Abstract
Measurement of hemoglobin A1c (A1C) has long been accepted as the best indicator of glucose control over time. Assays for A1C use technologies based on either charge differences (high-pressure liquid chromatography) or structure (boronate affinity or immunoassay combined with general chemistry). These technologies are generally employed in expensive laboratory instruments. More recently, A1C technology has been incorporated into point of care (POC) devices, allowing for immediate availability of A1C measurements, greatly facilitating diabetes care in both specialist and general practices. POC A1C tests should have acceptable performance, standardization to national reference, National Glycohemoglobin Standardization Program (NGSP) certification, simple operation without need for costly instrumentation, and Clinical Laboratory Improvement Amendments (CLIA) waiver. CLIA-waived POC technology includes Bio-Rad MicroMat™ II (distributed by Cholestech as GDX™) and the Axis-Shield Afinion,™ both of which utilize boronate affinity. The DCA 2000®+ utilizes combined immunoassay and general chemistry. These instruments cost $1000 to $3000 and require regular maintenance, making them appropriate only for high-volume physician offices. The newly improved A1CNow+™ also utilizes combined immunoassay and general chemistry, but the small, inexpensive, disposable monitor can be used by patients as well as by health care professionals. The new version of A1CNow+ has improved performance through recent introduction of automated solid state chemistry manufacturing, improved fluidics and automated assembly of the test cartridge, error-correcting software, and unitary meter calibration with factory calibration directly to the NGSP reference standard.
Keywords: A1C, CLIA waived, glycated hemoglobin, hemoglobin A1c, point of care
Introduction
Measurement of hemoglobin A1c (A1C) has long been accepted as the best indicator of glucose control over time.1 A recent estimate is that there are approximately 20.8 million children and adults in the United States with diabetes, of which approximately one-third remain undiagnosed.2 Diabetes is the fifth leading cause of death by disease in the United States and also contributes to higher rates of complications such as heart disease, blindness, kidney failure, and extremity amputation. The direct and indirect costs attributable to diabetes in 2002 were estimated at $132 billion.3
About 20% of people with diabetes are treated by diabetes specialists or specialty clinics. These patients are fortunate and are generally in good glycemic control. The rest (80% or more) are managed in the primary care setting, with an average A1C greater than 8% A1C.4 Patients under primary care are unlikely to benefit from advanced technologies such as continuous glucose monitoring in the near future. Advances in access to physician and patient A1C monitoring can benefit this largest group under management.
Background
Hemoglobin A1C is an irreversible complex that forms when glucose binds to hemoglobin. The percentage of A1C is directly related to the average glucose level over the past 6 to 8 weeks. Blood glucose levels from the past 30 days represent 50% of the total A1C result.1 The American Diabetes Association (ADA) recommends an A1C goal of <7% A1C,5 while the American Association of Clinical Endocrinology recommends a goal of <6.5%.6 Every 1% reduction in A1C lowers the risk of developing eye, kidney, and nerve disease by 40%.2 Point of care (POC) A1C provides immediate feedback, which has been shown to increase physician intervention, resulting in lower A1C values for patients.7 Despite the importance of A1C in management of diabetes, it is about 60% underutilized in the United States. There are approximately 24 million A1C tests done per year. Estimating 14.6 million people diagnosed with diabetes, there should be over 58 million tests done yearly (assuming four A1C tests per year, per patient).
