Abstract
Purpose
There are neural connections between the upper and lower limbs of humans that enable muscle activation in one limb pair (upper or lower) to modulate muscle activation in the other limb pair (lower or upper, respectively). The aims of this study were to extend previous findings regarding submaximal exercise to maximal effort exercise and determine if there is an ipsilateral or contralateral bias to the neural coupling during a rhythmic locomotor-like task.
Methods
We measured upper and lower limb muscle activity, joint kinematics, and limb forces in neurologically intact subjects (n = 16) as they performed recumbent stepping using different combinations of upper and lower limb efforts.
Results
We found increased muscle activation in passive lower limbs during active upper limb effort compared to passive upper limb effort. Likewise, increased muscle activation in passive upper limbs occurred during active lower limb effort compared to passive lower limb effort, suggesting a bidirectional effect. Maximal muscle activation in the active lower limbs was not different between conditions with active upper limb effort and conditions with passive upper limb movement. Similarly, maximal muscle activation in the active upper limbs was not different between conditions with active lower limb effort and conditions with passive lower limb movement. Further comparisons revealed that neural coupling was primarily from active upper limb muscles to passive ipsilateral lower limb muscles.
Conclusion
These findings indicate that interlimb neural coupling affects muscle recruitment during maximal effort upper and lower limb rhythmic exercise and provide insight into the architecture of the neural coupling.
Keywords: interlimb coupling, neural coupling, electromyography, coordination, bilateral deficit
INTRODUCTION
Humans naturally couple limb movements. It is easier to move two limbs in the same direction rather than in opposite directions or when using homologous muscles rather than non-homologous muscles (2, 20, 27). Humans also choose coordination patterns that maintain an integral frequency ratio between the upper limbs and lower limbs during rhythmic whole-body tasks like walking, swimming, and crawling (29). Muscle activation patterns (3, 10, 16) and reflex responses (4, 5, 9) during multi-joint and/or multi-limb tasks suggest that the coupled limb movements have a neural component. For example, during walking humans use shoulder muscles to help drive backward arm swing in-phase with the backward swing of the contralateral leg. When the arms are bound to restrict arm swing, there is still muscle activation in the shoulder muscles (3). A likely candidate for facilitating coordinated interlimb movements in humans is propriospinal connections between upper limb neural networks and lower limb neural networks (7, 33).
Studies have shown that adding upper limb movement or effort concurrently with lower limb movement during rhythmic tasks may improve lower limb muscle recruitment. We previously demonstrated that increased upper limb muscle activity resulted in greater muscle activation in passively moving legs of neurologically intact subjects performing recumbent stepping (15, 17). Kawashima and colleagues similarly examined the effects of resting, passive, and active arm swing on passive lower limb muscle activation during a reciprocal leg swinging task. They found that passive arm swing improved muscle activation patterns in passively moved lower limbs compared to a resting arms condition in incomplete spinal cord injured individuals (18). These two studies demonstrate that upper limb activation or afferent feedback can increase or improve lower limb muscle activation.
When examining muscle recruitment during multi-limb tasks, another neurally mediated interlimb effect is the so-called bilateral deficit (14). A bilateral deficit occurs when the force output of two bilateral limbs performing a task simultaneously is less than the sum of the force output of each single limb performing the same task. Deficits in muscle activation often parallel the deficits in force production (22, 28) and suggest that the bilateral deficit may represent a limitation in neuromuscular activation (16). Bilateral deficits occur in both the upper and lower body during isometric tasks (19, 21–24). Dynamic and/or multi- joint lower limb movements also typically produce bilateral deficits (25, 28, 30). Despite the common use of simultaneous upper and lower limb tasks for exercise (such as using elliptical trainers), no study has examined how maximum muscle activation is affected by simultaneous upper and lower limb rhythmic exercise compared to just upper or lower limb exercise.
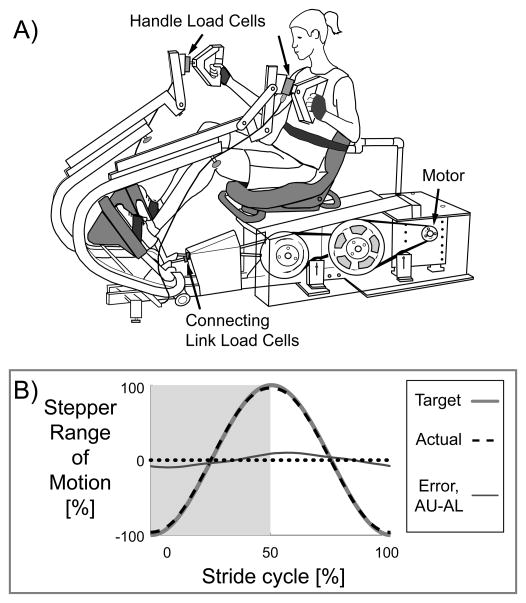
The general purpose of this study was to examine the effect of maximal voluntary upper limb muscle activation on lower limb muscle activation during a rhythmic task in neurologically intact humans. A secondary general purpose was to examine the same effect, but in the reverse direction (the effect of maximal voluntary lower limb muscle activation on upper limb muscle activation). We wanted to answer several specific questions. First, does maximal voluntary effort in the upper (or lower) limbs lead to increased muscle recruitment of passive lower (or upper) limbs during rhythmic movement? This question seeks to understand whether interlimb neural coupling can increase muscle recruitment of passively moving limbs. We hypothesized that active effort in the upper (or lower) limbs will increase muscle activation in passive lower (or upper) limbs. Second, does simultaneous upper and lower limb maximal effort produce more or less lower (or upper) limb muscle activation compared to only lower (or upper) limb maximal effort. This second question seeks to understand whether interlimb neural coupling can increase muscle recruitment during maximal effort. We hypothesized that simultaneous upper and lower limb maximal effort would produce greater lower limb muscle activation compared to only lower limb maximal effort. Third, does single upper (or lower) limb maximal effort produce more or less muscle activation in the ipsilateral or contralateral lower (or upper) limb? This third question seeks to understand whether interlimb neural coupling is more ipsilateral or contralateral in nature. We hypothesized that single limb effort would produce more muscle activation in the in-phase contralateral limb. To examine these questions, we studied subjects performing recumbent stepping similar to our previous works (15, 17), but modified the stepping device to allow maximal effort at a constant stepping frequency (Fig. 1A).
Figure 1.
A) Recumbent stepping machine with real-time computer-controlled resistance and force and position sensors (modified TRS 4000, NuStep Inc, Ann Arbor, MI). The handles and seat are adjustable. Velcro gloves, foot straps, and a torso belt help minimize unwanted movement. B) The machine provides smooth consistent stepping regardless of subject effort through a custom designed position control of a prescribed sine wave stepping profile. The group mean profile for the actual stepper position (black dashed line) closely follows the target sine wave (thick dark grey line). The thin grey line is the maximum error which occurred during the Active Upper & Active Lower condition. Dotted line is zero.
