Abstract
Sickness behaviour is an adaptive behavioural response to the activation of the innate immune system. It is mediated by brain cytokine production and action, especially interleukin-6 (IL-6). Polyunsaturated fatty acids (PUFA) are essential fatty acids that are highly incorporated in brain cells membranes and display immunomodulating properties. We hypothesized that a decrease in n-3 PUFA brain level by dietary means impacts on lipopolysaccharide (LPS)-induced IL-6 production and sickness behaviour. Our results show that mice exposed throughout life to a diet containing n-3 PUFA (n-3/n-6 diet) display a decrease in social interaction that does not occur in mice submitted to a diet devoid of n-3 PUFA (n-6 diet). LPS induced high IL-6 plasma levels as well as expression of IL-6 mRNA in the hippocampus and cFos mRNA in the brainstem of mice fed either diet, indicating intact immune-to-brain communication. However, STAT3 and STAT1 activation, a hallmark of IL-6 signalling pathway, was lower in the hippocampus of LPS-treated n-6 mice as compared to n-3/n-6 mice. In addition, LPS did not reduce social interaction in IL-6 knock-out (IL-6 KO) mice and failed to induce STAT3 activation in the brain of IL-6 KO mice. Altogether, these findings point to alteration in brain STAT3 as a key mechanism for the lack of effect of LPS on social interaction in mice fed with the n-6 PUFA diet. The relative deficiency of Western diets in n-3 PUFA could impact on behavioural aspects of the host response to infection.
Keywords: docosahexaenoic acid, arachidonic acid, STAT3, social interaction, LPS
Introduction
Activation of the innate immune system induces a sickness syndrome that includes a loss of interest in usual activities, lethargy and anorexia (Konsman et al., 2002). Sickness behaviour is mediated by cerebral action of peripherally released cytokines, especially interleukin-1 (IL-1), tumour necrosis factor α (TNFα) and IL-6 (Laye et al., 2000; Dantzer, 2001). IL-6 seems to be an absolute requisite for the full expression of sickness behaviour (Bluthe et al., 2000a; Konsman et al., 2000; Sparkman et al., 2006). Indeed, studies using transgenic mice deficient for cytokine genes demonstrated that whereas IL-1 can be replaced by TNF α in the brain to mediate sickness behaviour (Bluthe et al., 2000b), this is not the case for IL-6 (Bluthe et al., 2000a).
Binding of IL-6 to its receptor triggers the phosphorylation of receptor-associated Janus kinases (JAK), allowing the recruitment of signal transducer and activator of transcription (STAT) 1 and 3. STAT1 and 3 homo- or hetero-dimers then translocate into the nucleus, where they regulate transcription of target genes (Zhong et al., 1994; Hirano et al., 2000; Kamimura et al., 2003). Upon peripheral administration of IL-6 or bacterial endotoxin lipopolysaccharide (LPS), STAT3 is activated in astrocytes and brain endothelial cells (Gautron et al., 2002; Harre et al., 2002) and is thought to mediate IL-6 action in LPS-induced fever (Rummel et al., 2006).
Excessive peripheral and cerebral inflammatory cytokine response is associated with clinical complications among which cognitive dysfunction (Heyser et al., 1997), prolonged sickness behaviour (Combrinck et al., 2002), and depressive-like behaviour (Capuron et al., 2003), that are detrimental for the infected host. Based on these data, limiting the over-expression of brain proinflammatory cytokines seems crucial for protection against the adverse effects of cytokines in the brain. Dietary essential polyunsaturated fatty acids (PUFA) of the n-3 and n-6 series are potent regulators of cytokine production on which they exert opposing actions (Yaqoob, 2004; Calder, 2006). In particular, n-3 long-chain derivatives eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) down-regulate cytokine production in macrophages and microglia (Connor et al., 2007; Liuzzi et al., 2007; De Smedt-Peyrusse et al., 2008), while n-6 long-chain derivative arachidonic acid (AA, 20:4n-6) displays proinflammatory properties, particularly through the synthesis of eicosanoids (Tilley et al., 2001). Interestingly, it was demonstrated that anti-inflammatory properties of DHA targets proinflammatory cytokine signalling pathways in monocytes (Lee et al., 2004), T cells (Denys et al., 2001), and microglia (De Smedt-Peyrusse et al., 2008).
Brain cells membranes are particularly enriched in DHA and AA (Bourre et al., 1993; Salem and Niebylski, 1995). Because chronic dietary imbalance of n-3/n-6 PUFA leads to brain DHA decrease (Pifferi et al., 2005), such a diet could impair the expression and action of cytokines in the brain. Thus, we postulated that lower n-3 PUFA status in the brain, resulting from n-3 PUFA dietary deficiency from gestation to adulthood, may alter LPS behavioural effects by disturbing cerebral innate immune activation and action. Our attention was focused on IL-6 expression and signalling pathways as potential candidates for regulation by n-3 PUFA.
Materials and Methods
Animals and treatments
Animal experiments were carried out according to the Quality Reference System of INRA (1), the framework of the European Directives and the French Rules on Animal Experimentation. Every effort was made to minimize suffering and the number of animals used. Dietary intervention experiments were performed with CD1 Crl:CD-1 (ICR) IGS mice, that were obtained from a colony raised in the laboratory. After mating, CD1 females were fed either a diet containing 6% fat in the form of African peanut oil (rich in linoleic acid 18:2n-6) and rapeseed oil (rich in α-linolenic acid 18:3n-3) called n-3/n-6 diet or an isocaloric diet containing 6% fat in the form of only African peanut oil called n-6 diet (Aid et al., 2003) throughout gestation and lactation. Because during the pre-natal and early post-natal periods long-chain PUFA are actively accumulated in the brain (Martinez, 1992), the most efficient way for decreasing n-3 PUFA in the brain is to start giving n-6 diet during this period of life. The male offspring were submitted to the same diet as their dams throughout life and were housed in groups of ten per cage. They were approximately 8 weeks old when the behavioural and biochemical analyses were conducted. IL-6 knock out (IL-6 KO) mice (Kopf et al., 1994) back-crossed into a C57BL/6J background were kindly provided by Dr Bayard and colleagues at Institut Bugnard in Toulouse (Elhage et al., 2001). In experiments using IL-6 KO mice, C57BL/6J mice were used as WT controls.
For behavioural studies, LPS (escherichia coli, serotype 0127:B8, Sigma) was injected intraperitoneally (IP). A dose of 30 μg/kg was injected in CD1 mice fed with n-3/n-6 or n-6 diets while 60 μg/kg was injected in IL-6 KO and WT mice. These doses were selected for their ability to induce a non maximal decrease of social exploration in CD1 (data not shown) and in C57BL/6J mice (Bluthe et al., 2000b). Half the mice were injected with LPS whereas the other half received an injection of saline in a counterbalanced manner (according to mean baseline social exploration time for behaviour). For analysis of cytokine production and signalling in the brain, a dose of LPS (125 μg/kg) that reliably induces the synthesis of proinflammatory cytokines, was selected (Mingam et al., 2008). This dose induced the expression of several cytokines in the brain, including IL-1 and TNFα (data not shown). Because of the large number of mice required in these experiments and the difficulties to get enough male mice from the diet experiments, we decided to use the same dose of LPS (125 μg/kg) for cytokine measurement and signalling pathway analysis.