Laboratory assays for A1C use technology based on either charge differences [high-pressure liquid chromatography (HPLC)] or structure (boronate affinity or immunoassay combined with general chemistry). These technologies are generally part of expensive laboratory instruments and require trained laboratory personnel for operation. More recently, A1C has been incorporated into POC devices, allowing for immediate availability of A1C measurements, greatly facilitating diabetes care in both specialist and general practices. POC A1C tests should have acceptable performance, standardization to the national reference (National Glycohemoglobin Standardization Program, NGSP), NGSP certification, simple operation without the need for expensive instrumentation, and Clinical Laboratory Improvement Amendments (CLIA) waiver. The ADA recommends that A1C methods be NGSP certified.5
The purpose of the NGSP is to standardize glycated hemoglobin test results so that clinical laboratory results are comparable to those reported in the Diabetes Control and Complications Trial (DCCT) where relationships to mean blood glucose and risk for vascular complications have been established. A key component of the program is the Reference Laboratory Network. The network interacts with manufacturers of A1C methods to assist them first in standardizing their methods and then in providing comparison data for certification of traceability to the DCCT. A Central Primary Reference Laboratory sets the initial calibration and is responsible for monitoring backup Primary Reference Laboratories and Secondary Reference Laboratories (SRLs). The SRLs work directly with manufacturers to standardize their methods and provide comparison data for method certification in order to achieve the desired end result of comparability of fresh sample results with the DCCT reference. Proficiency testing data (from fresh sample surveys) are used to assess the effectiveness of method standardization.8 A 5-year report9 stated that most of the methods used in the United States are certified by the NGSP.
Congress passed the Clinical Laboratory Improvement Amendments in 1988, establishing quality standards for all laboratory testing to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test was performed. The CLIA 1988 law specified that laboratory requirements be based on the complexity of the test performed and established provisions for categorizing a test as waived. Tests may be waived from regulatory oversight if they meet certain requirements established by the statute.
In the regulations, waived tests were defined as simple laboratory examinations and procedures that are cleared by the Food and Drug Administration (FDA) for home use, employ methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible, or pose no reasonable risk of harm to the patient if the test is performed incorrectly.
Technology
Laboratory A1C Methods
Large laboratory analyzers typically run multiple batched samples. There are different methodologies, and the technology may be based on either charge (HPLC or ion-exchange chromatography) or structure (boronate affinity chromatography or immunoassay). They are costly to purchase and require trained personnel for operation.
POC A1C Methods
Point of care A1C methods are small instruments or unitized devices that run single samples. There are four POC, CLIA-waived devices cleared for use in the United States. As CLIA-waived devices, highly trained personnel are not required for operation. All four methods are NGSP certified.
Clinical Laboratory Improvement Amendments-waived POC technology includes the instrument-based Micromat™ II (Bio-Rad, Hercules, CA, distributed by Cholestech as GDX™), the Afinion™ (Axis-Shield, Oslo, Norway), and the DCA 2000®+ (Siemens Diagnostics, Tarrytown, NY). These instruments cost $1000 to $3000 and require regular maintenance, making them appropriate only for high-volume physician offices.
The GDX/Micromat II and the Afinion are both boronate affinity methods. The GDX/Micromat II is a 5-minute assay, with manual manipulation required by the operator for the entire test. The Afinion is a 3-minute assay and is a walk-away instrument. The DCA 2000+ utilizes immunoassay and general chemistry. It is a 6-minute assay and is also a walk-away instrument. All three instruments require an initial capital investment and minor maintenance.
The newly improved A1CNow+™ (Metrika, a member of Bayer Healthcare LLC, Sunnyvale, CA) also utilizes combined immunoassay and general chemistry, but the small, inexpensive, disposable monitor can be used by patients as well as by health care professionals. The new version of A1CNow+ has improved performance through recent advances in both manufacturing and design. The introduction of automated solid state chemistry manufacturing and automated assembly of the test cartridge has contributed to improved precision. The developments of error-correcting software and improved fluidics have also improved the product precision. Unitary meter calibration tied directly to the NGSP reference standard ensures that accuracy is not subject to a user calibration process.
A1CNow+ is a 5-minute assay. There is no initial instrument cost, as there is one monitor per 10 test cartridges. Once the end user utilizes the 10 test kit, the monitor is disposed. Therefore, there is no maintenance or calibration required, and the test is economical for both specialist and primary care with a retail cost to the health care professional of approximately $12 per test. It has also been FDA cleared for over-the-counter home use.
