Abstract
Background
Microdialysis is a sampling technique based on controlling the mass transfer rate of different-sized molecules across a semipermeable membrane. Because the dialysis process has minimal effects on the surrounding fluid, it is viewed as a tool for continuous monitoring of human metabolites. In diabetes treatment, microdialysis probes have been used as sampling systems coupled to a glucose biosensor but may struggle to obtain high recoveries of analytes, as the sampling housing, probes, and glucose sensors are fabricated as separate pieces and then assembled, resulting in a large dead volume, which limits sensing frequency. An in situ combination of a miniaturized microdialysis probe with an integrated glucose sensor could help solve some of these problems.
Method
The system was fabricated by bonding a 6-μm-thick polycarbonate track-etch membrane with 100-nm-diameter pores onto microfluidic channels with the electrochemical glucose sensing electrodes patterned within the microchannels.
Results
In vitro experiments demonstrating glucose microdialysis with continuous sensing were conducted. The permeability of glucose to the polycarbonate membrane with a 100-nm-diameter pore size was obtained to be 5.44 μm/s. Glucose recovery of 99% was observed using this microdialysis system at a perfusion flow rate of 0.5 μl/min. Experiments monitoring fluctuating glucose concentrations in the time domain at 99% recovery were also performed. The lag time was measured to be 210 seconds with 45 seconds contributed by mass transfer limitations and the rest from dead volume within the experimental setup.
Conclusion
The electrochemical sensing component was able to continuously track concentration changes in the reservoir. This system is expected to have the proper sensitivity to track physiologically relevant concentration changes of glucose with a lag time of less than 1 minute and minimal amplitude reduction for continuous glucose monitoring for diabetes treatment.
Keywords: CGMS, glucose sensing, microdialysis, microfluidics
Introduction
Diabetes mellitus is a clinically heterogeneous group of disorders characterized by elevated blood glucose levels resulting from deficiency in insulin secretion, insulin resistance, or a combination of both. The blood glucose concentration is regulated within a narrow range, 3.5 to 5 mM, in people who do not have diabetes, while the level in diabetic patients varies considerably, between 1 and 30 mM. In diabetic patients, the consistently high glucose levels result in long-term complications, including retinopathy, nephropathy, and neuropathy, which often leads to amputation of extremities. Studies have shown that intensive glycemic control with self-monitoring can reduce the long-term complication dramatically.1,2 Self-blood glucose monitoring can be achieved using a portable glucose monitor available in most pharmacies. The kit contains a glucose meter and its own reagent strips. Usually a drop of blood obtained through a finger prick is placed on the reagent strip. A finger prick can be done with a small lancet fixed in a spring-loaded device that punctures the fingertip quickly. The strip is then placed in the blood glucose monitor, which reads the blood glucose level usually through enzyme-linked electrochemistry.
However, one of the disadvantages of tight glycemic control with self-monitoring is an increase in the risk of hypoglycemic incidences, highlighting the need for a feedback-controlled insulin infusion system or “artificial pancreas.” One of the critical components of such a system is a continuous glucose monitoring system. Of particular interest is a microdialysis-based glucose sensing system.3–5
Microdialysis is a continuous sampling technique based on controlling the mass transfer rate of small molecules across a semipermeable membrane while excluding the larger ones. For biochemical monitoring, microdialysis systems are usually placed (inserted or implanted) inside the tissue of interest with an isotonic perfusion fluid flowing through the system and diffusional exchange occurring between the perfusate and the surrounding interstitial fluid (ISF). Because the dialysis process has a minimal effect on the surrounding fluid, it is viewed as a tool for continuous monitoring.6–12
The most common type of commercially available microdialysis probe is constructed as a concentric tube. These microdialysis probes have been used for diabetes treatment as a continuous monitoring system coupled to a glucose sensor but can struggle to obtain high recoveries of analytes due to a limited diffusional surface area and a relatively large diameter creating a long diffusional path length. The sampling probes and glucose sensors are also fabricated as separate pieces and then assembled, which can result in a large dead volume.7,11,13 An in situ combination of a miniaturized microdialysis probe with an integrated glucose sensor, allowing both a high glucose recovery and less system lag time (both due to system dead volume and mass transfer limitations) for accurate glucose sampling during continuous patient monitoring, could help solve these problems.
Currently there is a single continuous glucose monitor on the market that uses microdialysis technology. This is the Glucoday S, which is available only in Europe. A semipermeable dialysis fiber is inserted subcutaneously into the abdominal wall and perfused with glucose-free isotonic fluid. The system provides a reading every 3 minutes for 48 hours. Only one calibration is needed for the 48 hours. It has been reported to have better accuracy, precision, and long-term stability since foreign body reactions are avoided. A disadvantage is the instrumental time lag inherent to the microdialysis technique, estimated to be about 7 minutes.14
Microdialysis systems have recently been adapted to microfluidic technologies for sample preparation prior to sample analysis or for miniaturized biochemical probes. Pan and colleagues15 sandwiched a commercially available dialysis membrane between two microfluidic chips and adopted this approach for continuous glucose monitoring. Kirby and colleagues16 integrated microdialysis membranes with microfluidic channels by using an ultraviolet laser photopolymerization technique to lithographically define a patterned nanoporous dialysis membrane within a microfluidic channel. A final approach by Bohn and colleagues17 was to integrate microdialysis membranes with microfluidics and is based on direct bonding of microdialysis membranes with the microchannel structures.