Measurements of sickness behaviour
Twenty-four mice were used in this experiment according to diet and treatment (n-3/n-6 + SAL: n=6; n-3/n-6 + LPS: n=6; n-6 + SAL: n=6; n-6 + LPS: n=6). Beginning at 24 h prior to initiation of the experiment, mice were individually housed in polycarbonate transparent cages (26 × 20 × 14 cm). Behavioural observations were made using a video camera under red light illumination between 09:00h and 17:00h (dark phase). Sickness behaviour was measured by two criteria: decrease in the duration of social exploration of a juvenile mouse introduced for 4 min into the home cage of the mouse under test; and decrease in food intake. Social exploration was measured immediately before and after injection of LPS or saline (0, 2, 6 and 24 h or 0, 3, 6 and 24 h) using different juveniles on each occasion so as to maintain a high level of social exploration in normal conditions. Food was weighed on a top-loading balance accurate to 0.1g immediately after treatment and again 2, 6 and 24 h or 3, 6 and 24 h later. Behaviour was monitored only 2 to 3 h after treatment since this latency is necessary for development of the characteristic behavioural alterations of sickness (Bluthe et al., 2000b).
Plasma IL-6 levels
Mice were killed by decapitation 2 and 4 hours after LPS or saline treatment. Blood was collected on ice in 10 U/ml endotoxin-free EDTA, and plasma was stored at −80°C until IL-6 assay. IL-6 measurement was performed by ELISA according to manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
Brain cytokine expression
Quantitative PCR was performed to measure brain cytokine expression using the Applied Biosystems (Foster, CA) assay-on demand gene expression protocol as previously described (Mingam et al., 2008). Total RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase. cDNA was amplified by PCR where a target cDNA (IL-6, IL-1 or TNFα) and a reference cDNA (glucose-3 phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7900 detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as relative fold change (Mingam et al., 2008).
Cytokine signalling pathways
Western blot analysis of cytokine signalling pathways was performed according to previously published method (Gautron et al., 2006). Briefly, hippocampi were homogenized in lysis buffer (TRIS 20 mM pH 7,5, protease inhibitors cocktail (Sigma, Saint-Louis, MO, USA), 5 mM MgCl2, 1 mM DTT, 0,5 M EDTA,1 mM sodium orthovanadate (Na3VO4), 1 mM NaF). After centrifugation, protein concentration was determined using a BCA assay kit (Uptima, Montlucon, France). Equal amounts of proteins (60 μg) were loaded on SDS/PAGE gels (10%) and transferred onto PVDF membranes (Millipore, Billerico, MA, USA). Membranes were incubated overnight at 4°C with the following primary antibodies: anti-phospho-STAT3 (Tyr705), anti-Phospho-JAK2 (Tyr1007/1008) and anti-Phospho-p42/44 MAP kinase (Thr2002/Tyr204) (1/500, Cell Signaling Technology, Denvers, MA, USA); anti-STAT3, anti-STAT1 and anti-JAK2 (1/1000, SantaCruz Biotechnology, Santa-Cruz, CA, USA); anti-ERK1/2 (1/1000, Cell Signaling Technology, Denvers, MA, USA), and anti-actin (1/2500, Sigma, Saint-Louis, MO, USA). After washing, membranes were incubated with peroxidase-conjugated secondary anti-rabbit for 1 h (1/5000, Jackson ImmunoResearch laboratories, Westgrove, PA, USA). Between each revelation, membranes were incubated for 10 min at 70°C in stripping buffer (0.065 M TRIS pH6.7, 1%, SDS, 0.7% β-mercapto-ethanol) in order to erase the previous antibody. Staining was revealed with ECL-Plus Western blotting detection system (Perkin Elmer, Forest City, CA). Chemiluminescence was captured by a Syngene detection system and quantified by Gene Tools software (Syngene).
Immune-to-brain communication
Since the integrity of immune-to-brain communication is responsible for activation of the early response gene c-fos in the brain in response to LPS (Konsman et al., 2000), cFos immunohistochemistry was performed as previously described (Gautron et al., 2005). Briefly, 2 h after treatment, brains were fixed by intracardiac perfusion of saline followed by 4% paraformaldehyde (pH 9.5 at 10°C). Brains were postfixed for 4 h, and then cryoprotected in 30% sucrose in 0.1 M phosphate buffer (pH 7.4) for 24 h. Series of 6 coronal cryostat sections through the brainstem were collected in cold cryoprotectant (0.05 M phosphate buffer, 30% sucrose, 30% ethylene glycol) and stored at −20°C until immunohistochemistry.
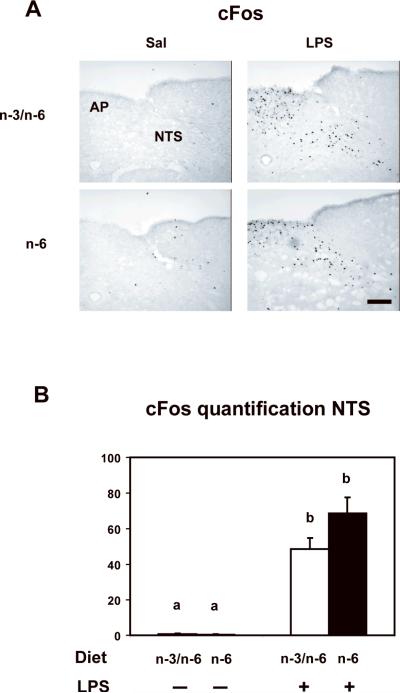
After a 45 min incubation of sections in TBS containing 0.3% Triton X-100 and 1% BSA, the first antibody (polyclonal rabbit anti-cFos, 1/2000,SantaCruz Biotechnology, Santa-Cruz, CA, USA) was added for 12 h at 4°C. After four rinses in TBS, sections were incubated for 30 min in 0.3% hydrogen peroxide, 2 h at room temperature with biotinylated donkey antisheep or biotinylated goat anti-rabbit immunoglobulins G (1/2000, Jackson ImmunoResearch laboratories, Westgrove, PA, USA) and streptavidin biotin-peroxidase complex (kit ABC Vectastain, PK6100, 1/1000, Vector laboratories, Burlingame, CA, USA) with interposed rinses. A peroxidase reaction product was developed using diaminobenzidine and the nickel-enhanced glucose oxidase method (Gautron et al., 2003). Sections were mounted on gelatine-coated slides, air dried and coverslipped. Slides were examined under a light microscope (Nikon E400) and cFos labelling patterns were compared between groups and within the same animal. cFos positive cells were counted by using the Image J software (image processing and analysis in java). Digital photomicrographs in Figure 3 were obtained by using a Nikon E400. Image editing software (Adobe Photoshop) was used to adjust only contrast and brightness.