Methods
Titles and abstracts relevant to A1C POC methods were retrieved in a search of MEDLINE, published in English, for the years of 2001–January 2007. Search terms included A1C, point of care, HbA1c, waived testing, DCA 2000, Provalis (the previous manufacturer for the Micromat II/GDx), Axis-Shield, Afinion, and A1cNow. No information was found for the Afinion instrument.
Manufacturing improvements have been made to A1cNow, and it was recently released as A1CNow+. The improvements are presented in this article. A1CNow+ field correlation studies were performed at two separate sites using whole blood samples. The A1CNow+ results were compared to a NGSP level II-certified laboratory (Sunnyvale, CA) HPLC method (Tosoh 2.2, Tosoh Bioscience, South San Francisco, CA).
Results
DCA 2000
The DCA 2000 has the longest history of the POC instruments, having been marketed since the late 1990s. Recent literature indicates good correlation of the DCA 2000 to HPLC methods (r = 0.939, p <0.000110 and r = 0.94, p <0.00111). Mean biases of the DCA 2000 to HPLC methods in the studies were positive: +0.36% A1C for one study10 and +0.2% A1C for the other study.11 A third study12 compared several POC tests and found a positive bias for the DCA 2000 as well (+0.12% A1C), with good correlation to the HPLC method (r = 0.98).
Micromat II/GDx
The Micromat II/GDx was originally released by Provalis Diagnostics as the Glycosal. A literature search returned minimal studies performed with this instrument. The two extensive studies that were reported both indicated that medical staff found the test not user-friendly because of the amount of dedicated time required for the test.12,13Correlation varied (r = 0.87 for one study12 and r = 0.773, p < 0.001 for the other study13). Mean biases were low, −0.31 and −1.27% A1C, respectively.
A1cNow
The original version of A1cNow, a fully integrated, single-use disposable device merging duplicate reagent strips with optics/electronics components (Figure 1), has been tested independently as well. Studies indicate that A1cNow is easy to use12,14 and that it correlates well with the standardized HPLC method (r = 0.97 for the first study12 and r = 0.94 for the second study14). A small study15 found A1cNow to be economical and clinically useful. One study found the DCA 2000 to be more accurate than this version of A1cNow.16 The design and capabilities of the single-use A1cNow have been described thoroughly elsewhere.17
Figure 1.
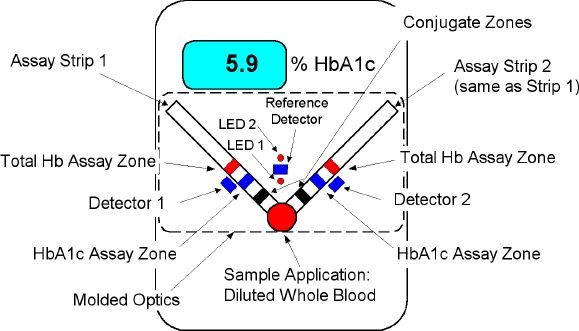
A1cNow disposable (first generation). Optic and test strip components are self-contained within the monitor.
Second-Generation A1CNow+ Improvements
The successor to A1cNow, named A1CNow+, was designed as a semidisposable product. Multiple (10) single-use test cartridges come packaged with a single finite-use monitor, allowing the user to obtain 10 A1C determinations before the A1CNow+ monitor must be discarded. Disposable test cartridges containing the reagent test strips can be inserted and removed from the monitor through a slot at its bottom (Figure 2). There have been no formatting changes to the reagent strips, and the test procedure has only been updated to facilitate insertion and removal of the test cartridge. Optical, electronic, and software updates have been implemented both to address the multiuse nature of the test system and to enhance its performance. The sample dilution kit (SDK) provided with the A1CNow+ system to date is identical to that used with the first-generation A1cNow product. A clinically proven and soon-to-be-launched enhancement to the A1CNow+ system is the Sampler, an integrated and easy-to-use replacement for the SDK (Figures 2 and 5).
Figure 2.