In this study, a novel on-chip microdialysis system, which addresses some of the problems with continuous glucose monitoring, is presented.18–20 The device is based on thin film fabrication, which is easily integrated with in situ biosensors, and direct polymer bonding on microfluidic channels and will ultimately be used as a microdialysis probe with direct contact between the dialysis membrane and the tissue of interest. Integration of a biosensor directly with the microdialysis system is designed to allow high recovery of analytes with a smaller diffusional surface area and lower flow rates, resulting in a less invasive, microdialysis probe that can track fluctuating blood glucose concentrations more accurately than existing systems. In addition, the large surface to microchannel volume ratio and short diffusional path will allow higher recoveries and faster equilibration times for higher frequency sampling rates.
Theory
Analytical Model of the Microdialysis System
The concentration gradient between the perfusate and the component of interest in the ISF (e.g., glucose) is the driving force needed to transport molecules to the lumen of the microdialysis probe. The concentration of the molecules of interest in the perfusion fluid at the output of the microdialysis probe is a representative of the ISF and can be correlated to the concentration within the ISF. The term, recovery, is defined as
| (1) |
where Cout is the outlet concentration and C∞ is the bulk concentration in the surrounding fluid.
For this microdialysis system (Figure 1), a two-compartment system is used with a reservoir channel and a perfusion flow channel. Molecules inside the reservoir are dialyzed across the microdialysis membrane. The mass transfer coefficient is assumed to remain constant, as K0, over the diffusion path. From mass transport theory and conservation theory, the system can be described mathematically as
| (2) |
where Cr,in and Cr,out are the inlet and the outlet concentrations of the polydimethylsiloxane (PDMS) reservoir channel. Cd,in and Cd,out are the dialysate inlet and outlet concentrations of the perfusion flow. Qr is the PDMS reservoir channel flow rate, and Qd is the perfusion flow rate. K0 is the overall molecular permeability, and Am is the diffusional surface area.
Figure 1.
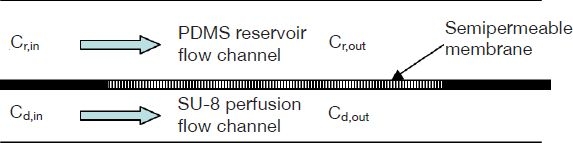
Schematic of the mass transfer model of the two-compartment microdialysis system.
In this work, Cd,in is always zero. Because the reservoir flow rate is much higher than the perfusion flow rate, Cr,in and Cr,out are assumed to be equal values, C∞, and Cd,out is simply Cout. In addition, when Qr is much larger than Qd (1/Qr ≪ 1/Qd), the 1/Qr can be ignored on the right side of the equation. Therefore, Equation (2) can be simplified as
| (3) |
where Q is the perfusion flow rate. The equation can be rearranged to represent recovery as a function of perfusion flow rate:
| (4) |
By plotting the left side of Equation (3), −ln(1-Cout/C∞), as a function of perfusion flow rate (1/Q), the permeability of the membrane to the molecules of interest (the slope as K0A) may be determined to characterize the system functionality.
Glucose Sensing
A glucose oxidase (GOX)-based sensor is described here to be integrated with the microdialysis system. The catalysis of glucose by glucose oxidase is
| (5) |
| (6) |
Hydrogen peroxide is produced as a product of the enzymatic reaction. As long as oxygen and enzyme are in excess of the glucose substrate (i.e., not rate limiting), the hydrogen peroxide concentration will increase linearly with increasing glucose concentration. In this work, a three electrode-modified Clark cell electrochemical sensor was chosen to be integrated with the microdialysis chip because it can be integrated easily using thin film-based fabrication. A platinum (Pt) electrode that is biased at +0.7 V versus a reference electrode (Ag/AgCl) can be used as a hydrogen peroxide detector by oxidizing hydrogen peroxide at the working electrode surface. In the three electrode configuration, the AgCl electrode is protected from consumption by an auxiliary electrode, which injects current into the electrolyte while holding the reference electrode at a constant potential but with no current flow. The oxidation current is then monitored by amperometry:
| (7) |
Method
The system is fabricated using a polycarbonate track-etch membrane (100-nm-diameter pore sizes, 6 μm thick, Whatman Inc.) directly bonded onto SU-8 microfluidic channels with the platinum sensing electrodes patterned and in the bottom of the microchannels.
