Abstract
Background
Currently, monitoring blood glucose levels for diabetic patients is invasive and painful, involving pricking the finger to obtain a blood sample three to four times daily. The need for frequent tests and pain involved with testing leads to poor compliance. In order to raise compliance, we propose to create an implantable electrochemical sensor array that would monitor glucose levels continuously.
Methods
Glucose sensor arrays were fabricated on gold electrodes on flexible polyimide sheets by photopolymerization of the biocompatible polymer poly(ethylene glycol) diacrylate (PEG-DA) to develop hydrogels and encapsulate the sensing elements. Using conventional silicon fabrication methods, arrays of five gold microdisk electrodes were fabricated using lift-off photolithography and sputtering techniques. A redox polymer was then attached electrostatically to the electrode, and glucose oxidase was entrapped inside the hydrogel on the array of electrodes by ultraviolet-initiated photopolymerization of PEG-DA.
Results
When the array of fabricated sensors was sampled together the elements behaved like one large electrode with peak current equivalent to the sum of individual array elements. The enzyme, glucose oxidase, catalyzed the oxidation of glucose and then exchanged electrons with the redox polymer in the hydrogel. The entrapped glucose oxidase was found to respond linearly to increasing glucose concentrations (0–360 mg/dl), as determined using cyclic voltammetry.
Conclusion
The fabricated microarray sensors were individually addressable and showed no cross talk between adjacent array elements as assessed using cyclic voltammetry. We have fabricated an array of glucose sensors on flexible polyimide sheets that exhibits the desired linear response in the biological range.
Keywords: biocompatible, cyclic voltammetry, glucose sensor, microarray
Introduction
One of the major challenges in the management of diabetes is the monitoring of glucose concentrations. A number of strategies for measuring blood glucose are continuously under development to allow pain-free, more frequent glucose monitoring. Some of the techniques include near-infrared spectroscopy,1,2 optical rotation,3 transdermal spectroscopic,4, 5 and interstitial fluid sampling,6, 7 among others. A major advantage of transdermal spectroscopic techniques is painless testing; however, their main drawback is measurement of glucose in a highly complex matrix of water, proteins, polysaccharides, and lipids. The result is a complex signal with the glucose measurement having to be extracted through other mathematical programs, hence introducing errors at every stage. Reverse iontophoresis8 and sonophoresis9 techniques for interstitial blood sampling have been studied. One advantage of these techniques is that a physiologically relevant fluid sample containing glucose at concentrations closely related to concentrations in blood is collected.
Another recent technique being explored uses calcium alginate microspheres to encapsulate glucose oxidase (GOX) and the film stabilized by multilayer polyelectrolyte coatings, but its biocompatibility has still to be evaluated.10 Development of implantable, accurate, reliable, continuous, and noninvasive glucose sensors would significantly improve the lives of patients with diabetes and decrease the long-term complications of persistent hyperglycemia and the immediate risks of hypoglycemia. The implantation of sensors can be performed either in subcutaneous tissue to measure glucose in interstitial fluid11–15 or intravenously.16 As with any foreign object introduced into the body, biocompatibility is one of the most important requirements of these sensors. Problems with implanted in vivo analytical sensors with potential sensitivity alterations arise during prolonged implantation. This is believed to be because of physiological and pathological changes at the sensor/tissue biointerface. This not only affects the sensor output but also can cause harmful side effects on the subject, e.g., thrombi formation after adsorption and activation of platelets for sensors implanted intravenously and fibrous capsule formation for those implanted in subcutaneous tissue.17 In this article, we used biocompatible poly(ethylene glycol) diacrylate (PEG-DA) hydrogel networks that have shown to provide an ideal three-dimensional environment18–21 to entrap both the redox polymer and the glucose oxidase on polyimide sheets. We used silicon microfabrication technologies to fabricate sensor arrays and used mid-ultraviolet (UV) photolithography to polymerize and pattern on micrometer scale three-dimensional redox polymer hydrogels containing glucose oxidase for glucose monitoring. The use of a microarray of electrodes can increase the reliability of the sensor through the use of redundancy.22 A second benefit of using an array is that elements of the array can be used to detect other analytes (i.e., creating a multianalyte sensor array) or to determine the background signal of the sample (i.e., quantification of interferant molecules).
