% homogenates of liver and kidney prepared in cold 0.25 M sucrose. The homogenates were centrifuged at 8500 rpm for 10 min at 4°C. Next 400 mL of cold extraction reagent (absolute ethanol/chloroform 62.5/37.5 v/v) were added into 250 mL of supernatant, mixed for 30 s, and centrifuged at 3000 rpm for 5 min at 4°C. The activity of SOD was measured in the aqueous phase using SOD assay kit from Calbiochem (San Diego, CA). One unit of enzyme activity is defined as the amount of enzyme that decreases the initial rate to 50% of its maximal value for the particular tissue being assayed.
Catalase (CAT) is also known as hydrogen peroxide oxidoreductase. To determine its activity, 10% homogenates of liver and kidney, prepared in 0.05 M phosphate buffer were used. CAT activity was determined according to Aebi (1984). Briefly, the reaction mixture consisted of 0.1 mL of supernatant and 0.01 mL of absolute ethanol. This was vortexed well and kept on ice (Ice Bucket, Fischer-Scientific, Pittsburg, PA, USA) for 30 min. Then, the tubes containing the sample and the blank were brought to room temperature and 0.01 mL of Triton X-100 was added and vortexed well till the whole tissue extract was completely dissolved. Next, 0.66 M H2O2 in phosphate buffer was prepared afresh at the time of reaction and 0.1 mL of this buffer containing H2O2 was added to the above reaction mixture, and the decrease in the absorbance was read in a spectrophotometer at 240 nm against a blank for 60 s. Care was taken to carry out the reactions in the dark. The results were expressed in nmoles of H2O2 metabolized/mg protein tissue. One unit corresponds to 1 nmol CAT per gram tissue.
Total Protein Determination
The concentration of total protein in the 10% homogenates (prepared in phosphate buffer, pH 7.4) of liver and kidney was estimated according to Lowry et al. (1951) method using bovine albumin (Sigma) as a standard.
Data Analysis and Statistics
Data were compared by ANOVA. Statistical analysis was performed using SAS for Windows 2003 package program. Using the Dunnett test, multiple comparisons were performed. All values were reported as means ± SD for all the experiments. The significance level was set at P < 0.05.