Figure 3.
n-6 PUFA diet does not impair LPS-induced cFos activation in the NTS. cFos was measured by immunohistochemistry in the NTS of mice fed with n-3/n-6 or n-6 diet, 2 hours after a peripheral injection with saline (−) or LPS (+). A) Photomicrographs of the distribution of cFos immunoreactivity in the brainstem (NTS and AP) (Scale bar represents 100 μm). B) Bar graphs summarizing the mean number of cFos-positive cells per section in the NTS of n-3/n-6 and n-6 mice 2 hours after peripheral injection of LPS. Data are given as the mean number of cFos positive cells per section ± SEM. Means with different letters are significantly different from each other (p<0.001).
Fatty acid analysis of phospholipid classes
Mouse cortices were homogenized on ice with 0.5 or 1 ml of NaCl solution (9 g/L) containing butylhydroxytoluene (0.02 g/L), and their total lipids were then extracted according to Folch et al. (Folch et al., 1957). Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were separated from total lipids by solid-phase extraction on a 500 mg aminopropyl-bonded silica column (BAKERBOND spe™ Amino, Baker, USA) as previously described (Pifferi et al., 2005). Briefly, neutral lipids were eluted with isopropanol/chloroform (1:2, vol/vol) mixture from the total lipid extract deposited beforehand on the silica cartridge. A mixture of diethylether/acetic acid (98:2, vol/vol) then eluted free fatty acids, and acetone eluted PC. After elution of sphingomyelin with acetonitrile/n-propanol mixtures (1:1, vol/vol), PE was finally eluted by methanol. After evaporation under nitrogen, PC and PE were transmethylated with 10% boron trifluoride (Fluka, Socolab, Paris, France) at 90°C for 20 min. Fatty acid methyl esters were injected into a Carlo Erba gas chromatograph (HRGC 5300, Fisons Instruments, Arcueil-Cachan, France) equipped with an on-column injector, a flame ionisation detector and a CP WAX 52 CB bonded fused-silica capillary column (50 m × 0.2 mm id with 0.20 μm film thickness) purchased from Chrompack (Les Ulis, France). Hydrogen was used as carrier gas at 1–2 ml/min flow rate. Fatty acid methyl esters with a C8 to C24 chain length and dimethyl acetals were identified by comparing equivalent chain lengths with those of commercial standards (Nu-Check-Prep Inc., Coger, Paris, France) and quantified with a computing integrator using the Nelson Analytical Program System (SRA, Gagny, France). Fatty acid composition was expressed as weight percentage.
Statistical Analyses
Behavioural data collected at time 0 were analyzed by a one-way ANOVA to confirm the lack of baseline difference between experimental groups. Data collected after treatments were analyzed by two- or three-way ANOVA with repeated measurements on the time factor. Between-group differences were determined by post hoc comparison with Fisher's least significant difference test. All results are summarized and presented as the mean ± SEM.
Results
n-6 PUFA diet decreases DHA in the brain
We first investigated the impact of the diets under study on brain n-3 and n-6 long-chain PUFA (Table 1). For this purpose, AA (20:4n-6), DPA n-6 (22:5n-6) and DHA (22:6n-3) were measured in cerebral cortex phospholipids (PC and PE). As expected, DHA was strongly decreased in both PC and PE of mice fed the n-6 diet as compared to n-3/n-6 mice, while DPA was increased. AA was not altered by the n-6 diet.
Table 1.
n-6 PUFA diet decreases DHA in brain phospholipids
| Weight % of total fatty acids | ||||||||
|---|---|---|---|---|---|---|---|---|
| PC | 20:4n–6 | 22:5n–6 | 22:6n–3 | PE | 20:4n–6 | 22:5n–6 | 22:6n–3 | |
| n-3/n-6 | 3.9±0.7 | 0.1±0.0 | 3.7±0.3 | 11.2±0.3 | 0.5±0.1 | 26.4±0.6 | ||
| n-6 | 5.0±1.0 | 1.9±0.1* | 1.3±0.3* | 12.7±1.2 | 13.8±2.1* | 11.1±0.9* | ||
AA (20:4n-6), DPA n-6 (22:5n-6) and DHA (22:6n-3) levels in cortex phosphatidylcholine (PC) and phosphatidylethanolamine (PE) of mice fed either the n-3/n-6 or the n-6 diet. Data are mean values ± SEM, expressed in weight percent of total fatty acids (n=4).
significant difference between n-3/n-6 and n-6 groups, p<0.001.
n-6 PUFA diet abrogates LPS-induced decrease in social interaction but not in food intake
The primary objective of this study was to determine whether the activation of the peripheral innate immune system with LPS induced a different profile of sickness behaviour in n-6 mice as compared to n-3/n-6 mice. For this purpose, social interaction and food intake were measured in n-6 and n-3/n-6 mice after intraperitoneal injection of LPS (30 μg/kg) or saline (Figure 1). The duration of baseline social exploration ranged from 90.2 to 111 sec and did not differ according to the diet at time 0. LPS decreased the duration of social exploration in a time-dependent manner (Figure 1A, treatment: (F(1,20)=15.4, P<0.001); time: (F(3,60)=4.3, P<0.01); treatment x time: (F(3,60)=38.8, P<0.001). The diet x treatment x time interaction was significant (F(3,60)=4.7, P<0.01). Post-hoc comparisons of individual group means revealed that LPS-induced decrease in social exploration was strongly attenuated 3 and 6 h after injection in n-6 mice as compared to n-3/n-6 mice (Figure 1A). LPS decreased food intake (F(1,20)=63.5, P<0.001) in a time-dependent manner (F(2,40)=120.5, P<0.001) and this effect did not differ according to the diet (Figure 1B).
Figure 1.
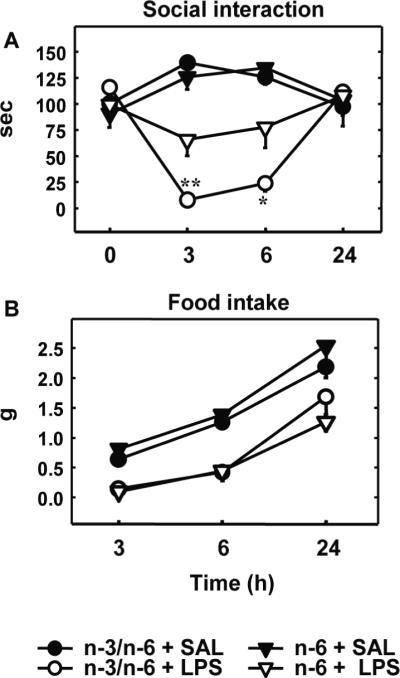
n-6 PUFA diet attenuates LPS-induced social interaction decrease, but not food intake
Different components of sickness behaviour were measured just before (time 0) and 3, 6 and 24h after IP LPS or saline (SAL). A) Duration of social exploration. Each symbol represents the mean ± SEM of six mice in each experimental group. * p<0.05, ** p<0.01, n-6 + LPS vs. n-3/n-6 + LPS. B) Food intake measured by weighing the wire cage tops at the indicated times.