A1CNow+ reusable test system. Optic components are contained in the monitor; test strip components are contained within the test cartridge. Monitor is disposed after 10 uses.
Figure 5.

A1CNow+ Sampler comparison to original sample dilution kit (SDK).
The new version of A1CNow+ has improved performance through the recent introduction of precision-enhancing software, automated solid state chemistry manufacturing, optimized test cartridge fluidics, and automated test cartridge assembly. Factory calibration continues to be done directly to the NGSP reference standard.
Precision-enhancing software reduces variation. The first-generation A1cNow product (single-use disposable) did not permit monitor calibration. Second-generation A1CNow+ allows for individual monitor responses to be measured, matched, and calibrated. Outlier monitors are eliminated from the production process (see Figure 3).
Software compensates for variation in strip development. Lateral flow uniformity is measured, and assay signals from each chemistry strip are adjusted according to a lot-specific algorithm each time a test is performed. The effect of random material and flow variation, traditionally a limiting factor in achieving high precision in lateral flow systems, is minimized. Precision is improved 10–30%. Additionally, multiple quality checks prevent the display of inaccurate results.
Automated solid state chemistry manufacturing. Instead of manufacturing dry chemistry strips in small batches of a few hundred each with extensive manual processing, ≃100,000 test strips are manufactured in a single continuous operation for the A1CNow+ product. Reduced process variation results in better precision and accuracy.
Optimized fluidics. Controlled transfer of the diluted sample to the test strips is critical for reproducible results. Normal variation in the volume added must not affect the displayed results. This control and tolerance have been achieved through the design and engineering of the test cartridge and sample pad system, as shown in Figure 4.
Automated production of the test cartridge. Replacement of manual processes with an automated assembly system provides increased test cartridge consistency, contributing to improved precision.
Factory calibration to the national reference standard (NGSP). Each monitor and test kit are factory calibrated. There are no steps required by the end user for calibration, resulting in a high degree of accuracy for every lot.
Figure 3.
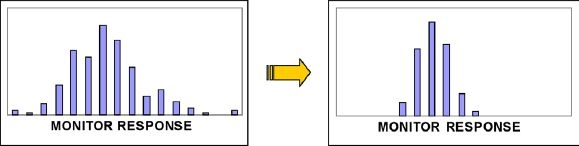
A1CNow+ monitor calibration enhances product accuracy.
Figure 4.
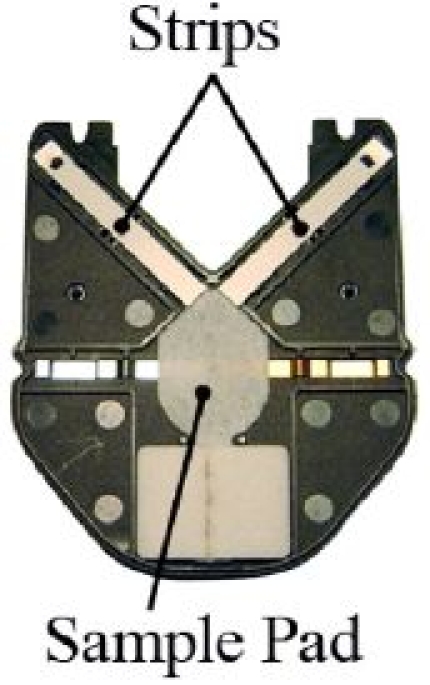
A1CNow+ test cartridge test strip and sample pad alignment.
The new version of A1CNow+ also has improved usability.
Only 5 minutes to results. Enhanced product performance enables the A1CNow+ product to report an accurate answer 3 minutes sooner than the original A1cNow product.
Clinically proven Sampler system reduces blood requirement to 5 µl while enhancing product usability. The Sampler system requires half of the blood that the original SDK system needed, making it easier on the patient. The “chemistry set” provided with the original SDK has been replaced by an ergonomically designed, self-contained Sampler system to maximize the simplicity and effectiveness of the blood dilution and transfer process (Figure 5). The Sampler further insulates the A1CNow+ result from the user's technique.