RESULTS
DNA Damage
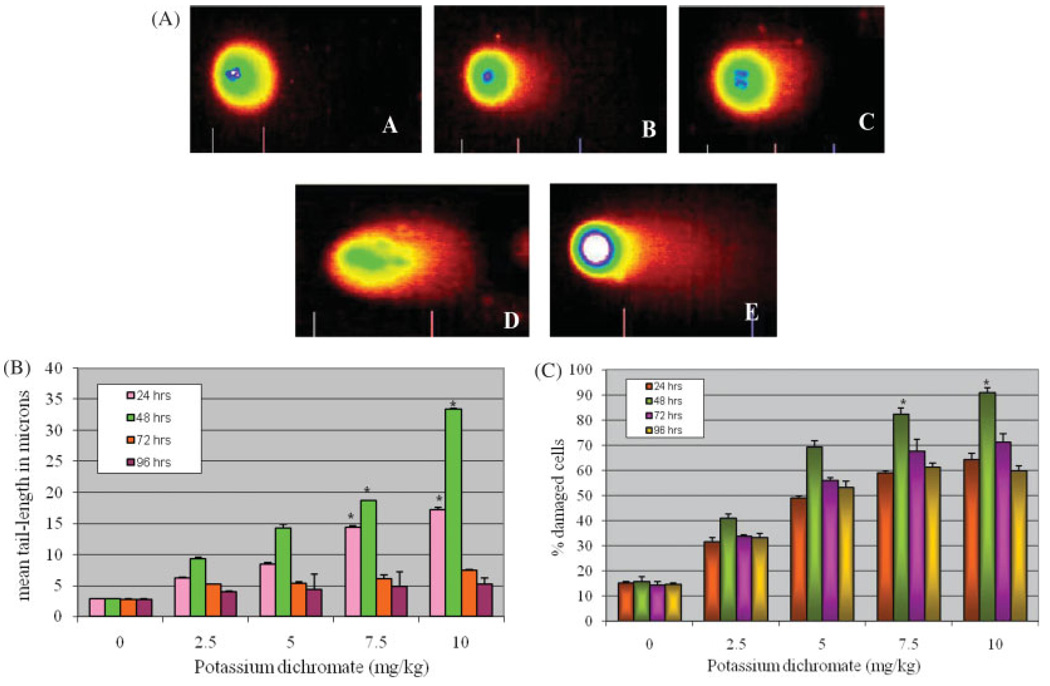
Comet tail length is an important parameter in evaluating the DNA damage. All the doses of potassium dichromate induced statistically significant increase in mean comet tail length [6.23–33.42 µM] indicating DNA damage when compared with controls [2.79 µM]. Maximum increase in mean comet tail length was observed at 10 mg/kg BW at 48 h posttreatment [33.42 µM]. The mean comet tail length showed a clear dose-dependent increase from 2.5 to 10 mg/kg BW. A gradual decrease in the tail lengths from 72 h post-treatment [3.74 µM] was observed by the 96 h and values had returned almost to control levels at all doses, indicating repair of the damaged DNA and/or loss of heavily damaged cells. The percentage of DNA damaged cells that were observed in chromium exposed leukocytes ranged from (42.3–85.7%) when compared with the control (15.3%). The results of DNA damage and percentage of DNA damaged cells are illustrated in Figure 1(A–C), respectively. Representative comet assay images of control leukocytes (A) and potassium dichromate treated leukocytes at 2.5 mg/kg (B); 5 mg/kg (C); 7.5 mg/kg (D); and 10 mg/kg (E) are presented in Figure 1. Genotoxicity as characteristized by the length of the comet tail and the percentage of DNA damage are represented in Figure 2 and Figure 3, respectively.
Fig. 1.
Single Cell Gel Electrophoresis assessment of potassium dichromate toxicity in peripheral blood leukocytes of Sprague-Dawley rats: (A) Representative Comet images of control (A), and 2.5 (B), 5.0 (C), 7.5 (D), and 10 mg/kg (E); (B) Effect of various doses of potassium dichromate on DNA migration at various time intervals; and (C) Percentages of DNA damaged cells observed in rat leukocytes treated with potassium dichromate at different time points. Each experiment was done in triplicate. Data were represented as means ± SDs. Statistical significance was indicated as (*) for P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
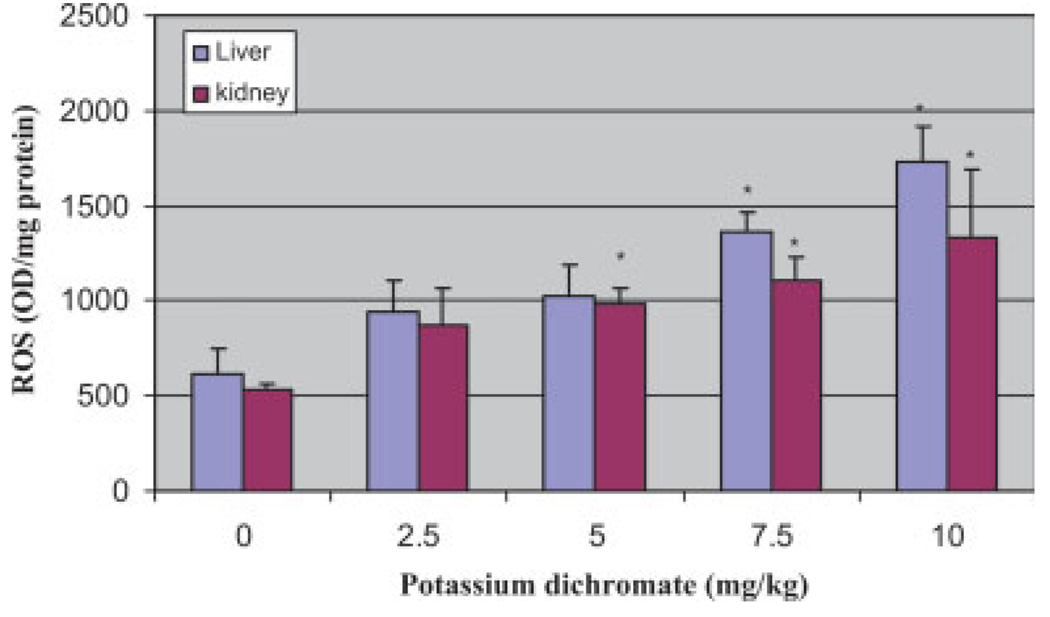
Fig. 2.