Materials and Methods
Glucose oxidase (EC 1.1.3.4, Type X-S, 128 units/mg solid from Aspergillus niger), ammonium hexachloroosmate(IV), 11-mercaptoundecanoic acid (MUA), poly(4-vinylpyridine), β-D-glucose, acetone, ammonium hexafluorophosphate, sodium dithionite, ether, 2-bromoethylamine hydrobromide, N,N-dimethylformamide (DMF), anion-exchange beads, hydrochloric acid (HCl), PEG-DA, and 2,2′-dipyridyl (bpy) were obtained from the Aldrich Chemical Company (Milwaukee, WI). Ethyl alcohol, ethylene glycol, and acetonitrile were obtained from Fisher Scientific Company (Pittsburgh, PA). DAROCUR, the photo initiator, was obtained from Ciba. All reagents, unless otherwise stated, were used as received. Polyimide sheets (1/16-in. thick) were purchased from McMaster Carr (New Brunswick, NJ). Phosphate-buffered saline consisted of 1.1 mM potassium phosphate monobasic, 3 mM sodium phosphate dibasic heptahydrate. Conductive silver epoxy was obtained from Ladd Research (Williston, VT). Ma-P 100, positive photoresist, and ma-D 330, photoresist developer, were purchased from MicroChem Corporation (Newton, MA). The polycationic redox polymer, poly[4-vinylpyridine osmium (bipyridine)2 chloride]-coethylamine (POs-EA), was synthesized according to a procedure described previously.12
Synthesis of POs-EA
Synthesis of POs-EA, an osmium-based polycationic redox polymer, was done following modifications of established protocols.23 Osmium (bipyridine)2 dichloride [Os(bpy)2Cl2] was synthesized according to a standard procedure with minor modifications.24 In brief, two equivalents of bipyridine (1440 mg) were mixed with one equivalent of ammonium hexachloroosmate(IV) (2000 mg) in 100 ml ethylene glycol. The mixture was heated to reflux for 45 minutes and then precipitated with supersaturated sodium dithionite. The precipitate was washed repeatedly with water and finally with ether.
Os(bpy)2Cl2 (0.988 g, 1.728 mmol) and poly(4-vinylpyridine) (0.860 g, 8.18 mequiv) were heated under nitrogen at reflux in 36 ml of ethylene glycol for 2 hours. The solution was then cooled down to room temperature, and 60 ml of DMF and 3.0 g of 2-bromoethylamine hydrobromide (14.6 mmol) were added and then stirred overnight at 45°C. A crude polymer precipitate was formed by pouring the solution into rapidly stirred acetone. The hygroscopic precipitate was collected and dissolved in water. The solution was filtered and precipitated as hexafluorophosphate (PF6−) salt by addition of a solution of ammonium hexafluorophosphate. The precipitate was dried in a vacuum at 40°C. Dry PF6− salt (0.98 g) was dissolved in 40 ml of acetonitrile, diluted with 100 ml of water, and stirred over 10.4 g of anion-exchange beads for 2 hours. The solution was filtered and evaporated under vacuum to ∼20 ml. Concentrated HCl was added to the solution to adjust to pH 2. The solution was then dripped into rapidly stirred acetonitrile. The precipitate that formed was filtered and dried in a vacuum oven. The pure product was analyzed.
Fabrication of Electrode Arrays on Polyimide Sheets
Polyimide sheets are a flexible and insulating material that is an ideal platform for electrode manufacturing. Square pieces of polyimide were cut and washed with ethanol before coating with positive photoresist (ma-P 100). A modified literature procedure was used to fabricate the electrode arrays.22 In brief, positive photoresist (ma-P 100) was deposited on square polyimide sheets, spin coated at 4000 rpm for 30 seconds, and then soft baked at 100°C for 5 minutes. These polymer sheets with dry photoresist were brought in close contact with the photo-mask and exposed to 365-nm UV light. The polymer sheets were then placed in the developer solution (ma-D 330) for 70 seconds to remove the portions of photoresist exposed to UV light. The sheets were then rinsed with distilled water.
The photoresist patterns were then sputter coated with 10 nm of an adhesion layer of chrome followed by a 150-nm layer of gold (done at Pennsylvania State University using the Lesker CMS-18 sputtering tool and by Lance Goddard Associates in Foster City, California). The photoresist was removed using acetone to lift off the excess chrome and gold from all nonpatterned areas. The result was distinct patterns of gold with 500-µm-diameter electrodes with 10-µm leads and 2.5 × 2.5-mm contact pads. Wires were attached to the contact pads using conductive silver epoxy resin (Ladds Research, Williston, VT).