n-6 PUFA modulates LPS-induced circulating and brain expression of IL-6
Because LPS-induced social interaction decrease did not develop in n-6 mice, we investigated whether peripheral and brain IL-6 expression was impaired in these mice. For this purpose, plasma levels of IL-6 and hypothalamic IL-6 mRNA were measured in n-3/n-6 and n-6 mice 2 and 4 hours after LPS (125 μg/kg) treatment (Figure 2). Very low levels of IL-6 were detected in the plasma of saline-treated mice whatever diet they were exposed to (Figure 2A). LPS treatment induced IL-6 release in both experimental groups. A three-way ANOVA revealed a significant effect of diet (F(1,21)=7.0, P<0.05), treatment (F(1,21)=184.0, P<0.001) and their interaction (F(1,21)=6.8, P<0.05). Post-hoc comparison of individual group means revealed that LPS-induced IL-6 was higher in n-6 compared to n-3/n-6 diet (Figure 2A). IL-6 mRNA expression measured in the hippocampus of saline-treated mice was low whatever diet they were exposed to (Figure 2B). LPS enhanced IL-6 mRNA expression in the hippocampus of both experimental groups; however the kinetic of IL-6 expression was different according to the diet as revealed by a three-way ANOVA. Indeed, a significant effect of treatment (F(1,43)=47.94, P<0.001), time (F(1,43)=4.45, P<0.05), treatment and time interaction (F(1,43)=4.3, P<0.05), diet and time interaction (F(1,43)=4.52, P<0.05) were revealed (Figure 2B). A trend for treatment, time and diet interaction was also measured (P=0.053). Post-hoc analysis showed a significant effect of the treatment time within the n-6 group (2 hours vs. 4 hours, P<0.01), while it was not the case for the n-3/n-6 group.
Figure 2.
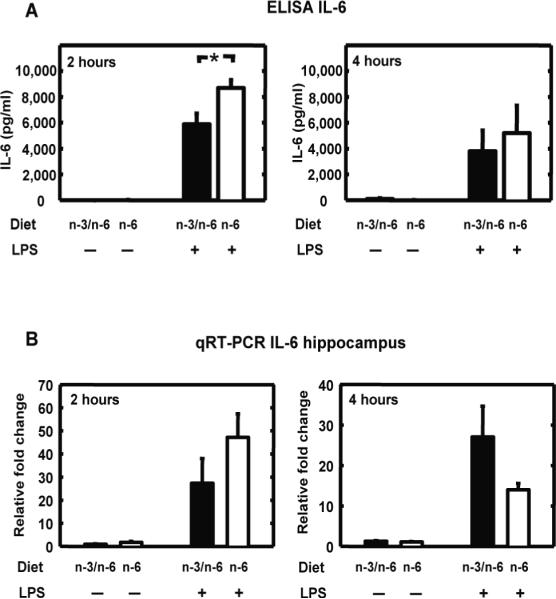
n-6 PUFA diet modulates LPS-induced circulating and brain expression of IL-6. IL-6 was measured in plasma and hippocampus of mice fed with n-3/n-6 or n-6 diet, 2 and 4 hours after a peripheral injection with saline (−) or LPS (+). A) IL-6 plasma level was measured by ELISA. Data are given as the mean level of IL-6 ± SEM (pg/ml). * p<0.005. B) IL-6 mRNA expression was measured by qRT-PCR in the hippocampus. Data are given as the mean level of relative fold change ± SEM.
Immune-to-brain communication is not blunted in n-6 mice
Because immune-to-brain communication via the vagus nerve plays a key role in the expression of brain cytokines in response to peripheral immune activation (Laye et al., 1995), we examined whether LPS-induced cFos activation in the primary projection area of the vagus nerve, the NTS, was altered in n-6 as compared to n-3/n-6 mice. As depicted in Figure 3, there was only weak cFos immunoreactivity in the NTS in saline-treated mice, regardless of the diet. LPS (125 μg/kg) induced a strong increase in cFos immunoreactivity in the NTS of n-3/n-6 and n-6 mice. A significant effect of the treatment was revealed (F(1,11)=54, P<0.001) with no effect of the diet or treatment x diet interaction.
n-6 PUFA diet reduces JAK2/STAT1/STAT3 activation in the brain
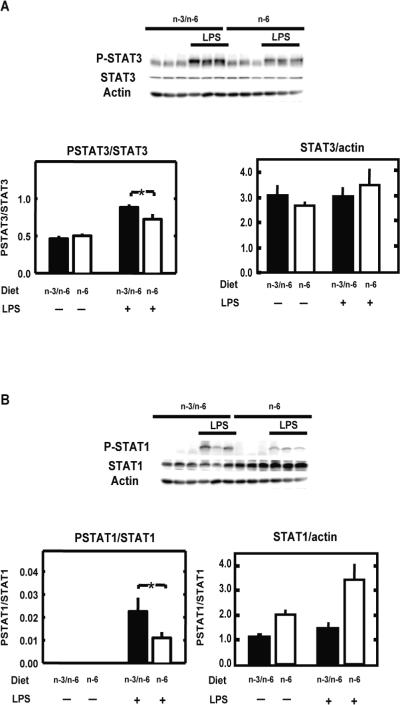
Since the alteration of LPS-induced sickness behaviour in n-6 mice was not associated with the absence of IL-6 production or with impairment of immune-to-brain activation, we investigated whether IL-6 signalling pathway was impaired in the hippocampus of these mice. STAT1 and STAT3 activation were first investigated because these transcription factors are involved in IL-6 effect on the brain (Gautron et al., 2002; Harre et al., 2002). Total STAT1, STAT3, their respective phosphorylated forms and actin were measured in the hippocampus of n-3/n-6 and n-6 mice by Western blot 2 hours after saline or LPS (125 μg/kg) (Figure 4). A significant effect of treatment (F(1,11)=15,4, P<0.01) and diet (F(1,11)=5.2, P=0.05) with no interaction, was measured for STAT1 expression as compared to actin expression. Such effects were not revealed for STAT3. LPS treatment enhanced the phosphorylation of both STAT3 and STAT1. A two-way ANOVA revealed no effect of diet factor but a significant effect of the treatment factor (F(1,8)=10.6, P<0.001) and diet x treatment interaction (F(1,8)=9.9, P<0.05) on STAT3 activation. Post-hoc comparison of individual group means revealed that LPS effect was higher in n-3/n-6 as compared to n-6 hippocampus (Figure 4A). Concerning STAT1 activation, a two-way ANOVA revealed a significant effect of the diet (F(1,8)=5.9, P<0.05) and treatment factors (F(1,8)=42.5, P<0.001) and their interaction (F(1,8)=5.9, P < 0.05). Post-hoc comparison of individual group means revealed that LPS effect on STAT1 phosphorylation was higher in n-3/n-6 as compared to n-6 diet (Figure 4B). The same results were observed in the hypothalamus (data not shown).