Effect of potassium dichromate on detection of ROS in the liver and kidney of Sprague-Dawley rats. Each experiment was done in triplicate. Data were represented as means ± SDs. Statistical significance was indicated as (*) for P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
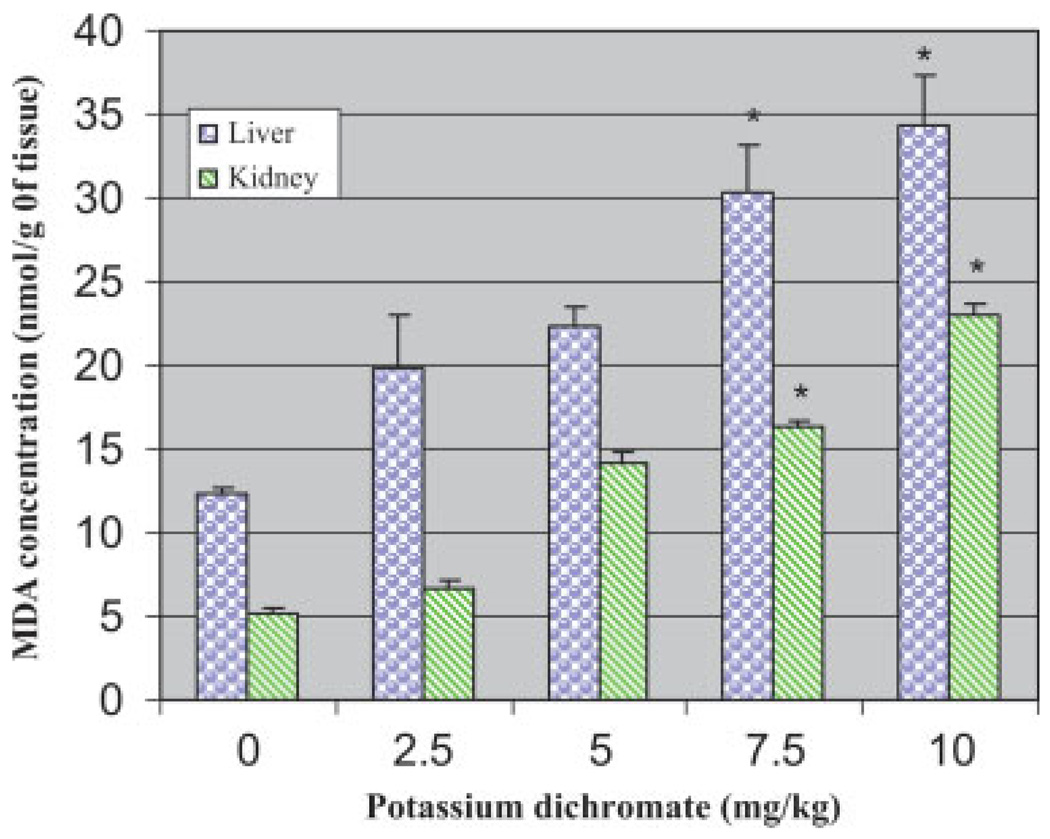
Fig. 3.
Effect of potassium dichromate on malondialdehyde concentration in liver and kidney of Sprague-Dawley rats. Each experiment was done in triplicate. Data were represented as means ± SDs. Statistical significance was indicated as (*) for P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
ROS Detection in Liver and Kidneys
ROS were determined in liver and kidney homogenates in control and treatment groups after administration of potassium dichromate to rats for 5 days. The administration of Cr (VI) to rats significantly enhanced the ROS level at four tested doses as compared with the control animals and increases were dose-dependent. However, the level of ROS in kidneys was lower compared with liver at all four dose levels. Figure 2 represents the results of ROS detection.