Immobilization and Pattering of Biosensor Films on Gold Surfaces
The electrode arrays were functionalized with a carboxylic end group by immersing in 2 mM MUA in ethanol for 20 minutes and then washing with ethanol. The electrodes were then dried under nitrogen. The thiol end group was chemisorbed to gold to provide an anchor. Next, the redox polymer was immobilized on the electrodes by depositing 2 µl of 10 mg/mL POs-EA solution on the electrode surface and left to cure overnight in the dark. Strong electrostatic interactions occur between the negative MUA and the positive POs-EA. Excess POs-EA was removed by washing with water and dried with nitrogen. The PEG-DA precursor was made by mixing 5 µl of a GOX solution (50 mg/ml in HEPES buffer), 5 µl PEG-DA, and 0.5 µl DAROCUR and then placed onto the electrode surface. UV light was then applied and PEG-DA hydrogels were formed on the five different electrode array elements (Figure 1).
Figure 1.
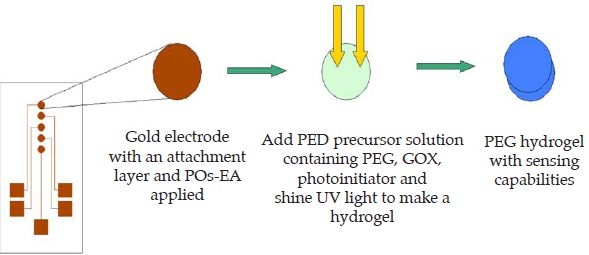
Schematic of fabrication of the sensor.
Results and Discussion
Amperometric biosensors based on redox polymer/enzyme complexes were shown to be miniaturizable and implantable.25–27 Previously, enzymes entrapped in redox hydrogels using photopolymerization were shown to retain their activity.28–32 Enzyme-containing redox polymer films were formed through the UV-initiated free radical cross-linking of a redox polymer and PEG-DA in each electrode array element (Figure 1). Each sensor array element after microfabrication was smooth and without any discontinuities. To determine whether the array elements had any defects or whether they functioned independently, cyclic voltammetry was done initially without the analyte (glucose) to evaluate the reproducibility of the microfabrication process and the stability of the film. The microfabrication process was quite reproducible as observed from almost overlaid cyclic voltammograms for each element of the array (not shown). Entrapment of the redox polymer, glucose oxidase, and PEG-DA was also uniform on all the electrodes, which is especially important when summing sensor signals of the arrays. The signal from all the array sensor elements mimics the behavior of one large sensor with an electrode area equal to the sum of all the electrode array elements. Figure 2 shows cyclic voltammograms of an increasing number of array elements. Measurements done after combination of different array members show increasing peak currents with increasing number of array elements combined. With such a sensor array, multiple analytes can be measured simultaneously and, through the introduction of redundancy, measurements can be derived from the average of the signal resulting from each array element. There was no cross talk between the array members.
Figure 2.
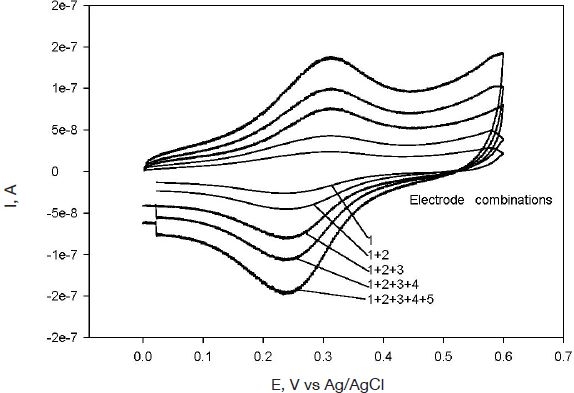
Cyclic voltammograms of different combinations of microarray electrodes at 20-mV/s scan rate.