Figure 4.
n-6 PUFA diet reduces LPS-induced STAT1 and STAT3 activity in hippocampus STAT3, its phosphorylated tyrosine-705 form and actin (A), and STAT1, its phosphorylated tyrosine-701 form and actin (B) were measured in hippocampi isolated from mice fed with n-3/n-6 or n-6 diet by Western blot (upper panel) and quantified (lower panel) 2 hours after a peripheral injection with saline (−) or LPS (+). Data are given as the mean level of the ratio of P-STAT3 or P-STAT1 to STAT3 or STAT1 respectively (lower left panel) and STAT3 or STAT1 to actin (lower right panel) ± SEM. * p<0.05.
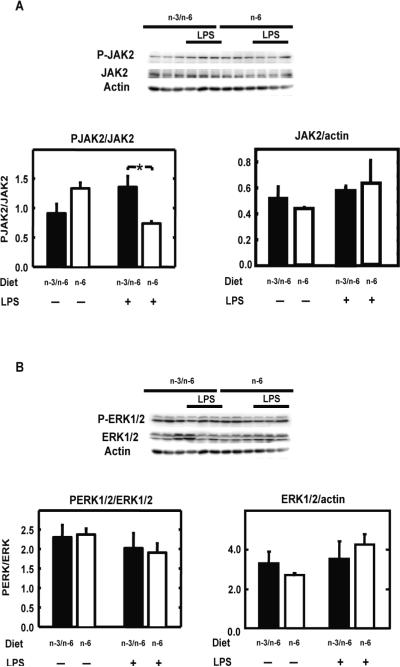
Because both JAK2 (Satriotomo et al., 2006) and ERK1/2 (Tancredi et al., 2000) have been linked to STAT3 activation in the brain, we further investigated whether these kinases were differentially regulated in the brain of n-3/n-6 and n-6 mice. Both kinases were expressed in the hippocampus of mice (Figure 5). JAK2 and ERK1/2 expression as compared to actin expression was not different in n-6 and n-3/n-6 mice hippocampus (Figure 5A and 5B). There was no effect of the diet nor treatment on PJAK2/JAK2 but a diet x treatment interaction (F(1,8)=15.4, P<0.01). Post-hoc comparison of individual group means revealed that LPS increased PJAK2/JAK2 only in n-3/n-6 mice whereas it decreased PJAK2/JAK2 in n-6 mice (Figure 5A). There was no effect of the diet, treatment and their interaction on the phosphorylation of ERK1/2 (Figure 5B).
Figure 5.
n-6 PUFA diet reduces LPS-induced JAK2 activation but does not affect ERK1/2 phosphorylation in hippocampus
A) JAK2, its phosphorylated tyrosine-1007/1008 form and actin (A), and ERK1/2, its phosphorylated tyrosine-42/44 form and actin (B) were measured in hippocampi isolated from mice fed with n-3/n-6 or n-6 diet by Western blot (upper panel) and quantified (lower panel) 2 hours after a peripheral injection with saline (−) or LPS (+). Data are given as the mean level of the ratio of P-JAK2 or P-ERK1/2 to JAK2 or ERK1/2 respectively (lower left panel) and JAK2 or ERK1/2 to actin (lower right panel) ± SEM. * p<0.05.
LPS-induced sickness behaviour and hippocampal STAT3 activation do not develop in IL-6 KO mice
Our results demonstrated that LPS-induced decrease in social exploration and hippocampal IL-6 signalling pathway are altered in n-6 mice. We therefore analyzed whether IL-6 was required for LPS effect on sickness behaviour and STAT3 activation in the hippocampus. For this purpose, we used IL-6 KO mice (Kopf et al., 1994). We first measured LPS-induced sickness behaviour in WT and IL-6 KO mice (Figure 6). The duration of baseline social exploration (time 0 in Figure 6A) ranged from 93.0 to 115 sec and did not differ according to strain (WT vs. KO) and treatment (SAL vs. LPS). LPS (60 μg/kg) decreased the duration of social exploration in a time-dependent manner (treatment: (F(1,13)=12.2, P<0.01); time: (F(3,39)=9.0, P<0.001); treatment x time: (F(3,39)=11.4, P<0.001)). However, the effect of LPS differed according to the strain factor (F(1,13)=6.6, P<0.05), time x strain interaction (F(3,39)=11.4, P<0.001), and treatment x time x strain interaction (F(3,39)=8.4, P<0.001). Post-hoc comparisons of individual group means revealed that the LPS-induced reduction in social exploration observed in WT mice at 3 and 6 h post-treatment was completely absent in IL-6-deficient mice (Figure 6A). A three-way (strain x treatment x time) ANOVA on food intake revealed a significant effect of treatment (F(1,13)=73.0, P<0.001), time (F(2,26)=,196.2, P<0.001), and their interaction (F(2,26)=15.4, P<0.001) together with a significant strain x treatment x time interaction (F(2,26)=3.5, P<0.05). Post-hoc comparison of individual group means revealed that LPS reduced food intake 2 and 6 h after treatment in WT and IL-6 KO mice in the same manner as compared to saline injected mice (P<0.05) (Figure 6B).
Figure 6.
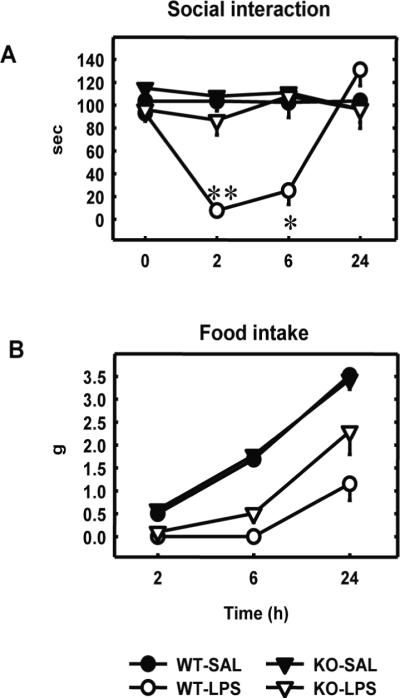
IL-6 KO mice exhibit alteration of LPS-induced sickness behaviour
Sickness behaviour was measured just before (time 0) and 2, 6 and 24 h after peripheral injection with saline (SAL) or LPS in IL-6 deficient (KO) mice and their wild-type controls (WT). A) Duration of social exploration. Each symbol represents the mean ± SEM of 5 (WT-SAL), 3 (WT-LPS), 5 (KO-SAL) and 4 (KO-LPS) mice. * p<0.05, ** p<0.01, KO-LPS vs. WT-LPS. B) Food intake measured by weighing the wire tops at the indicated times. Each value represents the mean ± SEM.
Because LPS-induced decrease in social exploration was abolished in IL-6 KO mice, we further measured STAT3 activation in the hippocampus of these mice 2 hours after LPS treatment (125 μg/kg) (Figure 7) A two-way ANOVA on PSTAT3/STAT3 revealed a significant effect of strain (F(1,9)=40.0, P=0.001), treatment (F(1,9)=43.0, P<0.001) and their interaction (F(1,9)=8.8, P<0.05). Post-hoc comparison of individual group means revealed that LPS induced an increase of PSTAT3/STAT3 in WT but not in IL-6 KO mice (Figure 7).
Figure 7.