METHODS
Subjects
Sixteen healthy female subjects (age range 18–29 yrs) participated in this study. We could only test subjects who did not exceed an upper limit of the stepper’s power output. Subjects provided informed written consent, and the University of Michigan Medical School Institutional Review Board approved the protocol and consent form. The study complied with the Declaration of Helsinki.
Computer-controlled Recumbent Stepper
We have modified a commercially available recumbent stepper (TRS 4000, NuStep Inc., Ann Arbor, MI) to have computer-controlled real-time resistance and force measuring capabilities (Fig. 1A). We use RT Lab Solo software (Opal-RT, Montreal, Quebec, Canada) to customize control of the servomotor (Kollmorgen, Radford, VA) that powers the recumbent stepper. We use the position sensor in the servomotor to measure the kinematics of the recumbent stepper. The stepper has one degree of freedom and thus needs just one position sensor to describe its kinematics.
For this study, we programmed the recumbent stepper to follow a prescribed sine-wave position profile (Fig. 1B) to produce smooth consistent stepping at a set frequency (75 BPM, equivalent to the stepping frequency of walking at 1.25 m/s). The harder the subjects pushed or pulled, the more resistance they encountered to maintain a constant stepping frequency and minimize error. The largest errors occurred in the Active Upper & Active Lower condition, reaching an average peak error of 10 ± 3 % (mean ± standard deviation) of the stepper range of motion and an average error of 5.7 ± 1.7% across the cycle. Some design limitations made it difficult to further reduce the error, but all data collected were within < 1% of the target stepping frequency. To measure the force each arm contributed to the stepping motion, we mounted a single axis load cell (Strainsert, West Conshohocken, PA) in each handle. We also mounted a single axis load cell (LCWD-1000, Omegadyne, Sunbury, OH) in the connecting link of each handle-pedal unit to measure the total force each contralateral upper-lower limb pair supplied to the stepping kinetics. We found that the sum of the handle and pedal torques equaled the handle-pedal unit torque. Based on this, we could determine the force contribution of each lower limb to the stepping motion.
Protocol
Subjects performed recumbent stepping using different combinations of upper (U) and lower (L) limb effort, being either active (A) or passive (P). Active effort was always with maximal effort, and passive effort was with minimal effort or as relaxed as possible. Subjects could choose any combination of pushing and pulling to drive the stepping motion, unless specifically told to just push or just pull (for the single limb conditions).
We used Velcro gloves to attach the hands to the handles, and foot straps to attach the feet to the pedals during passive conditions. This adaptation allowed subjects to be as passive as possible because they did not have to actively hold the handles or keep their feet on the pedals throughout the stepping motion. A torso strap minimized torso movement during stepping. The seat position was set so that the subject’s knees were near full extension but could not lock out. The position of each handle was adjusted for subject comfort (Fig. 1A). For each condition, we collected fifteen seconds of data, approximately six to eight complete stride cycles. The order of the conditions was pseudo-randomized for each subject. There was an average of one minute of rest between conditions.
Question 1: Does Maximal Effort in the Upper (or Lower) Limbs Increase Muscle Activation in Passive Lower (or Upper) Limbs?
To answer our first question, we compared lower limb muscle activation between Passive Upper & Passive Lower [PU-PL] and Active Upper & Passive Lower [AU-PL] to determine if maximum voluntary effort in the upper limbs leads to greater muscle activation in the passive lower limbs. We also looked at the reverse direction and compared Passive Upper & Active Lower [PU-AL] to Passive Upper & Passive Lower [PU-PL] to determine if maximum voluntary effort in the lower limbs leads to greater muscle activation in the passive upper limbs.
Question 2: Does Simultaneous Upper and Lower Limb Maximal Effort Increase Lower (or Upper) Limb Muscle Activation Compared to Only Lower (or Upper) Limb Maximal Effort?
To answer our second question, we compared lower limb muscle activation between Active Upper & Active Lower [AU-AL] and Passive Upper & Active Lower [PU-AL] conditions. We also compared upper limb muscle activation between Active Upper & Active Lower [AU-AL] and Active Upper & Passive Lower [AU-PL] conditions. These comparisons will determine if simultaneous upper and lower limb maximal effort produces more or less muscle activation compared to only lower (or upper) limb maximal effort.
Question 3: Does Single Limb Maximal Effort Increase Muscle Activation in the Ipsilateral or Contralateral Limb?
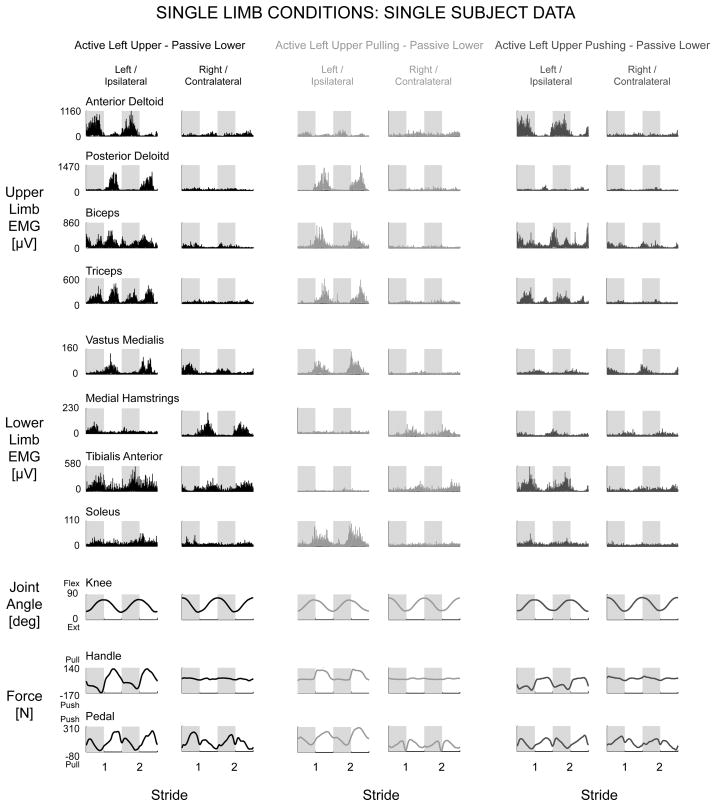
To answer our third question, we examined the effect of single upper limb effort on muscle activation of passive lower limbs to determine if there was more muscle activation in the ipsilateral or contralateral passive lower limb. We tested conditions of just left upper limb effort, Active Left Upper & Passive Lower and just right upper limb effort, Active Right Upper & Passive Lower. For these conditions, subjects were instructed to push and pull using just their left (or right) upper limb, while the right (or left) upper limb was as passive as possible. We also tested conditions instructing subjects to just pull with a single upper limb, Active Left Upper Pulling & Passive Lower and Active Right Upper Pulling & Passive Lower. Similarly, we tested conditions instructing subjects to just push with a single upper limb, Active Left Upper Pushing & Passive Lower and Active Right Upper Pushing & Passive Lower. By focusing active effort of a single upper limb to just pulling (or pushing), we could potentially observe how specific upper limb muscle groups affect passive lower limb muscle activation. During these conditions, the device drove the stepping motion for the portion of the stride when the subject was instructed to be passive until reaching the portion of the stride when the subject was instructed to push (or pull) with the specified single upper limb. To examine bidirectionality, we also tested a Passive Upper & Active Left Lower and a Passive Upper & Active Right Lower condition to examine whether any ipsilateral or contralateral coupling also occurred in the lower to upper direction.