The formal potential of this redox polymer was approximately 0.3 V (Ag/AgCl), in agreement with previous results described in the literature.33 Anodic and cathodic peaks were observed at 0.32 and 0.25 V (Ag/AgCl) at 20 mV/s with a peak separation of approximately 60 mV, indicating electrochemical reversibility. The absolute value of the ratio of the anodic-to-cathodic peak currents was unity, also indicating chemical reversibility. Figure 3 shows cyclic voltammograms of one element of the microarray at different scan rates. Plots of peak current versus scan rate were linear at scan rates 0.5–50 mV/s. At high scan rates (above 50 mV/s) a deviation from linearity was observed (not shown). A plot of peak current (ip) versus square root of scan rate (ν½) yielded a straight line (Figure 4). The dependence of the current ip on the square root of the scan rate, ν½, is an important diagnostic criterion for establishing the type of reaction mechanism by cyclic voltammetry.34 This dependence suggests that the osmium layer is sufficiently thick and/or the electron transfer between osmium moieties is slow, leading to the system to mimic semi-infinite linear diffusion. Because the method of fabrication is a layering technique (MUA, POs-EA, and then the PEG-DA hydrogel), the film can be thought of as sufficiently thick; the electron transfer can also be hindered by attachment of the PEG-DA hydrogel to the layers. Because the timescales used for the cyclic voltammetry experiments lay on the lower end of the scan rates, the electron transfer should be sufficiently fast and reversible for the experiments.
Figure 3.
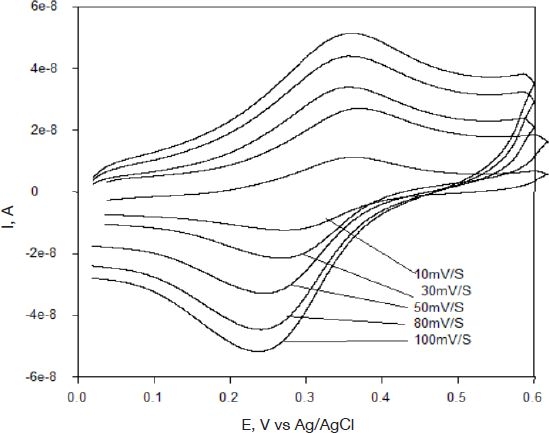
Cyclic voltammograms of one element of the microarray at different scan rates.
Figure 4.
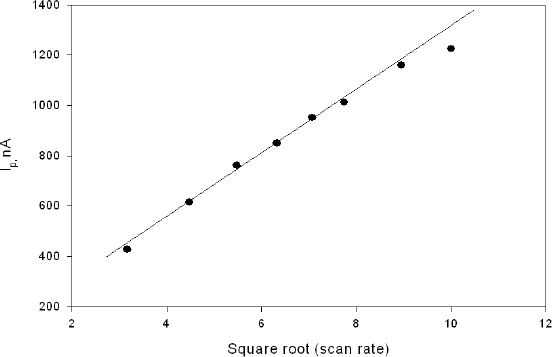
Scan rate dependence of film containing GOX, redox polymer, and PEG-DA in phosphate buffer.
Properly cross-linked GOX and POs-EA molecules are expected to retain the activity of glucose oxidase and to present signal stability. Signal stability would degrade if the enzyme were to leach from the hydrogel and was not seen in the fabricated sensors. Figure 5 shows cyclic voltammograms of five combined elements of the microarrays in buffer containing 0, 90, 180, and 360 mg/dl glucose solutions. In the presence of increasing glucose concentration, the current is found to increase. The rate of this catalytic reaction is proportional to the concentration of glucose present. Flavin adenine dinucleotide of glucose oxidase GOX(FAD) reacts with β-D-glucose to form a reduced form GOX(FADH2) and gluconic acid and hydrogen peroxide inside the hydrogel. The reduced form of GOX(FADH2) is in turn oxidized by the electrochemically generated Os3+ form of the redox polymer, setting up a catalytic pathway that produces an enhanced oxidation peak.
Figure 5.
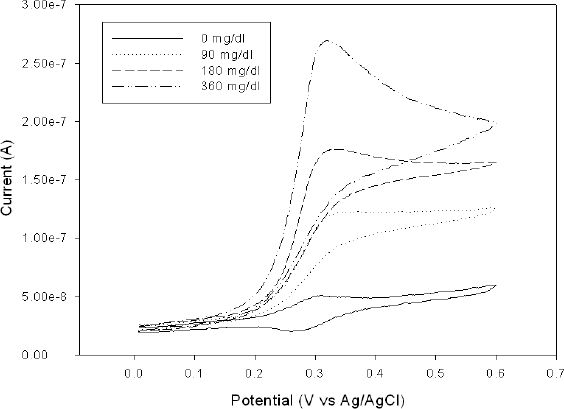
Cyclic voltammograms of the collective response of five sensor elements at different glucose concentrations.