MDA Concentration in Liver and Kidneys
One of the methods for evaluating LPO is measurement of the circulating MDA concentration. Potassium dichromate exposure significantly (P < 0.05) increased the concentration of MDA in both liver (19.8–34.3 nmol/g liver) and kidneys (6.7–22.9 nmol/g kidney) when compared with the control (12.3-liver; 5.22-kidney). Results are illustrated in Figure 3. The increase in MDA concentration in both liver and kidneys was found to be dose-dependent, indicating a gradual increase with increasing dose of potassium dichromate. However, the liver exhibited more oxidative stress than the kidneys.
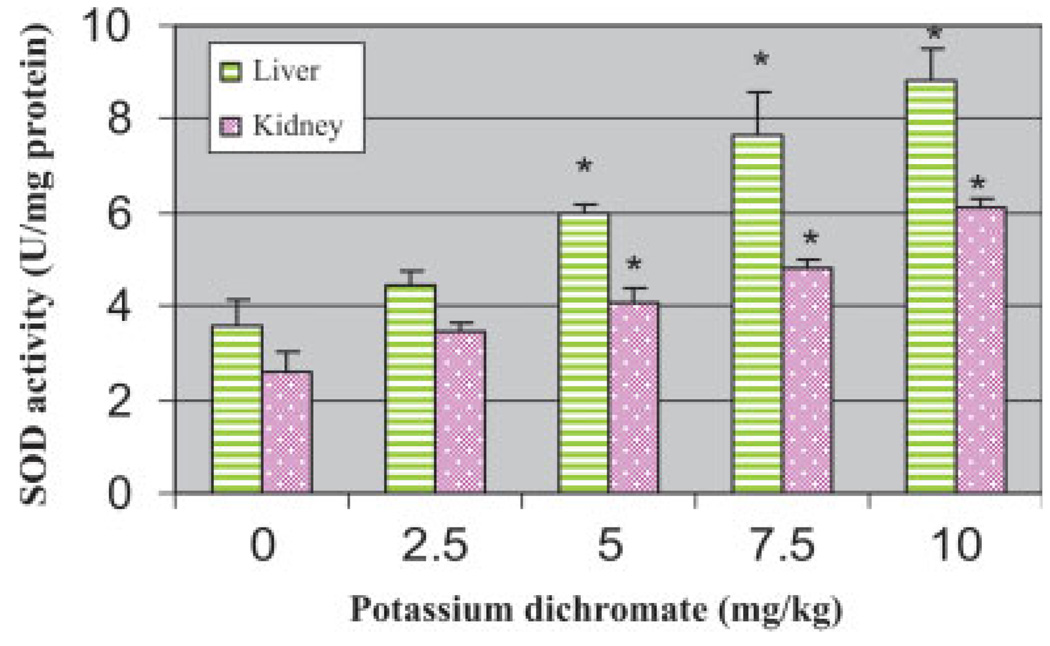
SOD Activity in Liver and Kidneys
SOD activity was determined in 10% homogenates of liver and kidneys of Sprague-Dawley rats. There was a significant increase in the activity of SOD in potassium dichromate treated rats compared with their respective controls in both organs. The results of the SOD activity are represented in Figure 4. A similar trend of a significant increase in SOD activity was found in both liver [4.43–8.82 U/mg of protein] and kidney [3.44–6.09 U/mg of protein] homogenates; however, the liver exhibited more enzymatic activity than the kidneys.
Fig. 4.