Data Acquisition and Analysis
Two computer systems sampled all of the data signals at 1,000 Hz. We used a common data signal sampled in both systems to synchronize the data offline.
Electromyography (EMG)
We measured muscle activity from sixteen muscles, four muscles on each limb, using a surface electromyography system with an EMG bandwidth of 20–450 Hz (Delsys, Boston, MA). On each lower limb, we measured muscle activity from the vastus medialis (VM), medial hamstrings (MH), tibialis anterior (TA), and soleus (SO). On each upper limb, we measured muscle activity from the anterior deltoid (AD), posterior deltoid (PD), biceps brachii (BB), and triceps brachii (TB). We cleaned each electrode site with rubbing alcohol, placed the electrode sensor over the muscle belly along the long axis, and then secured the electrode with tape. To further minimize mechanical artifact, we wrapped excess loose electrode wires to the limbs with elastic foam wrap. We processed the EMG data with a second order high-pass Butterworth filter (cutoff frequency 20 Hz) with zero phase lag to attenuate low frequency components such as mechanical artifact. We then full wave rectified the EMG data signals.
Joint Angles
We measured joint angles of the ankles, knees, and hips on both legs and the elbows of both arms using twin-axis electrogoniometers placed along the sagittal plane (Biometrics Ltd, Ladysmith, VA). The electrogoniometers were zeroed with the limbs in the anatomically neutral position. We processed the joint angle data with a second order low-pass Butterworth filter (cutoff frequency 6 Hz) with zero phase lag.
Kinetics
We calculated the forces each hand and foot contributed to the stepping motion via single axis load cells (Fig. 1). We measured the force exerted by each hand through a load cell mounted in the handle and the total force exerted by each contralateral hand-foot pair through a load cell mounted in a connecting link on the machine. We filtered this force data using a second order low-pass Butterworth filter (cutoff frequency 6 Hz) with zero phase lag. Using the measured forces and moment arm relationships, we calculated the torques associated with each handle and handle-pedal unit. To determine the pedal torque, we subtracted the handle torque from the handle-pedal unit torque. We then divided the pedal torques by the pedal moment arm to find the pedal forces.
Calculation of Mean Profiles
To compare EMG patterns between conditions, we calculated group EMG mean profiles over a stride cycle for each condition. The beginning and end of each stride corresponded with the left lower limb and right upper limb at full extension as indicated from the position data. We first calculated an intra-subject EMG mean profile for a stride cycle per condition. We then calculated a group EMG mean profile for each condition by averaging all of the intra-subject EMG mean profiles for that condition. We used the same procedure for the joint angle and force mean profiles.
Calculation of EMG Root-Mean-Square (RMS)
To compare EMG amplitudes across conditions, we calculated a group averaged (n=16) normalized EMG root-mean-square (RMS) for each muscle and condition. The data for left and right muscles were analyzed independently. For each muscle, we calculated EMG RMS during the half of the stride when the muscle was concentrically contracting. We calculated an intra-subject average EMG RMS for each muscle per condition. We then normalized the lower limb EMG RMS amplitudes for each muscle (left VM, MH, SO and TA; right VM, MH, SO and TA) to the intra-subject average EMG RMS for the Passive Upper & Active Lower condition. Likewise, we normalized upper limb EMG RMS amplitudes for each muscle (left AD, PD, BB and TB; right AD, PD, BB and TB) to intra-subject average EMG RMS for the Active Upper & Passive Lower condition. We then averaged the intra-subject EMG RMS values to calculate the group EMG RMS value for each muscle per condition.
Statistical Analysis
For each question, we used a repeated measures analysis of variance (rmANOVA) to determine if there were significant differences between conditions for each lower (or upper) limb muscle. For example, for question 1, we performed the rmANOVA looking at only the passive lower limb muscles for the Passive Upper & Passive Lower and Active Upper & Passive Lower conditions. If the rmANOVA showed a significant difference among conditions, we used a Tukey’s honestly significant difference (THSD) post hoc test to determine which condition(s) were significantly different (P < 0.05). For the single limb conditions, we used a Chi-Squared test of association (P < 0.05) to determine if there was a significant difference in the number of muscles that had significant increases in EMG RMS with ipsilateral versus contralateral limb effort.
RESULTS
Question 1: Does Maximal Effort in the Upper (or Lower) Limbs Increase Muscle Activation in Passive Lower (or Upper) Limbs?
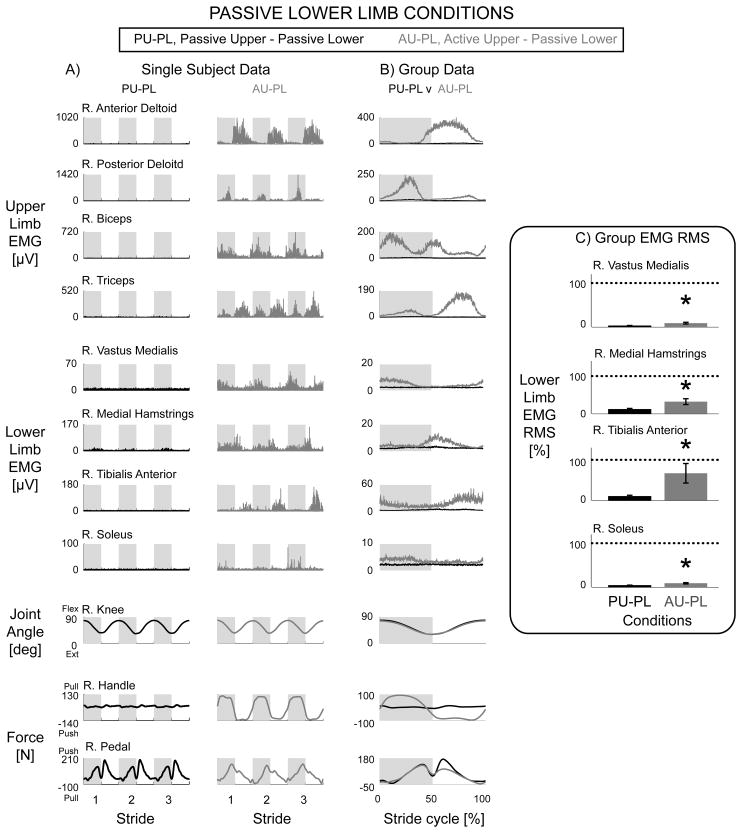
Active upper limb effort resulted in increased muscle activation of the passive lower limbs. In a representative single subject, the Passive Upper & Passive Lower condition had minimal muscle activation in the passive lower limbs while the Active Upper & Passive Lower condition had greater amplitudes and more distinct bursts in the passive lower limb muscle activation (Fig. 2A, black vs. grey respectively). When looking at the group average of all the subjects, the data showed the same effects as in the representative single subject data (Fig. 2B). The Active Upper & Passive Lower condition had significantly greater muscle activity than the Passive Upper & Passive Lower condition for the bilateral vastus medialis, medial hamstrings, tibialis anterior, and soleus (Fig. 2C, * over grey bars, THSD P < 0.05). The knee joint angle data were similar for the two conditions, Passive Upper & Passive Lower and Active Upper & Passive Lower (Fig. 2A & 2B). The handle and pedal forces indicated that subjects performed the stepping conditions as instructed, with the handle forces being significantly different and the pedal forces being similarly minimal. The small pushing pedal forces indicated the pedal pushing against the subject’s passive foot (Fig. 2A & 2B).