The electrons are transferred from the enzyme to the redox polymer, shuttled between the redox sites in a self-exchange reaction until being transferred to the electrode surface. The catalytic current produced is proportional to the glucose concentration. Glucose oxidase is securely trapped in the hydrogel network and glucose diffuses through to access the glucose oxidase sites. Photopolymerization of the acrylate end groups of the PEG-DA by the photoinitiator DAROCUR occurs and entraps the glucose oxidase enzyme on the redox polymer film. We have yet to find out how varying the thickness of the hydrogel affects the glucose movement in and out of the hydrogel and how the catalytic signal is affected. We believe that the thickness of the hydrogel network ultimately affects the communication of enzyme and the redox polymer. Hence optimum thickness will be very important in this sensor.
Figure 6 indicates that the sensor signal is linearly dependent on the concentration of glucose (0–360 mg/dl) with an R2 value of 0.99. The calculated sensitivities for this sensor are 1.782 μA/(cm2 mM) for the whole array and 0.357 μA/(cm2 mM) for each electrode. Previously reported sensitivities for the same gold microelectrodes without a biocompatible hydrogel were lower [0.26 μA/(cm2 mM)] than for this sensor.22
Figure 6.
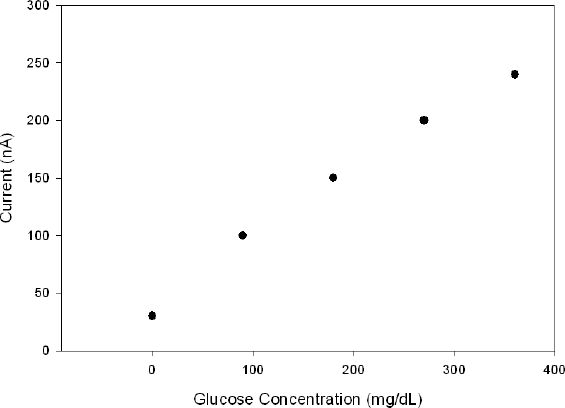
Collective response of five sensor elements to increasing glucose concentration.
Conclusions
We have fabricated a glucose sensor array on flexible polyimide sheets. Using conventional silicon microfabrication methods, we have initially formed a five-element microelectrode array consisting of gold microdisks. Biocompatible enzyme-containing redox polymer films on five microarrays were formed through the UV-initiated free radical cross-linking of a redox polymer and PEG-DA. These microarray sensors were individually addressable and were without discontinuities. There was no cross talk between adjacent members. When sampled together the microarray electrodes behaved as one large electrode with peak current equivalent to the sum of the individual elements of the array, which is especially important when diagnosing any array element failure. We have shown catalysis of glucose oxidation resulting from glucose oxidase enzyme exchanging electrons with redox polymer in PEG-DA hydrogel. We are exploring other parameters such as the extent of free radical cross-linking to ensure optimum catalytic performance of the enzyme and also electron exchange between the enzyme and the redox polymer.
Acknowledgements
We thank the United States Department of the Army for funding of this project. The view, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as a position, policy, decision, or endorsement of the Federal Government.