Assay Performance
Recent correlation studies utilizing the latest generation of A1CNow+ were performed, which included comparison to a level II NGSP-certified laboratory. A1CNow+ testing was performed at potential customer sites. Whole blood samples were sent to the NGSP level II-certified laboratory in Sunnyvale, California for A1C testing using the Tosoh 2.2 HPLC analyzer (Tosoh Bioscience). Data from the two sites were combined to provide a larger statistical number. Linear regression analysis was performed, and assessment of agreement, in the manner of Bland–Altman, was calculated to assess the limits according to NGSP. Even though this was not a true NGSP protocol, the assessment of agreement is utilized to determine the total error of the assay. Data are provided in Figure 6 and Table 1.
Figure 6.
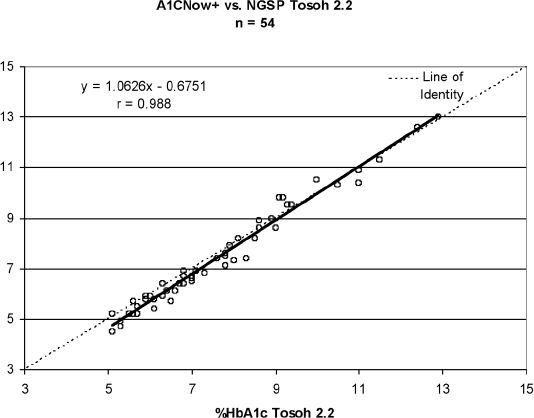
A1CNow+ correlation to a standardized NGSP laboratory; two sites were combined. Total n = 54, y = 1.06 – 0.68, r = 0.988, SEE = 0.32.
Table 1.
Linear Regression, Assessment of Agreement, and Bias Statistics
| Data set | N | Assessment of agreement 95% CI (% HbA1c) | Slope | Intercept | r | Mean bias (% HbA1c) |
|---|---|---|---|---|---|---|
| A1CNow+ vs NGSP Site 1 | 30 | −0.89 to +0.55 | 1.08 | −0.75 | 0.99 | −0.17 |
| A1CNow+ vs NGSP Site 2 | 24 | −0.24 to +0.31 | 1.04 | −0.51 | 0.99 | −0.24 |
| A1CNow+ vs NGSP Combined sites | 54 | −0.87 to +0.47 | 1.06 | −0.68 | 0.988 | −0.20 |
Precision testing was performed on two lots of material at the manufacturer site. Normal and high patient samples were utilized, and testing was performed over 5 days. Multiple tests were performed morning and afternoon, giving a total of 80 results per level, per lot. Results are presented in Table 2.
Table 2.
Precision (Two Lots A1CNow+)
| Lot | % A1C | % coefficient of variation | Number of replicates |
|---|---|---|---|
| 1 | 5.1 | 2.74 | 80 |
| 1 | 9.4 | 4.02 | 80 |
| 2 | 5.2 | 3.61 | 80 |
| 2 | 9.1 | 3.86 | 80 |
Conclusion
Point of care A1C as reviewed in this publication is emerging as an important tool for diabetes care. The DCA 2000+ is accessible, accurate, and easy to use, but is limited to high-volume clinics because of instrument costs. The Micromat II/GDX is accessible and accurate, but is not as easy to use as it requires manual manipulation. A1CNow+ is accessible, accurate, and easier to use than ever with the new Sampler system, while remaining affordable to both primary and specialist practices.
Abbreviations
- A1C
hemoglobin A1c
- ADA
American Diabetes Association
- CLIA
Clinical Laboratory Improvement Amendments
- DCCT
Diabetes Control and Complications Trial
- FDA
Food and Drug Administration
- HPLC
high-pressure liquid chromatography
- NGSP
National Glycohemoglobin Standardization Program
- POC
point of care
- SDK
sample dilution kit
- SRLs
Secondary Reference Laboratories
References
- 1.Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. 3rd. Philadelphia (PA): WB Saunders; 1999. [Google Scholar]
- 2. CDC National Diabetes Fact Sheet, United States, 2005.