Figure 2.
Data from the right limbs for the passive lower limb conditions, Passive Upper & Passive Lower (black) and Active Upper & Passive Lower (grey). Representative single subject data (A) and group EMG mean profiles (B) show minimal EMG activity in the Passive Upper & Passive Lower condition while the Active Upper & Passive Lower condition had rhythmic bursts of EMG. Group EMG RMS amplitudes with standard error bars (C) for Active Upper & Passive Lower were significantly greater than Passive Upper & Passive Lower for the vastus medialis, medial hamstrings, tibialis anterior, and soleus muscles (* significantly different, AU-PL > PU-PL, THSD P < 0.05). Dotted line equals 100%. Note different y-axes between A and B. The knee joint angle profiles and passive pedal forces were similar between the two conditions. Handle forces during the Active Upper & Passive Lower condition had a clear increase in pulling force during the upper limb flexing (and lower limb extending) phase and pushing force during the upper limb extending (and lower limb flexing) phase.
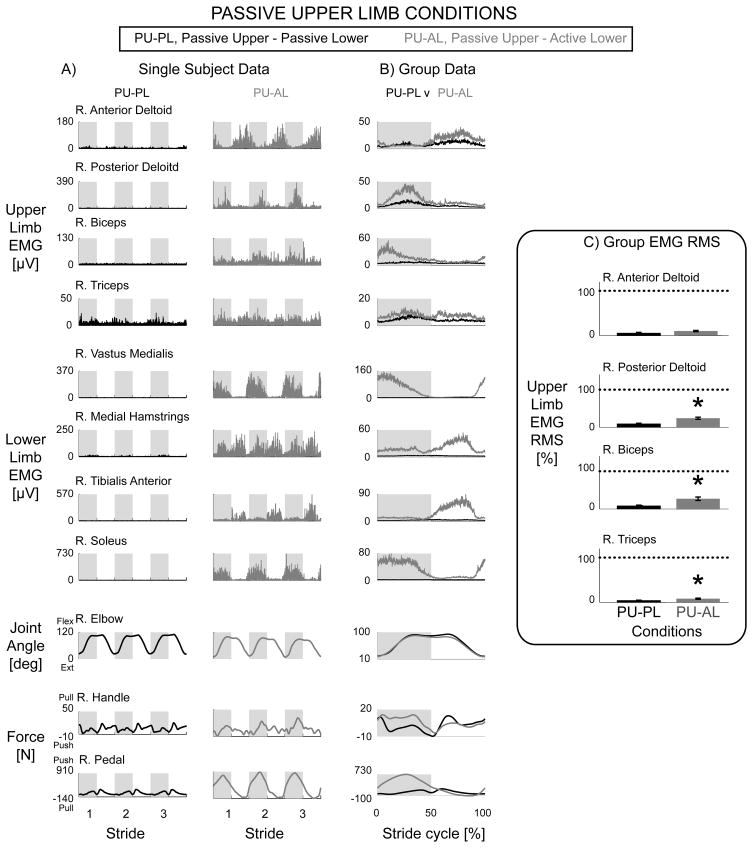
Similarly, lower limb muscle activation resulted in increased muscle activation of passive upper limb muscles. Representative single subject data and group mean profiles showed burst-like muscle activity in the passive upper limbs during the Passive Upper & Active Lower condition compared to the minimal muscle activity during the Passive Upper & Passive Lower condition (Fig. 3A & 3B, grey vs. black lines). Passive Upper & Active Lower EMG RMS amplitudes were significantly greater than Passive Upper & Passive Lower EMG RMS amplitudes for the bilateral posterior deltoid, biceps brachii, and triceps brachii muscles (Fig. 3C * over grey bars, THSD P < 0.05). The elbow joint angle, handle force, and pedal force data again indicated that subjects performed the stepping conditions as instructed (Fig. 3A & 3B) as the handle forces were similar while the pedal forces were different.
Figure 3.
Data from the right limbs for the passive upper limb conditions, Passive Upper & Passive Lower (black) and Passive Upper & Active Lower (grey). Representative single subject data (A) and group EMG mean profiles (B) showed a rhythmic burst-like pattern in passive upper limbs during the Passive Upper & Active Lower condition compared to the Passive Upper & Passive Lower condition. Group EMG RMS amplitudes with standard error bars (C) for Passive Upper & Active Lower were significantly greater than Passive Upper & Passive Lower for the posterior deltoid, biceps, and triceps but not the anterior deltoid (* significantly different, PU-AL > PU-PL, THSD P < 0.05). Dotted line equals 100%. Note different y-axes between A and B. The elbow joint angle profiles and passive handle forces were similar between the two conditions. The pedal force profiles for Passive Upper & Active Lower showed a large increase in lower limb pushing force during the lower limb extending phase of the stride cycle.
Question 2: Does Simultaneous Upper and Lower Limb Maximal Effort Increase Lower (or Upper) Limb Muscle Activation Compared to Only Lower (or Upper) Limb Maximal Effort?