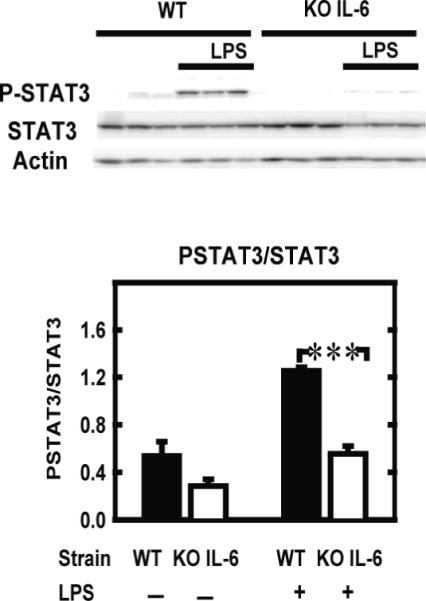
LPS fails to induce STAT3 activation in the hippocampus of IL-6 KO mice STAT3 and its phosphorylated tyrosine-705 form were measured in hippocampi isolated from IL-6 KO and WT mice by Western blot (upper panel) and quantified (lower panel) 2 hours after a peripheral injection with saline (−) or LPS (+). Data are given as the mean level of the ratio of P-STAT3 to STAT3 ± SEM of 3 independent experiments. *** p<0.001.
Discussion
The present results show that long term exposure to n-6 diet as compared to n-3/n-6 diet decreased brain DHA levels. Upon peripheral immune challenge with LPS, n-6 mice displayed an attenuated decrease in social exploration whereas IL-6 expression was strongly induced in both plasma and hippocampus. We therefore investigated whether IL-6 signalling pathway was altered in the brain. Our results revealed that in n-6 mice, LPS-induced STAT1, STAT3 and JAK2 activation was also attenuated as compared to n3/n-6 mice. No decrease in social interaction and no activation of STAT3 were developed in IL-6 deficient mice, suggesting that IL-6 activation of STAT3 is positively linked to LPS effect on social interaction. All together, these data can be interpreted to suggest that when brain DHA level is decreased as a consequence of n-3 PUFA deficiency, although IL-6 expression is still induced by LPS, sickness behaviour is down-regulated via the impairment of IL-6 signalling in the brain.
We observed that LPS-induced decrease in social interaction was fully expressed in n-3/n-6 but strongly attenuated in n-6 mice. This was not the case for LPS-induced decrease in food intake. Contradictory results have been observed previously concerning the effect of n-3 enriched diet on sickness behaviour. Whereas dietary supplementation with n-3 long-chain PUFA for 6 weeks did not affect LPS-induced lethargy and anorexia (Kozak et al., 1997), it partially reversed LPS-induced behavioural depression and IL-1-induced anxiety (Watanabe et al., 2004; Song et al., 2006). In these studies, the attenuated behavioural effect of LPS by n-3 PUFA cannot be explained by its effect on peripheral cytokine production. The n-3 fattyacid-rich diet augmented serum TNFα but not IL-1, in response to intraperitoneal injection of LPS (Watanabe and Okuyama, 1991; Kozak et al., 1997). Similarly, humans supplemented for 3–4 weeks with fish oil (rich in n-3 long-chain PUFA) did not display any fever after LPS injection despite similar induction of TNFα production in the plasma (Michaeli et al., 2007). Our results showing that n-3 deprived mice do not develop social interaction decrease in response to LPS are puzzling. It could be viewed either as a benefit for the individual, because sickness behaviour is reduced and that the general activity is maintained, or as a poorly adapted response of the host to the infection. Indeed, behavioural modifications induced by the activation of the innate immunity are commonly viewed as an adaptive way to promote recovery from an infection (Hart, 1988; Dantzer, 2001). Behavioural symptoms of sickness correspond to a motivational state competing with other motivated activities (Aubert, 1999; Renault and Aubert, 2006). Among the non-specific symptoms of inflammation, the loss of interest in social environment could limit the risk of infection of the other members of the social group (Hart, 1990). As a result, possible contamination could be bypassed by a decreased physical contact toward sick individuals (Hart, 1990). The maintained social interaction in response to LPS in n-6 mice could therefore favour physical contact and the spread of the disease, suggesting a non adapted behaviour. However, that point remains to be determined.
We previously demonstrated that sickness behaviour induced by peripheral activation of the innate immune system is mediated by brain cytokine expression and action (Laye et al., 1995; Laye et al., 2000). In particular, the effect of LPS on food intake depends on brain IL-1 (Bluthe et al., 2000b) and TNFα (Porter et al., 2000) whereas the decrease in social exploration is dependent on IL-1 and IL-6, TNFα plays a role only in the absence of IL-1 (Bluthe et al., 2000b). We confirmed in the present study that IL-6 deficient mice experience less severe depression in social behaviour than wild-type controls in response to LPS (Bluthe et al., 2000a). These findings point to brain IL-6 action as a key factor in mediating LPS effect on social interaction but not on food intake. An obvious causal factor for the decreased effect of LPS on social interaction in n-6 mice was the possibility of a lower IL-6 production both at the periphery and in the brain in response to LPS. By measuring circulating and brain IL-6 production in LPS-treated mice, we demonstrated that IL-6 is not deficient and if anything overproduced in mice fed the n-6 as compared to n-3/n-6 diet. The decreased brain DHA could account for IL-6 increased production in the brain of n-6 mice 2 hours after LPS. Indeed, we have previously demonstrated that DHA potently down-regulates LPS-induced cytokine expression in microglia (De Smedt-Peyrusse et al., 2008). IL-6 is directly targeted by DHA that down-regulates its expression in macrophages (Moon and Pestka, 2003). Such an anti-inflammatory effect has been linked to DHA inhibitory action on AKT-dependent phosphorylation and subsequent binding of CREB/ATF1 to the IL-6 promoter (Jia et al., 2006). Our results further reinforce the idea that DHA displays anti-inflammatory properties in the brain (Yaqoob, 2004; De Smedt-Peyrusse et al., 2008).
Because in the current study high circulating and hippocampal IL-6 was not sufficient to inhibit social interaction in n-6 mice, we also explored whether n-3 dietary deficiency would interfere with the vagal afferent pathway. Vagal afferences that converge to NTS are responsive to peripheral LPS (Konsman et al., 2000). Neurons in the NTS project to brain areas mediating sickness behaviour, limbic structures (Konsman et al., 2000) including the hippocampus (Rutecki, 1990). Vagus nerve is involved in mediating LPS-induced social interaction decrease, but not food intake impairment (Laye et al., 1995; Schwartz et al., 1997; Konsman et al., 2000). Therefore, if n-3 dietary deficiency blunted the vagal afferent pathway, it might explain why n-6 mice did not display social interaction decrease in response to LPS. Our results demonstrate that cFos immunoreactivity in the NTS was similarly increased in n-6 and n-3/n-6 mice after LPS injection. This result indicates that n-6 diet did not interfere with the LPS-activated vagal afferent pathway. Because vagal nerve activation has been shown to be involved in hippocampal cytokine production (Laye et al., 1995), this result is consistent with our observation that LPS induced IL-6 production in the hippocampus of n-6 mice.