Abbreviations
- bpy
2,2'-dipyridyl
- DMF
N,N-dimethylformamide
- FAD
flavin adenine dinucleotide
- GOX
glucose oxidase
- HCl
hydrochloric acid
- MUA
11-mercaptoundecanoic acid
- Os(bpy)2Cl2
osmium(bipyridine)2 dichloride
- PEG-DA
poly(ethylene glycol) diacrylate
- PF6−
hexafluorophosphate
- POs-EA
poly[vinyl pyridine osmium (bipyridine)2chloride]-coethylamine
- UV
ultraviolet
References
- 1.Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, Robinson PL. Noninvasive glucose monitoring in diabetic patients: a preliminary evaluation. Clin Chem. 1992 Sep;38(9):1618–1622. [PubMed] [Google Scholar]
- 2.Heise HM, Marbach R, Koschinsky T, Gries FA. Noninvasive blood glucose sensors based on near-infrared spectroscopy. Artif Organs. 1994 Jun;18(6):439–447. doi: 10.1111/j.1525-1594.1994.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 3.March WF, Rabinovitch B, Adams R, Wise JR, Melton M. Ocular glucose sensor. Trans Am Soc Artif Intern Organs. 1982;28:232–235. [PubMed] [Google Scholar]
- 4.Klonoff DC. Noninvasive blood glucose monitoring. Diabetes Care. 1997 Mar;20(3):433–437. doi: 10.2337/diacare.20.3.433. [DOI] [PubMed] [Google Scholar]
- 5.Koschinsky T, Heinemann L. Sensors for glucose monitoring: technical and clinical aspects. Diab Metab Res Rev. 2001 Mar–Apr;17(2):113–123. doi: 10.1002/dmrr.188. [DOI] [PubMed] [Google Scholar]
- 6.Glikfeld P, Hinz RS, Guy RH. Noninvasive sampling of biological fluids by iontophoresis. Pharm Res. 1989 Nov;6(11):988–990. doi: 10.1023/a:1015957816254. [DOI] [PubMed] [Google Scholar]
- 7.Kimura J. Noninvasive blood glucose concentration monitoring method with suction effusion fluid by ISFET biosensor. Appl Biochem Biotechnol. 1993 Apr–May;41(1-2):55–58. doi: 10.1007/BF02918529. [DOI] [PubMed] [Google Scholar]
- 8.Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002 Jan–Feb;18(Suppl 1):S49–53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- 9.Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Bioelectron. 2001 Dec;16(9-12):621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 10.Brown JQ, Srivastava R, McShane MJ. Encapsulation of glucose oxidase and an oxygen-quenched fluorophore in polyelectrolyte-coated calcium alginate microspheres as optical glucose sensor systems. Biosens Bioelectron. 2005 Jul 15;21(1):212–216. doi: 10.1016/j.bios.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JG, Schmidtke DW, Quinn CP, Fleming TF, Bernacky B, Heller A. Continuous amperometric monitoring of glucose in a brittle diabetic chimpanzee with a miniature subcutaneous electrode. Proc Natl Acad Sci USA. 1998 May 26;95(11):6379–6382. doi: 10.1073/pnas.95.11.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindra DS, Zhang Y, Wilson GS, Sternberg R, Thevenot DR, Moatti D, Reach G. Design and in vitro studies of a needle-type glucose sensor for subcutaneous monitoring. Anal Chem. 1991 Sep 1;63(17):1692–1696. doi: 10.1021/ac00017a008. [DOI] [PubMed] [Google Scholar]
- 13.Linke B, Kerner W, Kiwit M, Pishko M, Heller A. Amperometric biosensor for in vivo glucose sensing based on glucose oxidase immobilized in a redox hydrogel. Biosens Bioelectron. 1994;9(2):151–158. doi: 10.1016/0956-5663(94)80107-x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson GS, Zhang Y, Reach G, Moatti-Sirat D, Poitout V, Thevenot DR, Lemonnier F, Klein JC. Progress toward the development of an implantable sensor for glucose. Clin Chem. 1992 Sep;38(9):1613–1617. [PubMed] [Google Scholar]
- 15.Gifford R, Kehoe JJ, Barnes SL, Kornilayev BA, Alterman MA, Wilson GS. Protein interactions with subcutaneously implanted biosensors. Biomaterials. 2006 Apr;27(12):2587–2598. doi: 10.1016/j.biomaterials.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Armour JC, Lucisano JY, McKean BD, Gough DA. Application of chronic intravascular blood glucose sensor in dogs. Diabetes. 1990 Dec;39(12):1519–1526. doi: 10.2337/diab.39.12.1519. [DOI] [PubMed] [Google Scholar]