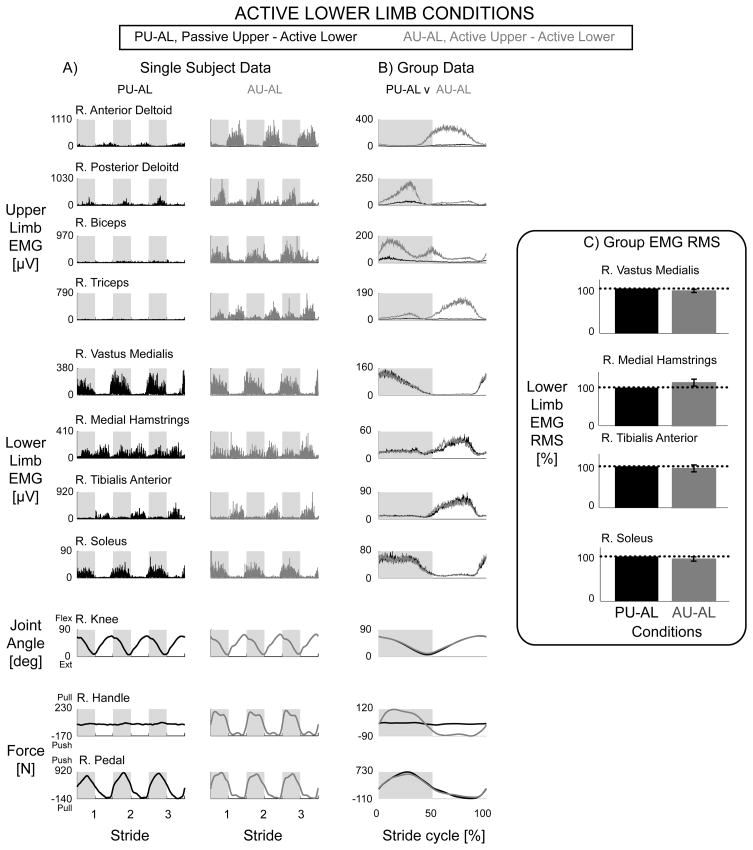
During simultaneous upper and lower limb maximal effort, there was no difference in muscle activation amplitudes compared to only upper limb maximal effort or only lower limb maximal effort. Representative single subject data and group EMG mean profiles showed that muscle activation in the active lower limbs for the Passive Upper & Active Lower and Active Upper & Active Lower conditions were similar in amplitude and shape (Fig. 4A & 4B, black vs. grey respectively). There were no significant differences in lower limb EMG RMS between the Passive Upper & Active Lower and Active Upper & Active Lower conditions (Fig. 4C, rmANOVA P > 0.05). Similarly, there were no significant differences in upper limb EMG RMS between Active Upper & Passive Lower and Active Upper & Active Lower (rmANOVA P > 0.05). The knee joint angle, handle force, and pedal force data indicated that subjects performed the stepping conditions as instructed (Fig. 4A & 4B).
Figure 4.
Data from the right limbs for active lower limb conditions, Passive Upper & Active Lower (black) and Active Upper & Active Lower (grey). Representative single subject data (A) and group EMG mean profiles (B) showed no observable differences in active lower limb muscle activity during Passive Upper & Active Lower and Active Upper & Active Lower conditions. Group EMG RMS amplitudes with standard error bars (C) were not significantly different between Passive Upper & Active Lower and Active Upper & Active Lower (rmANOVA P > 0.05). Dotted line equals 100%. Note different y-axes between A and B. The knee joint angle profiles and active lower limb forces were similar between the two conditions (Fig. 4A & 4B). There were large increases in lower limb pushing force during the lower limb extending phase of the stride cycle. The handle forces were minimal during the Passive Upper & Active Lower condition and had increases in pulling and pushing forces during the Active Upper & Active Lower condition (Fig. 4A & 4B).
Question 3: Does Single Limb Maximal Effort Increase Muscle Activation in the Ipsilateral or Contralateral Limb?
Single upper limb active effort resulted in increased muscle activation in the passive ipsilateral lower limb compared to the contralateral lower limb. Representative single subject data (Fig. 5, black) and group EMG mean profiles (Fig. 6A, black) for the Active Left Upper & Passive Lower condition showed clear rhythmic burst activity in the ipsilateral vastus medialis, ipsilateral tibialis anterior, ipsilateral soleus, and contralateral medial hamstrings. When focused on just pulling during the Active Left Upper Pulling & Passive Lower condition, the EMG data had clear rhythmic burst activity in the ipsilateral vastus medialis, ipsilateral soleus, and contralateral medial hamstrings (Fig. 5 & 6A, light grey). When focused on just pushing during the Active Left Upper Pushing & Passive Lower condition, the EMG data had clear rhythmic burst activity in the ipsilateral tibialis anterior (Fig. 5 & 6A, dark grey). The knee joint angle, handle force, and pedal force data indicated that subjects performed the stepping conditions as instructed (Fig. 5 & 6A).
Figure 5.
Representative single subject data from single left upper limb conditions, Active Left Upper & Passive Lower (black), Active Left Upper Pulling & Passive Lower (light grey), and Active Left Upper Pushing & Passive Lower (dark grey). Overall, more muscle activation occurred in the passive ipsilateral lower limb muscles compared to the passive contralateral lower limb muscles, except for the contralateral medial hamstrings. Same y-axes for each row.
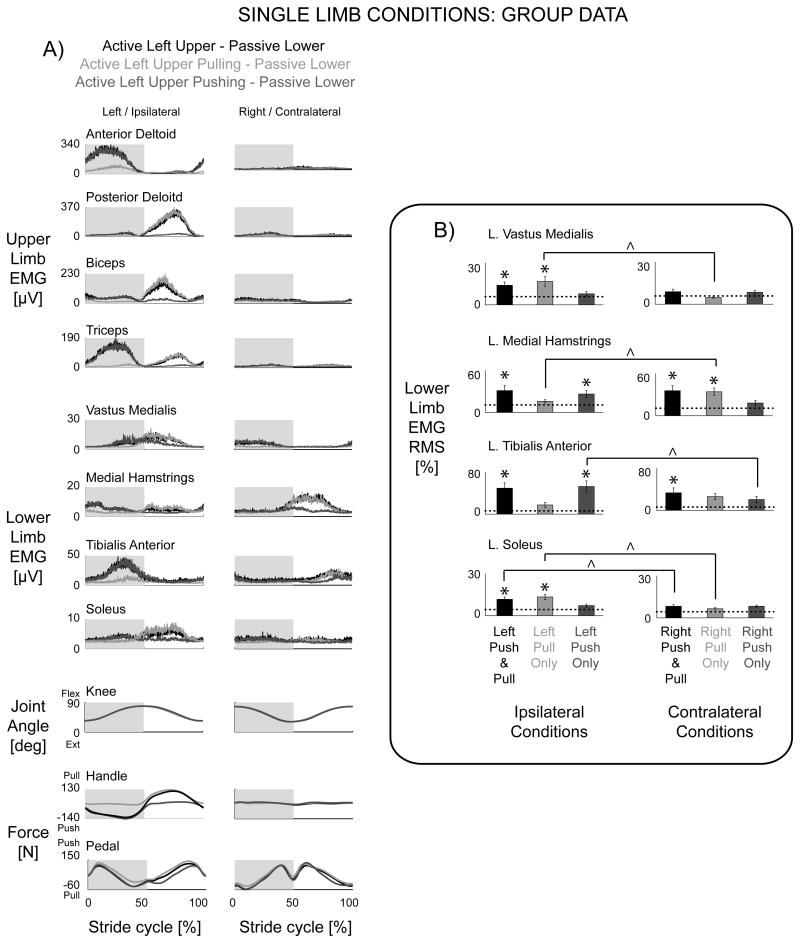
Figure 6.