The current study shows that in n-6 mice, LPS induced IL-6 expression in both blood and hippocampus, while social interaction was not decreased. These results are puzzling because IL-6 is a prerequisite for LPS mediation of sickness behaviour (Bluthe et al., 2000a; Sparkman et al., 2006) as further confirmed in our study using IL-6 KO mice. We therefore hypothesized that the lack of effect of exposure to n-6 diet on LPS-induced decrease in social exploration could be due to an impairment of brain IL-6 action. Our attention was focused on JAK/STAT pathway because this signalling pathway is necessary to mediate inflammatory effect of IL-6 in the brain (Harre et al., 2003; Sanz et al., 2008). Moreover, if this signalling pathway is activated by other cytokines such as IL-10 or leukaemia inhibitory factor (O'Shea et al., 2002), our results obtained in IL-6 KO mice demonstrate that LPS-induced STAT3 in the hippocampus is dependent on IL-6. Our hypothesis was evaluated through the measurement of ERK1/2, JAK2 and STAT1 and STAT3 activation in the brain. LPS and IL-6 have already been shown to activate brain STAT3 signalling pathways (Gautron et al., 2002; Harre et al., 2002). The measurement of these signalling pathways in the hippocampus revealed a strong decrease in LPS-induced JAK2, STAT1 and STAT3 phosphorylation in n-6 mice as compared to n-3/n-6 mice. This effect was not observed on ERK1/2 activation that is rather activated in the brain by IL-1 (Nadjar et al., 2005). The use of different doses of LPS for behavioural (30 to 60 μg/kg) and biochemical (125 μg/kg) analysis cannot rule out the induction of signalling pathways that are not induced by the doses used in behavioural experiments. By using IL-6 KO mice we demonstrated that LPS-induced STAT3 activation in the hippocampus is dependent on IL-6. In support of this interpretation, the pharmacological blockade of JAK2 and STAT3 phosphorylation was shown to significantly decrease the IL-6-mediated infarct volume, number of apoptotic cells and neurological deficits following transient middle cerebral artery occlusion (Satriotomo et al., 2006). It is therefore likely that the consequences of prolonged exposure to the n-6 diet on LPS-induced sickness behaviour were caused by impaired JAK2/STAT3/STAT1 activation in the brain.
Many biochemical processes conspire to regulate the JAK-STAT pathway, including loss of receptor numbers at the surface or increase of endogenous inhibitory systems. Interestingly, the administration of the active form of IL-6 receptor in the brain augments the behavioural effects of IL-6 (Schobitz et al., 1995). In our work, we did not identify the mechanisms underlying the blocking effect of n-3 PUFA dietary deficiency on IL-6/JAK/STAT activity. Brain DHA decrease could be responsible for the IL-6 receptor signalling decrease measured in hippocampus of LPS-treated n-6 mice. In rodents, n-3 PUFA dietary deficiency is responsible for a drastic decrease of DHA and an increase of AA metabolism in the brain (Aid et al., 2003; Rao et al., 2007). Increased AA metabolism linked to n-3 PUFA deprivation is associated with an increase in the whole-body content of prostaglandins (Logue et al., 2000). AA is an important component of membrane phospholipids and is released acutely in response to cytokines (Yellaturu and Rao, 2003; Rosenberger et al., 2004). Upon release, AA is either metabolized by cyclooxygenase (COX) or by lipoxygenase to produce respectively prostaglandins (PG) or hydroperoxyeicosatetraenoic acids, or is reincorporated into membrane phospholipids. Preliminary results obtained by our group indicate a trend for a higher expression of COX-2 in the hippocampus of n-6 as compared to n-3/n-6 mice after LPS treatment (data not shown), suggesting higher AA metabolism in these mice. The higher availability of AA in the brain of n-6 mice could be involved in the impaired STAT3 activation in the hippocampus of these mice. Interestingly some prostaglandins, namely 15d-PGJ2, have been reported to abrogate STAT3 activation in IL-6-treated endothelial cells and lymphocytes (Park et al., 2003; Kim et al., 2005). However, whether or not these prostaglandins are involved in STAT3 decrease measured in n-6 mice remains to be investigated.
In conclusion, n-3 PUFA dietary deficiency, which lowers brain incorporation of DHA, may contribute to the lack of behavioural response to bacterial endotoxin by uncoupling IL-6 from its signalling pathway. In particular, the lack of STAT3 activation that leads to an impairment of IL-6 action in the brain might help explain why n-6 mice did not show diminished social interaction after peripheral LPS challenge. Because sickness behaviour is considered to be an adaptive response of the host to the infection, the present results emphasize that the dietary ratio of n-6 to n-3 fatty acids may be a significant factor for modulating host defence strategies. This is of particular interest because the n-6 fatty acids account for the majority of polyunsaturated fatty acids provided in Western diets (Simopoulos, 1991).
Acknowledgements
Supported by INRA, CNRS, University Bordeaux 2, the Région Aquitaine (20030305001A2Bbis and 20051203008AB to SL) and the National Institutes of Health Grants (MH071349 to RD). A.M. is a stipend of France Alzheimer and Société Française de Nutrition. The authors gratefully thank Caroline Nevoit for her excellent technical assistance (Institut Bugnard, Toulouse, France).