Effect of Potassium dichromate on the activity of SOD in liver and kidney of Sprague-Dawley rats. Each experiment was done in triplicate. Data were represented as means ± SDs. Statistical significance was indicated as (*) for P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
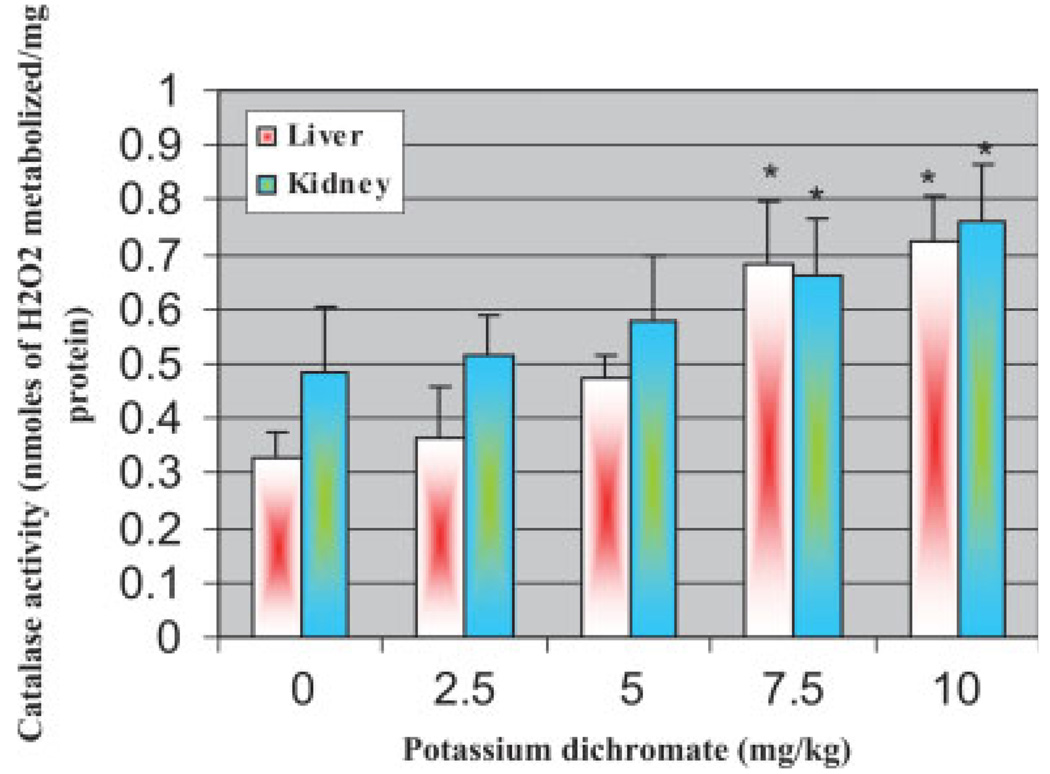
CAT Activity in Liver and Kidneys
Potassium dichromate treatment showed an increase in the activity of CAT in both liver and kidney homogenates. The highest doses (7.5 and 10 mg/kg) showed a significant increase in the activity of CAT in both organs when compared with the control. However, the trend was found to be different, that is, kidney exhibiting a slightly more CAT activity than the liver. The CAT activity was expected to rise in response to the tissue trauma. Results of CAT activity are illustrated in Figure 5.
Fig. 5.
Effect of potassium dichromate on the activity of catalase in liver and kidney of Sprague-Dawley rats. Each experiment was done in triplicate. Data were represented as means ± SDs. Statistical significance was indicated as (*) for P < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The present study was undertaken to assess the extent of DNA damage in peripheral blood leukocytes and the oxidative status of liver and kidneys during exposure to various doses of potassium dichromate. For this purpose, the level of ROS, the activities of antioxidant enzymes such as SOD, CAT, and the concentration of MDA as an indicator of LPO were determined in both organs. We have selected liver and kidneys in our investigation because these are major target organs of toxicity. In addition, the liver is primary organ of biotransformation of xenobiotic compounds. It contains metabolizing enzymes that change most toxicants to less toxic and more water-soluble but sometimes can lead to bioactivation. Liver is often important in tests of oxidative stress because LPO is a major cause of liver lesions. Kidneys on the other hand filter, detoxify, or bioactivate toxicants (Reed, 1985; Plaa et al., 1997). They are susceptible to the effects of oxidative stress or stress in general because of their dependence on osmotic pressure and a concentration gradient. The kidneys are also very important because they are the sites of peroxisomes, which store the enzyme CAT (Hook and Goldstein, 1993).