A) Group data for single left upper limb conditions, Active Left Upper & Passive Lower (black), Active Left Upper Pulling & Passive Lower (light grey), and Active Left Upper Pushing & Passive Lower (dark grey). The dotted line is Passive Upper & Passive Lower. Except for the contralateral medial hamstrings, group EMG mean profiles showed more burst-like patterns in passive ipsilateral lower limb muscles with single upper limb effort. The passive pedal force profiles for all three conditions were minimal. There was a large pulling handle force during the upper limb flexing (and lower limb extending) phase for Active Left Upper Pulling & Passive Lower and a large pushing handle force during the upper limb extending (and lower limb flexing) phase for Active Left Upper Pushing & Passive Lower. B) Group EMG RMS amplitudes with standard error bars indicate ipsilateral coupling of muscle activation with single upper limb effort (* significantly different from Passive Upper & Passive Lower, ^ significantly different between left and right upper limb conditions).
The EMG RMS amplitudes of the ipsilateral vastus medialis, medial hamstrings, tibialis anterior, and soleus muscles were greater during the Active Left Upper & Passive Lower condition compared to Passive Upper & Passive Lower (Fig 6B* Left Push & Pull column, THSD P < 0.05). The left medial hamstrings and tibialis anterior EMG RMS were also significantly greater during the contralateral Active Right Upper & Passive Lower condition compared to the Passive Upper & Passive Lower amplitudes (Fig 6B* Right Push & Pull column, THSD P < 0.05). In the Pull Only instructions, Active Left Upper Pulling & Passive Lower resulted in significantly greater EMG RMS amplitudes in the ipsilateral vastus medialis and soleus compared to Passive Upper & Passive Lower (Fig. 6B* Left Pull Only column, THSD P < 0.05). The left medial hamstrings had significantly greater EMG RMS during the contralateral Active Right Upper Pulling & Passive Lower condition compared to Passive Upper & Passive Lower (Fig. 6B* Right Pull Only column, THSD P < 0.05). In the Push Only instructions, the Active Left Upper Pushing & Passive Lower condition resulted in significantly greater EMG RMS amplitudes in the ipsilateral tibialis anterior and medial hamstrings compared to the Passive Upper & Passive Lower condition (Fig. 6B* Left Push Only column, THSD P < 0.05).
Comparing left versus right single limb conditions, left upper limb effort resulted in significantly greater EMG RMS in the left vastus medialis and soleus during Pull Only instructions compared to right upper limb effort (Fig. 6B^ Pull Only columns, THSD P < 0.05). Right upper limb effort resulted in significantly greater EMG RMS in the left medial hamstrings during the Pull Only instructions compared to left upper limb effort (Fig. 6B^ Pull Only columns, THSD P < 0.05). Left upper limb effort corresponded to greater EMG RMS in the left tibialis anterior during Push Only instructions compared to right upper limb effort (Fig. 6B^ Push Only columns, THSD P < 0.05). Left soleus EMG RMS amplitudes were significantly greater during the Left Push & Pull condition compared to the Right Push & Pull condition (Fig. 6B^ Push & Pull columns, THSD P < 0.05). Right upper limb effort conditions also showed more ipsilateral coupling with passive right lower limb muscle activation (not shown). A Chi-squared test revealed a significant association of ipsilateral upper limb effort with a greater number of significant increases in passive lower limb EMG RMS amplitudes compared to contralateral upper limb effort (P < 0.05). This ipsilateral coupling was also evident in the lower to upper direction. Passive upper limb muscle activation patterns and EMG RMS amplitudes showed more significant increases with ipsilateral single lower limb effort conditions (Chi-squared test P < 0.05).
DISCUSSION
We found three novel features of interlimb coupling on muscle activation during rhythmic movement in neurologically intact individuals in this study. Our first finding was a bidirectional coupling of muscle activation between upper and lower limbs. Maximum voluntary effort in the upper limbs increased passive muscle activation in the lower limbs. Likewise, maximum voluntary effort in the lower limbs increased passive muscle activation in the upper limbs. Our second finding was that interlimb neural coupling did not increase or decrease the total muscle recruitment possible during maximally activated lower (or upper) limbs. When subjects exerted simultaneous maximum upper and lower limb effort, there was neither facilitation nor inhibition of muscle activation compared to exclusively upper limb effort or exclusively lower limb effort. Our third finding was an ipsilateral coupling of muscle activation between upper and lower limbs. Maximum voluntary effort in a single upper limb increased muscle activation more in the ipsilateral lower limb than the contralateral limb. This ipsilateral effect was also bidirectional. Single lower limb active effort also resulted in greater muscle activation in the ipsilateral passive upper limb (not shown). These results provide a more thorough understanding of the features and limitations of interlimb coupling of muscle activation between upper limbs and lower limbs.
The general result that active effort in one limb pair resulted in increased muscle activation in passive muscles in the other limb pair was robust. It was consistent with our previous studies (15, 17), held for single upper limb active effort conditions (Figs. 5 & 6), and was bidirectional (i.e. upper to lower, Fig. 2, and lower to upper, Fig. 3). This bidirectional effect agrees with studies examining the role of arm movement on cutaneous reflexes in the legs and the role of leg movement on reflexes in the arms (4, 12, 34). The increases in passive muscle activation with active effort in another limb(s) were small but significant. This agrees with an arm-leg cycling study that demonstrated a nontrivial subtle effect of arm cycling on lower limb reflexes even in the presence of the more dominant effect of leg cycling on lower limb reflexes (4).
The single limb conditions revealed a surprising ipsilateral, rather than contralateral, coupling of muscle activation. The ipsilateral coupling was evident in both directions, upper to lower (Fig. 5 & 6) and lower to upper. This ipsilateral coupling is interesting because one might suspect contralateral coupling between the upper and lower limbs. Reflex studies suggest that contralateral upper to lower limb coupling may be more prevalent during rhythmic movements compared to ipsilateral upper to lower limb coupling, though the relative strengths are uncertain in humans (31, 33). The nervous system also naturally prefers in-phase movements (isodirectional) of ipsilateral limbs rather than anti-phase movements (2, 27). This suggests that there would be a preference for contralateral coupling during recumbent stepping because the contralateral upper limb moves in-phase with the lower limb while the ipsilateral upper limb moves anti-phase with the lower limb. Despite the anti-phase movements of the ipsilateral limbs, we found a greater increase in muscle activation in the passive ipsilateral lower (or upper) limb during single active upper (or lower) limb effort (Figs. 5 & 6). The preference for ipsilateral coupling of wrist and ankle flexion has been attributed to coupled corticospinal drive rather than afferent signals associated with limb movements (1, 5). Based on reflex studies, we might expect to see a preference for contralateral coupling if spinal mechanisms are dominant. Our results suggest that supraspinal drive may be more dominant compared to spinal mechanisms during a maximal effort rhythmic upper and lower limb task.