- 17.Sieminski AL, Gooch KJ. Biomaterial-microvasculature interactions. Biomaterials. 2000 Nov;21(22):2233–2241. doi: 10.1016/s0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 18.Heo J, Thomas KJ, Seong GH, Crooks RM. A microfluidic bioreactor based on hydrogel-entrapped E. coli: cell viability, lysis, and intracellular enzyme reactions. Anal Chem. 2003 Jan 1;75(1):22–26. doi: 10.1021/ac0259717. [DOI] [PubMed] [Google Scholar]
- 19.Hill-West JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci USA. 1994 Jun 21;91(13):5967–5971. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn CP, Pathak CP, Heller A, Hubbell JA. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995 Mar;16(5):389–396. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 21.Desai NP, Hubbell JA. Biological responses to polyethylene oxide modified polyethylene terephthalate surfaces. J Biomed Mater Res. 1991 Jul;25(7):829–843. doi: 10.1002/jbm.820250704. [DOI] [PubMed] [Google Scholar]
- 22.Revzin AF, Sirkar K, Simonian A, Pishko MV. Glucose, lactate, and pyruvate biosensor arrays based on redox polymer/oxidoreductase nanocomposite thin-films deposited on photolithographic ally patterned gold microelectrodes. Sens Act B. 2002 Jan;81(2-3):359–368. [Google Scholar]
- 23.Gregg BA, Heller A. Redox polymer-films containing enzymes.1. A redox-conducting epoxy cement: synthesis, characterization, and electrocatalytic oxidation of hydroquinone. J Phys Chem. 1991 Jul;95(15):5970–5975. [Google Scholar]
- 24.Denisevich P, Abruna HD, Leidner CR, Meyer TJ, Murray RW. Electropolymerization of vinylpyridine and vinylbipyridine complexes of iron and ruthenium: homopolymers, copolymers, reactive polymers. Inorg Chem. 1982;21(6):2153–2161. [Google Scholar]
- 25.Quinn CP, Pishko MV, Schmidtke DW, Ishikawa M, Wagner JG, Raskin P, Hubbell JA, Heller A. Kinetics of glucose delivery to subcutaneous tissue in rats measured with 0.3-mm amperometric microsensors. Am J Physiol. 1995 Jul;269(1 Pt 1):E155–61. doi: 10.1152/ajpendo.1995.269.1.E155. [DOI] [PubMed] [Google Scholar]
- 26.Csoregi E, Quinn CP, Schmidtke DW, Lindquist SE, Pishko MV, Ye L, Katakis I, Hubbell JA, Heller A. Design, characterization, and one-point in vivo calibration of a subcutaneously implanted glucose electrode. Anal Chem. 1994 Oct 1;66(19):3131–3138. doi: 10.1021/ac00091a022. [DOI] [PubMed] [Google Scholar]
- 27.Schmidtke DW, Pishko MV, Quinn CP, Heller A. Statistics for critical clinical decision making based on readings of pairs of implanted sensors. Anal Chem. 1996 Sep 1;68(17):2845–2849. doi: 10.1021/ac9602027. [DOI] [PubMed] [Google Scholar]
- 28.Revzin A, Russell RJ, Yadavalli VK, Koh WG, Deister C, Hile DD, Mellott MB, Pishko MV. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir. 2001 Sep 4;17(18):5440–5447. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- 29.Russell RJ, Axel AC, Shields KL, Pishko MV. Mass transfer in rapidly photopolymerized poly(ethylene glycol) hydrogels used for chemical sensing. Polymer. 2001 May;42(11):4893–4901. [Google Scholar]
- 30.Simonian AL, Revzin A, Wild JR, Elkind J, Pishko MV. Characterization of oxidoreductase-redox polymer electrostatic film assembly on gold by surface plasmon resonance spectroscopy and Fourier transform infrared-external reflection spectroscopy. Anal Chim Acta. 2002 Aug;466(2):201–212. [Google Scholar]
- 31.Pishko MV, Revzin A, Simonian AL. Mass transfer in amperometric biosensors based on nanocomposite thin films of redox polymers and oxidoreductases. Sensors. 2002 Mar;2(3):79–90. [Google Scholar]
- 32.Russell RJ, Sirkar K, Pishko MV. Preparation of nanocomposite poly(allylamine)-poly(ethylene glycol) thin films using Michael addition. Langmuir. 2000 Apr;16(8):4052–4054. [Google Scholar]
- 33.Pishko MV, Michael AC, Heller A. Amperometric glucose microelectrodes prepared through immobilization of glucose-oxidase in redox hydrogels. Anal Chem. 1991 Oct;63(20):2268–2272. doi: 10.1021/ac00020a014. [DOI] [PubMed] [Google Scholar]
- 34.Bard AJ, Faulkner LR. Electrochemical method: fundamentals and applications. 2nd. New York: Wiley and Sons; 1980. [Google Scholar]