In this study, we observed that there was a significant increase in the level of ROS in both the organs, however, liver exhibited higher level than the kidneys. ROS have been implicated in the toxicity of chromium (VI) by several authors (Sugiyama, 1992; Bagchi et al., 1997; O’Brien et al., 2003). Their formation with subsequent cellular damage is considered as the common molecular mechanism of Cr (VI)-induced toxicity. According to this hypothesis, chromium (VI) itself is not a cytotoxic agent but rather an oxygen free radical generator through cellular reduction to chromium (VI) (Miesel et al., 1995). Chromium reduction intermediates are believed to react with hydrogen peroxide to form the hydroxyl radical (HO) (Kadiiska et al., 1994), which may finally attack proteins, DNA, and membranes lipids thereby disrupting cellular functions and integrity (Bagchi et al., 1997).
In the present study, measuring the circulating MDA in homogenates of liver and kidneys of potassium dichromate-exposed and control rats assessed LPO. In both liver and kidneys, there was a significant increase in the concentration of MDA after 5 days of potassium dichromate administration. These results are in accordance with those obtained by (Bagchi et al., 1995, 1997) who detected oxidative lipid metabolites in the urine of Cr (VI) treated rats. The increase observed in LPO may be because of the formation of HO through a Fenton/Haber-Weiss reaction, catalyzed by chromium. This radical is capable of abstracting a hydrogen atom from a methylene group of polyunsaturated fatty acids enhancing LPO. However, Cr (VI) induced markedly higher levels of LPO in liver compared with kidneys. This was an unexpected finding as the liver was thought to have more antioxidant defense because of its metabolic role in detoxification.
The frequency of single-strand breaks showed a clear dose-related increase up to 10 mg/kg bodyweight in our investigation. Maximum DNA damage was observed at 48 h posttreatment when compared with the controls. Similar results were reported with other heavy metals in rodents using comet assay (Dana Devi et al., 2001; Wang et al., 2006). Cr (VI) itself is not reactive to DNA, however, the chromium metabolites radicals produced during reduction can subsequently attack macromolecules and lead to multiform DNA damages, for example, strand breakage, DNA–protein cross-links, DNA–DNA cross-links, Cr–DNA adducts and base modifications in cells. Especially DNA strand breaks are mainly ascribed to the ROS (Wang et al., 2006). However, at later time intervals, from 72 h posttreatment onward, a gradual decrease in mean comet tail lengths of all doses was observed, showing a time-dependent decrease in the DNA damage. Replication arrest may be a common response of DNA polymerase to DNA–Cr lesions and provide a plausible mechanism for the inhibition of DNA synthesis and S-phase cell-cycle delay that occurs in mammalian cells treated with genotoxic hexavalent chromium (Bridgewater et al., 1998). Single cell gel electrophoresis (comet assay) is a highly sensitive technique to evaluate single strand breaks and alkali labile sites in DNA of individual cells.
The activities of the antioxidant enzymes SOD and CAT were increased by chromium treatment in both the liver and kidneys. Since SOD catalyzes the dismutation of superoxide anion to H2O2, which is in turn the substrate of CAT, this fact could explain the observed increment of the two enzyme activities. It has been reported that CAT and SOD inhibited chromate-induced DNA strand breaks in cultured cell (Sugiyama, 1992). As these enzymes have a protective role against oxygen free radical-induced damage, their induction can be understood as an adaptive response to oxidative stress. Sengupta et al. (1990) also observed similar results in rats exposed to hexavalent chromium. Increased generation of superoxide radicals could lead to LPO. SOD activity also reflects the intensity of the stress because of toxic action. Heavy metals are known to increase the biochemical stress in the organisms because of deterioration of metabolic cascade (Hudecova and Ginter, 1992).
In summary, the current study demonstrates that intraperitoneal administration of Cr (VI) to the rats during 5 days could induce DNA damage in peripheral blood lymphocytes and oxidative stress in liver and kidneys. Both liver and kidney showed defense against chromium-induced oxidative stress by enhancing their antioxidant enzymes. However, the liver was found to exhibit a higher antioxidant defense than the kidney. ROS may play an essential role in DNA damage induced by Cr (VI) in vivo. Our results show that the use of thiobarbituric acid reactive substances as a marker of oxidative stress should be complemented with antioxidant parameters both in liver and kidneys namely SOD and CAT. The results support an involvement of the oxidative damage pathway in the mechanism of toxicity of chromium. An understanding of the behavior of these antioxidant enzymes can aid in the understanding of chromium-induced toxicity.