It is likely that a combination of neural mechanisms contribute to our observations of increases in passive muscle activation. One possible contributor is spinal connections between upper limb neural networks and lower limb neural networks (7, 33). These spinal connections allow information about muscle activation in one limb to modulate interlimb reflexes, coordination, and muscle activation of other limbs in both neurologically intact individuals and individuals with spinal cord injury (4, 8, 9, 11–13, 18, 29). Another possible contributor is sensory feedback from one or more moving limbs that modulates neural activity in another limb (6). Stretch reflexes, however, do not appear to contribute to our results because the timing and shape of the increased muscle activation during the passive limb conditions were similar to the timing and shape of the active limb conditions (15). Because recumbent stepping has been shown to have similar neural control to walking (26, 32), locomotor commands from central pattern generators and/or supraspinal centers and the propriospinal pathways that modulate locomotor commands are also possible neural mechanisms for our findings. Another possible contributor is descending supraspinal drive that results in “cross talk” and unintended muscle activation (2, 10). Postural adjustments may also have a role in our observation of increased passive muscle activation; however, we stabilized the torso as much as possible using a torso strap. We previously tested torso stabilization with multiple straps but still observed increases in passive muscle activation. Regardless, anticipated postural demands required to stabilize the body during the maximal effort tasks cannot be excluded as a possible contributor. Future studies would need to use more extensive electrophysiological measurements to further delineate the possible pathways involved.
An important result of this study was that adding simultaneous maximal upper limb effort with maximal lower limb effort did not enhance lower limb muscle activation in neurologically intact subjects. The presumed spinal connections that contributed to increased passive muscle activation were not a significant factor when lower limbs were maximally activated. During the simultaneous upper and lower limb condition, most subjects were able to attain but not exceed the muscle activation levels of the only upper limbs or only lower limbs conditions (Fig. 4). The mean force results (not shown) paralleled our EMG RMS results showing that simultaneous upper and lower limb effort did not produce a significant decrease in handle or pedal forces compared to the conditions with only upper limb or only lower limb effort. This agrees with a previous study that showed no difference between unilateral and bilateral force production in a static task for an upper limb and contralateral lower limb pair (14). These results suggest that a phenomenon like a bilateral deficit does not occur during a simultaneous maximum effort upper and lower limb rhythmic task of non-homonymous muscles. Our active lower limb results (Fig. 4) also compliment an arm and leg cycling study that demonstrated no significant differences in lower limb EMG during a combined arm and leg cycling task to only leg cycling (4). This also suggests that humans are able to generate maximum voluntary muscle activation and force production during rhythmic tasks whether using both upper and lower limbs, only upper limbs, or only lower limbs.
A limitation of this study was the subject’s ability and motivation to perform the task as instructed. Because the device was always moving, there was no incentive to do any work during passive conditions. There was no reason to “cheat” and use the passive limbs to aid the active limbs. On the other hand, because the device was always moving, the only motivation for full effort was to comply with the instructions given. When subjects used maximal effort, the resistance increased to maintain the specified stepping frequency and the subject had to do more work. For our experiment, we only provided subjects with verbal encouragement to use maximal effort or stay relaxed during the data collections. We chose not to provide some form of biofeedback such as a display showing handle and pedal forces generated during the collection. Giving subjects biofeedback could allow subjects to voluntarily negate any underlying natural neural coupling between the upper and lower limbs through increased supraspinal activity.
Another limitation with this experiment was that we did not examine what happens at submaximal effort. There is still the possibility that upper limb exertion can increase muscle activation in lower limbs during submaximal exercise despite our results at maximal efforts. Transcranial magnetic stimulation could be used to determine if there is an excitatory interlimb coupling during submaximal muscle activation that results in less supraspinal descending neural drive to the lower limbs with upper limb exertion (35). Understanding the role of upper limb effort on lower limb muscle activation at submaximal efforts has potential rehabilitation implications. This is of interest because therapeutic exercise and activities of daily living are often performed at submaximal levels. Perhaps incorporating active effort from unimpaired limbs may increase or improve muscle activation patterns in impaired limbs during whole body rhythmic tasks such as walking.
We conducted this study to answer questions about the effect of interlimb neural coupling on muscle activation. We found that maximum voluntary effort in the upper (or lower) limbs did increase muscle recruitment during passive rhythmic movement of the lower (or upper) limbs. Single limb effort conditions revealed a stronger ipsilateral coupling of muscle activation compared to contralateral coupling. We also found that simultaneous maximum upper and lower limb effort produced neither more nor less muscle activation compared to exclusively upper limb effort or exclusively lower limb effort in the active limbs. These results showed that active effort in one limb(s) can influence muscle activation in other unintended passive limbs during a rhythmic locomotor-like task in neurologically intact individuals. However, the presumed excitatory neural coupling did not enhance maximum activation. There are several factors that may contribute to this ipsilateral facilitatory neural coupling, including interlimb spinal neural connections that provide pathways for muscle activation from one limb(s) to contribute to muscle recruitment in another limb. These results support the existence of neural connections between the upper limbs and lower limbs and demonstrate that muscle activation in one limb(s) increases muscle activation in another limb(s) during a whole body rhythmic task. Further studies examining the role of upper limb effort on lower limb muscle activation patterns will provide more insight into the potential of integrating upper limb effort during lower limb rehabilitation.
Acknowledgments
We would like to thank Catherine Kinnaird, Pei-Chun Kao, and the rest of the members of the University of Michigan Human Neuromechanics Laboratory for help with data collections. This work was supported in part by Award Number F31 NS056504 from the National Institute of Neurological Disorders And Stroke and Award Number 2293-01 from the Paralyzed Veterans of America Spinal Cord Research Foundation. The results of the present study do not constitute endorsement by American College of Sports Medicine.
Funding Sources: National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) F31 NS056504 Fellowship. Paralyzed Veterans of America Spinal Cord Research Foundation Grant 2293-01.