- 3.Hogan P, Dall T, Nikolov P. American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26(3):917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 4.Phillips LS, Ziemer DC, Doyle JP, Barnes CS, Kolm P, Branch WT, Caudle JM, Cook CB, Dunbar VG, El-Kebbi IM, Gallina DL, Hayes RP, Miller CD, Rhee MK, Thompson DM, Watkins C. An endocrinologist-supported intervention aimed at providers improves diabetes management in a primary care site: improving primary care of African Americans with diabetes (IPCAAD) 7. Diabetes Care. 2005 Oct;28(10):2352–2360. doi: 10.2337/diacare.28.10.2352. [DOI] [PubMed] [Google Scholar]
- 5.Standards of Medical Care in Diabetes 2007. American Diabetes Association. Diabetes Care. 2007;30:S4–41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 6.American Association of Clinical Endocrinology Diabetes Guidelines. Endocr Pract. 2002;8:S1. [Google Scholar]
- 7.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999 Nov;22(11):1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 8. www.NGSP.org.
- 9.Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. NGSP Steering Committee. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001 Nov;47(11):1985–1992. [PubMed] [Google Scholar]
- 10.Hadjadj S, Duengler F, Barriere M, Mauco G, Coisne D, Warnier F, Sosner P, Torremocha F, Herpin D, Marechaud R. Determination of HbA1c concentrations in patients with acute myocardial infarction: comparison of the DCA 2000 device with the HPLC method. Diabetes Metab. 2005 Jun;31(3 Pt 1):290–294. doi: 10.1016/s1262-3636(07)70196-9. [DOI] [PubMed] [Google Scholar]
- 11.Tamborlane WV, Kollman C, Steffes MW, Ruedy KJ, Dongyuan X, Beck RW, Chase P, Fox LA, Wilson DM, Tsalikian E. The Diabetes Research in Children Network (DirecNet) Study Group. Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes. 2005 Mar;6(1):13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 12.St John A, Davis TM, Goodall I, Tonsend MA, Price CP. Nurse-based evaluation of point-of-care assays for glycated haemoglobin. Clin Chim Acta. 2006 Mar;365(1-2):257–263. doi: 10.1016/j.cca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz KL, Monsur JC, Bartoces MG, West PA, Neale AV. Correlation of same-visit HbA1c test with laboratory-based measurements: A Metro-Net study. BMC Fam Pract. 2005 Jul 13;6:28. doi: 10.1186/1471-2296-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klonoff DC, Bergenstal RM, Cole TG, Bohannon NJV, Ammirati EB, Blatt JM, Irvin BR, Stivers CR, Clark AL. Clinical evaluation of a rapid A1C test (A1cNow) for home use. Point Care. 2006;5(3):116–120. [Google Scholar]
- 15.Sicard DA, Taylor JR. Comparison of point-of-care HbA1c test versus standardized laboratory testing. Ann Pharmacother. 2005 Jun;39(6):1024–1028. doi: 10.1345/aph.1E504. [DOI] [PubMed] [Google Scholar]
- 16.Fox L, Dontchev M, Ruedy K, Beck R, Kollman C, Messer L, Coffey J, Wilson D, Doyle E, Tamborlane W, Steffes M DirecNet Study Group. Relative inaccuracy of the A1cNow in children with type 1 diabetes. Diabetes Care. 2007 Jan;30(1):135–137. doi: 10.2337/dc06-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stivers CR, Baddam SR, Clark AL, Ammirati EB, Irvin BR, Blatt JM. A miniaturized self-contained single-use disposable quantitative test for hemoglobin A1c in blood at the point of care. Diabetes Technol Ther. 2000 Winter;2(4):517–526. doi: 10.1089/15209150050501916. [DOI] [PubMed] [Google Scholar]