Acknowledgments
Contract grant sponsor: The National Institutes of Health – RCMI.
Contract grant number: 1G12RR13459.
The authors would like to thank Dr. Ronald Mason Jr., President, Dr. Abdul K. Mohamed, Dean Emeritus, and Dr. Mark Hardy, CSET Dean, Jackson State University, for their technical and administrative support in this project.
REFERENCES
- Aebi HE. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Hassoun EA, Bagchi M, Muldoon D, Stohs SJ. Oxidative stress induced by chronic administration of sodium dichromate (Cr VI) to rats. Comp Biochem Physiol. 1995;110C:281–287. doi: 10.1016/0742-8413(94)00103-h. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ. Induction of oxidative stress by chronic administration of sodium dichromate (chromium VI) and cadmium chloride (cadmium II) to rats. Free Rad Biol Med. 1997;22:471–478. doi: 10.1016/s0891-5849(96)00352-8. [DOI] [PubMed] [Google Scholar]
- Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning: An overview of current status. Drug Saf. 1990;5:243–251. doi: 10.2165/00002018-199005040-00002. [DOI] [PubMed] [Google Scholar]
- Brattin WJ, Glende EA, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Bridgewater LC, Manning FC, Patierno SR. Arrest of replication by mammalian DNA polymerases alpha and beta caused by chromium-DNA lesions. Mol Carcinog. 1998;23:201–206. [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit Rev Toxicol. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Connett PH, Wetterhahn KE. Metabolism of carcinogenic chromate by cellular constituents. Struct Bond. 1983;54:93–124. [Google Scholar]
- Costa M. Toxicity and carcinogenicity of Cr (VI) in animal models and humans. Crit Rev Toxicol. 1997;27:431–442. doi: 10.3109/10408449709078442. Review. [DOI] [PubMed] [Google Scholar]
- Dana Devi K, Rozati R, Saleha Banu B, Jamil K, Grover P. In vivo genotoxic effect of potassium dichromate in mice leukocytes using comet assay. Food Chem Toxicol. 2001;39:859–865. doi: 10.1016/s0278-6915(01)00019-9. [DOI] [PubMed] [Google Scholar]
- Dayan AD, Paine AJ. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum Exp Toxicol. 2001;20:439–451. doi: 10.1191/096032701682693062. [DOI] [PubMed] [Google Scholar]
- De Flora S, Wetterhahn KE. Mechanisms of chromium metabolism and genotoxicity. Life Chem Rep. 1989;7:169–244. [Google Scholar]
- De Flora S, Bagnasco M, Serra D, Zanacchi P. Genotoxicity of chromium compounds: A review. Mutat Res. 1990;238:99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- De Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. Review. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutase. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- Gambelunghe A, Piccinini R, Ambrogi M, Villarini M, Moretti M, Marchetti C, Abbritti G, Muzi G. Primary DNA damage in chrome-plating workers. Toxicology. 2003;188:187–195. doi: 10.1016/s0300-483x(03)00088-x. [DOI] [PubMed] [Google Scholar]
- Giles AR. Guidelines for the use of animals in biomedical research. Thromb Haemostasis. 1987;58:1078–1984. [PubMed] [Google Scholar]
- Goulart M, Batoreu MC, Rodrigues AS, Laires A, Rueff J. Lipoperoxidation products and thiol antioxidants in chromium exposed workers. Mutagenesis. 2005;20:311–315. doi: 10.1093/mutage/gei043. [DOI] [PubMed] [Google Scholar]
- Gumbleton M, Nicholls PJ. Dose-response and time-response biochemical and histological study of potassium dichromate-induced nephrotoxicity in the rat. Food Chem Toxicol. 1988;26:37–44. doi: 10.1016/0278-6915(88)90039-7. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- Gutteridge JMC, Quinlan GJ. Malondialdehyde formation from lipid peroxides in thiobarbituric acid test. The role of lipid radicals, iron salts and metal chelator. J Appl Biochem. 1983;5:293–299. [PubMed] [Google Scholar]