References
- 1.Baldissera F, Borroni P, Cavallari P, Cerri G. Excitability changes in human corticospinal projections to forearm muscles during voluntary movement of ipsilateral foot. J Physiol. 2002;539:903 – 11. doi: 10.1113/jphysiol.2001.013282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldissera F, Cavallari P, Civaschi P. Preferential coupling between voluntary movements of ipsilateral limbs. Neurosci Lett. 1982;34(1):95–100. doi: 10.1016/0304-3940(82)90098-2. [DOI] [PubMed] [Google Scholar]
- 3.Ballesteros ML, Buchthal F, Rosenfalck P. The Pattern of Muscular Activity during the Arm Swing of Natural Walking. Acta Physiol Scand. 1965;63:296–310. doi: 10.1111/j.1748-1716.1965.tb04069.x. [DOI] [PubMed] [Google Scholar]
- 4.Balter JE, Zehr EP. Neural coupling between the arms and legs during rhythmic locomotor-like cycling movement. J Neurophysiol. 2007;97(2):1809–18. doi: 10.1152/jn.01038.2006. [DOI] [PubMed] [Google Scholar]
- 5.Cerri G, Borroni P, Baldissera F. Cyclic h-reflex modulation in resting forearm related to contractions of foot movers, not to foot movement. J Neurophysiol. 2003;90(1):81–8. doi: 10.1152/jn.00030.2003. [DOI] [PubMed] [Google Scholar]
- 6.Collins DF, McIlroy WE, Brooke JD. Contralateral inhibition of soleus H reflexes with different velocities of passive movement of the opposite leg. Brain Res. 1993;603(1):96–101. doi: 10.1016/0006-8993(93)91303-a. [DOI] [PubMed] [Google Scholar]
- 7.Dietz V. Do human bipeds use quadrupedal coordination? Trends Neurosci. 2002;25(9):462–7. doi: 10.1016/s0166-2236(02)02229-4. [DOI] [PubMed] [Google Scholar]
- 8.Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995;37(5):574–82. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- 9.Dietz V, Fouad K, Bastiaanse CM. Neuronal coordination of arm and leg movements during human locomotion. Eur J Neurosci. 2001;14(11):1906–14. doi: 10.1046/j.0953-816x.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrijevic MR, McKay WB, Sarjanovic I, Sherwood AM, Svirtlit L, Vrbova G. Co-activation of ipsi- and contralateral muscle groups during contraction of ankle dorsiflexors. Journal of the Neurological Sciences. 1992;109(1):49–55. doi: 10.1016/0022-510x(92)90092-y. [DOI] [PubMed] [Google Scholar]
- 11.Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord. 2004;42(1):14–23. doi: 10.1038/sj.sc.3101542. [DOI] [PubMed] [Google Scholar]
- 12.Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol. 2003;90(5):2850–61. doi: 10.1152/jn.00531.2003. [DOI] [PubMed] [Google Scholar]
- 13.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77(2):797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 14.Howard JD, Enoka RM. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J Appl Physiol. 1991;70(1):306–16. doi: 10.1152/jappl.1991.70.1.306. [DOI] [PubMed] [Google Scholar]
- 15.Huang HJ, Ferris DP. Neural coupling between upper and lower limbs during recumbent stepping. J Appl Physiol. 2004;97(4):1299–308. doi: 10.1152/japplphysiol.01350.2003. [DOI] [PubMed] [Google Scholar]
- 16.Jakobi JM, Chilibeck PD. Bilateral and unilateral contractions: possible differences in maximal voluntary force. Can J Appl Physiol. 2001;26(1):12–33. doi: 10.1139/h01-002. [DOI] [PubMed] [Google Scholar]
- 17.Kao PC, Ferris DP. The effect of movement frequency on interlimb coupling during recumbent stepping. Motor Control. 2005;9(2):144–63. doi: 10.1123/mcj.9.2.144. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima N, Nozaki D, Abe MO, Nakazawa K. Shaping appropriate locomotive motor output through interlimb neural pathway within spinal cord in humans. J Neurophysiol. 2008;99(6):2946–55. doi: 10.1152/jn.00020.2008. [DOI] [PubMed] [Google Scholar]
- 19.Koh TJ, Grabiner MD, Clough CA. Bilateral deficit is larger for step than for ramp isometric contractions. J Appl Physiol. 1993;74(3):1200–5. doi: 10.1152/jappl.1993.74.3.1200. [DOI] [PubMed] [Google Scholar]
- 20.Meesen R, Wenderoth N, Temprado J, Summers J, Swinnen S. The coalition of constraints during coordination of the ipsilateral and heterolateral limbs. Experimental Brain Research. 2006;174(2):367–75. doi: 10.1007/s00221-006-0471-1. [DOI] [PubMed] [Google Scholar]
- 21.Oda S, Moritani T. Maximal isometric force and neural activity during bilateral and unilateral elbow flexion in humans. Eur J Appl Physiol Occup Physiol. 1994;69(3):240–3. doi: 10.1007/BF01094795. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsuki T. Decrease in human voluntary isometric arm strength induced by simultaneous bilateral exertion. Behav Brain Res. 1983;7(2):165–78. doi: 10.1016/0166-4328(83)90190-0. [DOI] [PubMed] [Google Scholar]
- 23.Schantz PG, Moritani T, Karlson E, Johansson E, Lundh A. Maximal voluntary force of bilateral and unilateral leg extension. Acta Physiol Scand. 1989;136(2):185–92. doi: 10.1111/j.1748-1716.1989.tb08651.x. [DOI] [PubMed] [Google Scholar]
- 24.Secher NH, Rube N, Elers J. Strength of two- and one-leg extension in man. Acta Physiol Scand. 1988;134(3):333–9. doi: 10.1111/j.1748-1716.1988.tb08500.x. [DOI] [PubMed] [Google Scholar]
- 25.Simon AM, Ferris DP. Lower limb force production and bilateral force asymmetries are based on sense of effort. Exp Brain Res. 2008;187(1):129–38. doi: 10.1007/s00221-008-1288-x. [DOI] [PubMed] [Google Scholar]
- 26.Stoloff RH, Zehr EP, Ferris DP. Recumbent stepping has similar but simpler neural control compared to walking. Exp Brain Res. 2007;178(4):427–38. doi: 10.1007/s00221-006-0745-7. [DOI] [PubMed] [Google Scholar]
- 27.Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3(5):348–59. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- 28.Vandervoort AA, Sale DG, Moroz J. Comparison of motor unit activation during unilateral and bilateral leg extension. J Appl Physiol. 1984;56(1):46–51. doi: 10.1152/jappl.1984.56.1.46. [DOI] [PubMed] [Google Scholar]
- 29.Wannier T, Bastiaanse C, Colombo G, Dietz V. Arm to leg coordination in humans during walking, creeping and swimming activities. Exp Brain Res. 2001;141(3):375–9. doi: 10.1007/s002210100875. [DOI] [PubMed] [Google Scholar]
- 30.Weir JP, Housh DJ, Housh TJ, Weir LL. The effect of unilateral eccentric weight training and detraining on joint angle specificity, cross-training, and the bilateral deficit. J Orthop Sports Phys Ther. 1995;22(5):207–15. doi: 10.2519/jospt.1995.22.5.207. [DOI] [PubMed] [Google Scholar]
- 31.Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc Sport Sci Rev. 2005;33(1):54–60. [PubMed] [Google Scholar]
- 32.Zehr EP, Balter JE, Ferris DP, Hundza SR, Loadman PM, Stoloff RH. Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J Physiol. 2007;582(Pt 1):209–27. doi: 10.1113/jphysiol.2007.133843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10(4):347–61. doi: 10.1177/1073858404264680. [DOI] [PubMed] [Google Scholar]
- 34.Zehr EP, Haridas C. Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp Brain Res. 2003;149(2):260–6. doi: 10.1007/s00221-003-1377-9. [DOI] [PubMed] [Google Scholar]
- 35.Zehr EP, Klimstra M, Johnson EA, Carroll TJ. Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci Lett. 2007;419(1):10–4. doi: 10.1016/j.neulet.2007.03.045. [DOI] [PubMed] [Google Scholar]