Glossary
Abbreviations
- AA
arachidonic acid
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- ERK
extracellular signal-regulated kinase
- IL
interleukin
- JAK
Janus kinase
- KO
knock-out
- LA
linoleic acid
- LPS
lipopolysaccharide
- NTS
nucleus tractus solitarius
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- STAT
signal transducer and activator of transcription
- TNF
tumour necrosis factor
- WT
wild type
- α-LNA
α-linolenic acid
References
- Aid S, Vancassel S, Poumes-Ballihaut C, Chalon S, Guesnet P, Lavialle M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J Lipid Res. 2003;44:1545–1551. doi: 10.1194/jlr.M300079-JLR200. [DOI] [PubMed] [Google Scholar]
- Aubert A. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev. 1999;23:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol Behav. 2000a;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000b;12:4447–4456. [PubMed] [Google Scholar]
- Bourre JM, Bonneil M, Clement M, Dumont O, Durand G, Lafont H, Nalbone G, Piciotti M. Function of dietary polyunsaturated fatty acids in the nervous system. Prostaglandins Leukot Essent Fatty Acids. 1993;48:5–15. doi: 10.1016/0952-3278(93)90003-f. [DOI] [PubMed] [Google Scholar]
- Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr., Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Laye S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105:296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- Denys A, Hichami A, Khan NA. Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase (ERK1/ERK2) signaling in human T cells. J Lipid Res. 2001;42:2015–2020. [PubMed] [Google Scholar]
- Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gautron L, Chaigniau M, Laye S. Specific localization of signal transducer and activator of transcription 1 immunoreactivity in oxytocin neurons of the rat hypothalamus. Brain Res. 2003;994:260–264. doi: 10.1016/j.brainres.2003.09.061. [DOI] [PubMed] [Google Scholar]
- Gautron L, De Smedt-Peyrusse V, Laye S. Characterization of STAT3-expressing cells in the postnatal rat brain. Brain Res. 2006;1098:26–32. doi: 10.1016/j.brainres.2006.04.115. [DOI] [PubMed] [Google Scholar]
- Gautron L, Lafon P, Chaigniau M, Tramu G, Laye S. Spatiotemporal analysis of signal transducer and activator of transcription 3 activation in rat brain astrocytes and pituitary following peripheral immune challenge. Neuroscience. 2002;112:717–729. doi: 10.1016/s0306-4522(02)00115-x. [DOI] [PubMed] [Google Scholar]
- Gautron L, Mingam R, Moranis A, Combe C, Laye S. Influence of feeding status on neuronal activity in the hypothalamus during lipopolysaccharide-induced anorexia in rats. Neuroscience. 2005;134:933–946. doi: 10.1016/j.neuroscience.2005.03.063. [DOI] [PubMed] [Google Scholar]
- Harre EM, Roth J, Gerstberger R, Hubschle T. Interleukin-6 mediates lipopolysaccharide-induced nuclear STAT3 translocation in astrocytes of rat sensory circumventricular organs. Brain Res. 2003;980:151–155. doi: 10.1016/s0006-8993(03)02923-8. [DOI] [PubMed] [Google Scholar]
- Harre EM, Roth J, Pehl U, Kueth M, Gerstberger R, Hubschle T. Selected contribution: role of IL-6 in LPS-induced nuclear STAT3 translocation in sensory circumventricular organs during fever in rats. J Appl Physiol. 2002;92:2657–2666. doi: 10.1152/japplphysiol.00822.2001. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hart BL. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci Biobehav Rev. 1990;14:273–294. doi: 10.1016/s0149-7634(05)80038-7. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Jia Q, Zhou HR, Shi Y, Pestka JJ. Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J Nutr. 2006;136:366–372. doi: 10.1093/jn/136.2.366. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rho YH, Choi SJ, Lee YH, Cheon H, Um JW, Sohn J, Song GG, Ji JD. 15-Deoxy-delta12,14-PGJ2 inhibits IL-6-induced Stat3 phosphorylation in lymphocytes. Exp Mol Med. 2005;37:179–185. doi: 10.1038/emm.2005.24. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kozak W, Soszynski D, Rudolph K, Conn CA, Kluger MJ. Dietary n-3 fatty acids differentially affect sickness behavior in mice during local and systemic inflammation. Am J Physiol. 1997;272:R1298–1307. doi: 10.1152/ajpregu.1997.272.4.R1298. [DOI] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Laye S, Bluthe RM, Kent S, Combe C, Medina C, Parnet P, Kelley K, Dantzer R. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am J Physiol. 1995;268:R1327–1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Latronico T, Rossano R, Viggiani S, Fasano A, Riccio P. Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells--implications for complementary multiple sclerosis treatment. Neurochem Res. 2007;32:2184–2193. doi: 10.1007/s11064-007-9415-9. [DOI] [PubMed] [Google Scholar]
- Logue JA, Howell BR, Bell JG, Cossins AR. Dietary n-3 long-chain polyunsaturated fatty acid deprivation, tissue lipid composition, ex vivo prostaglandin production, and stress tolerance in juvenile Dover sole (Solea solea L.) Lipids. 2000;35:745–755. doi: 10.1007/s11745-000-0581-3. [DOI] [PubMed] [Google Scholar]
- Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–138. doi: 10.1016/s0022-3476(05)81247-8. [DOI] [PubMed] [Google Scholar]
- Michaeli B, Berger MM, Revelly JP, Tappy L, Chiolero R. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr. 2007;26:70–77. doi: 10.1016/j.clnu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon Y, Pestka JJ. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J Nutr Biochem. 2003;14:717–726. doi: 10.1016/j.jnutbio.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Combe C, Busquet P, Dantzer R, Parnet P. Signaling pathways of interleukin-1 actions in the brain: anatomical distribution of phospho-ERK1/2 in the brain of rat treated systemically with interleukin-1beta. Neuroscience. 2005;134:921–932. doi: 10.1016/j.neuroscience.2005.04.035. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- Pifferi F, Roux F, Langelier B, Alessandri JM, Vancassel S, Jouin M, Lavialle M, Guesnet P. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J Nutr. 2005;135:2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- Porter MH, Hrupka BJ, Altreuther G, Arnold M, Langhans W. Inhibition of TNF-alpha production contributes to the attenuation of LPS-induced hypophagia by pentoxifylline. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2113–2120. doi: 10.1152/ajpregu.2000.279.6.R2113. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr., Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Renault J, Aubert A. Immunity and emotions: lipopolysaccharide increases defensive behaviours and potentiates despair in mice. Brain Behav Immun. 2006;20:517–526. doi: 10.1016/j.bbi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1316–1326. doi: 10.1152/ajpregu.00301.2006. [DOI] [PubMed] [Google Scholar]
- Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31(Suppl 2):S1–6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr., Niebylski CD. The nervous system has an absolute molecular species requirement for proper function. Mol Membr Biol. 1995;12:131–134. doi: 10.3109/09687689509038508. [DOI] [PubMed] [Google Scholar]
- Sanz E, Hofer MJ, Unzeta M, Campbell IL. Minimal role for STAT1 in interleukin-6 signaling and actions in the murine brain. Glia. 2008;56:190–199. doi: 10.1002/glia.20602. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- Schobitz B, Pezeshki G, Pohl T, Hemmann U, Heinrich PC, Holsboer F, Reul JM. Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6 in vivo. Faseb J. 1995;9:659–664. doi: 10.1096/fasebj.9.8.7768358. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Plata-Salaman CR, Langhans W. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1 beta in rats. Am J Physiol. 1997;273:R1193–1198. doi: 10.1152/ajpregu.1997.273.3.R1193. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Song H, Zhu L, Picardo CM, Maguire G, Leung V, Connelly PW, Ng DS. Coordinated alteration of hepatic gene expression in fatty acid and triglyceride synthesis in LCAT-null mice is associated with altered PUFA metabolism. Am J Physiol Endocrinol Metab. 2006;290:E17–E25. doi: 10.1152/ajpendo.00597.2004. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin- 6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on endotoxin-induced hepatitis in mice. Lipids. 1991;26:467–471. doi: 10.1007/BF02536074. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kanada S, Takenaka M, Hamazaki T. Dietary n-3 fatty acids selectively attenuate LPS-induced behavioral depression in mice. Physiol Behav. 2004;81:605–613. doi: 10.1016/j.physbeh.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Yaqoob P. Fatty acids and the immune system: from basic science to clinical applications. Proc Nutr Soc. 2004;63:89–104. doi: 10.1079/PNS2003328. [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Rao GN. Cytosolic phospholipase A2 is an effector of Jak/STAT signaling and is involved in platelet-derived growth factor BB-induced growth in vascular smooth muscle cells. J Biol Chem. 2003;278:9986–9992. doi: 10.1074/jbc.M211276200. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]