- Halliwell B. Oxygen radicals: A common sense look at their nature and medical importance. Med Biol. 1984;62:71–77. [PubMed] [Google Scholar]
- Henrotin Y, Deby-Dupont G, Deby C, Franchimont P, Emerit I. Active oxygen species, articular inflammation and cartilage damage. EXS. 1992;62:308–322. doi: 10.1007/978-3-0348-7460-1_31. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Collen D. Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis. 1998;137 Suppl:S33–S38. doi: 10.1016/s0021-9150(97)00305-5. [DOI] [PubMed] [Google Scholar]
- Hook JB, Goldstein JR. Target Organ Toxicology Series. 2nd ed. Washington, DC: Taylor & Francis; 1993. Toxicology of the kidney; p. 576. [Google Scholar]
- Hudecova A, Ginter E. The influence of ascorbic acid on lipid peroxidation in guinea pigs intoxicated with cadmium. Food Chem Toxicol. 1992;30:1011–1015. doi: 10.1016/0278-6915(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Xiang QH, Mason RP. In vivo free radical generation by chromium(VI): An electron spin resonance spin-trapping investigation. Chem Res Toxicol. 1994;7:800–805. doi: 10.1021/tx00042a013. [DOI] [PubMed] [Google Scholar]
- Kantola M, Sarranen M, Vanha PT. Selenium and glutathione peroxidase in seminal plasma of men and bulls. J Reprod Fertil. 1988;83:785–794. doi: 10.1530/jrf.0.0830785. [DOI] [PubMed] [Google Scholar]
- Kasprzak KS. Possible role of oxidative damage in metal induced carcinogensis. Cancer Inv. 1995;13:411–430. doi: 10.3109/07357909509031921. [DOI] [PubMed] [Google Scholar]
- Kim E, Na KJ. Nephrotoxicity of sodium dichromate depending on the route of administration. Arch Toxicol. 1991;65:537–541. doi: 10.1007/BF01973713. [DOI] [PubMed] [Google Scholar]
- Knight JA. Reactive oxygen species and the neuro-degenerative disorders. Ann Clin Lab Sci. 1997;27:11–25. [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radical Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- Miesel R, Kroger H, Kurpisz M, Wesser U. Induction of arthritis in mice and rats by potassium peroxochromate and assessment of disease activity by whole blood chemiluminescence and 99mpertechnetate-imaging. Free Radic Res. 1995;23:213–227. doi: 10.3109/10715769509064035. [DOI] [PubMed] [Google Scholar]
- Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s Biochemistry. 21st ed. Englewood Cliffs, NJ: Prentice Hall; 1988. pp. 138–139. [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Norseth T. The carcinogenicity of chromium—Review. Environ Health Perspect. 1981;40:121–130. doi: 10.1289/ehp.8140121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: Role of cellular response, repair and recovery mechanisms. Mutat Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Plaa LG, Hewitt RW. Target Organ Toxicology Series. 2nd ed. Washington, DC: Taylor & Francis; 1997. Toxicology of the liver; p. 431. [Google Scholar]
- Reed DJ. Cellular defense mechanisms against reactive metabolites. In: Anders MW, editor. Bioactivation of Foreign Compounds. Orlando: Academic Press; 1985. pp. 71–108. [Google Scholar]
- Sengupta T, Chattopadhyay D, Ghosh N, Das M, Chatterjee GC. Effect of chromium administration on glutathione cycle of rat intestinal epithelial cells. Ind J Exp Biol. 1990;28:1132–1135. [PubMed] [Google Scholar]
- Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium (VI) and its relationship to carcinogenesis. J Toxicol Environ Health. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36:1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Sugiyama M. Role of physiological antioxidants in Cr (VI) induced cellular injury. Free Radic Biol Med. 1992;12:397–407. doi: 10.1016/0891-5849(92)90089-y. [DOI] [PubMed] [Google Scholar]
- Wang XF, Xing ML, Shen Y, Zhu X, Xu LH. Oral administration of Cr(VI) induced oxidative stress. DNA damage and apoptotic cell death in mice. Toxicology. 2006;228:16–23. doi: 10.1016/j.tox.2006.08.005. [DOI] [PubMed] [Google Scholar]