In order to create a reservoir environment with the concentration continuously being changed for testing the time resolution of the device, a stacked system was developed (Figure 2). There are three main parts of the described device: the microdialysis monitoring chip, a PDMS reservoir channel piece, and a PDMS mixer.
Figure 2.
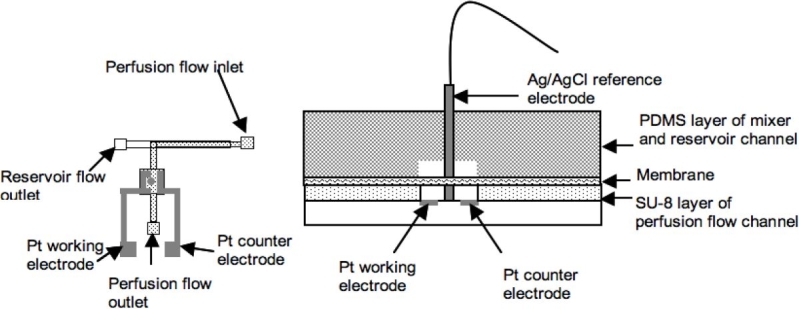
Schematic of the stacked microdialysis system with glucose sensing electrodes.
Fabrication
The Pt sensing electrodes were first fabricated using a lift-off technique on clean glass slides by sputtering a 100-Å-thick titanium/1000-Å platinum layer onto a 2-μm-thick Shipley 1818 photoresist layer patterned by optical lithography. The areas of the working and the counter electrodes were 2.94 and 2.1 mm2. The contact pads were 2 × 2 mm, which was designed to be large enough for easy wire connection. Access holes for the fluidic connection within the device were drilled by estimation after defining the electrodes.
The SU-8 2010 negative photoresist was the material chosen for the fluidic channels. The microfluidic channel was determined to be 15 μm thick after the standard SU-8 photolithographic fabrication procedure. A polycarbonate track-etch membrane was chosen as the microdialysis membrane. The membrane and the device were treated with oxygen plasma for 90 seconds (300 mTorr, 50 W). Before applying the membrane on the SU-8 fluidic channels, the device was wetted with a clean cotton wiper followed by a lamination bond. The device was next placed on a hot plate of 120°C for 30 minutes to enhance the bonding, which subsequently resulted in a strong nonleaking bond. The diffusional area of the system was about 7 mm2.
Even though the ultimate goal of this research was to create a device in direct contact with a tissue of interest, in this work, a second fluidic channel was needed in order to create a reservoir channel for testing the device with a changing reservoir concentration efficiently. The reservoir was fabricated in polydimethylsiloxane silicone rubber by casting the PDMS over a patterned SU-8 mold in a standard soft lithography procedure21 for the molding of 20-μm-deep reservoir fluidic channels.
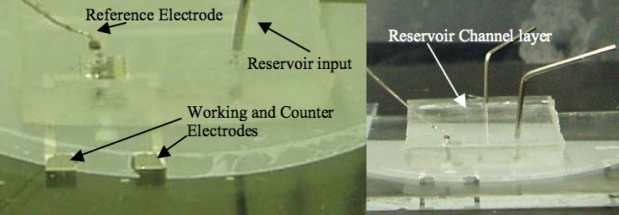
The last step to complete this system was to assemble the Ag/AgCl pellet electrode (0.8 mm diameter, A-M systems) with the chip. The pellet was inserted into the glucose sensing area directly (Figure 2). Two pictures of an actual device are shown in Figure 3.
Figure 3.
Photographs of the actual glucose microdialysis chip. Pt electrodes are on the bottom of the device covered with the SU-8 microfluidic channel layer, the polycarbonate membrane, and the PDMS reservoir channel layer.
The same soft lithography technique used to fabricate the reservoir channel was also utilized to build a supportive PDMS micromixer for controlling the input concentration into the reservoir, which was then bonded with clean glass to close the open surface. The strategy to enhance mixing was to reduce the diffusional length by splitting the flow stream into n substreams and rejoining them. Therefore the total mixing time was decreased by a factor of n2.22 Figure 4 shows the geometry of the mixer. The width of the mixing channel was 200 μm and split into five substreams, resulting in a 40-μm diffusional path length.
Figure 4.
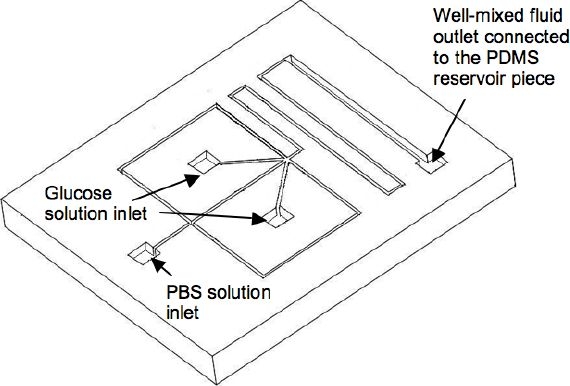
Geometry of the micromixer.
Experimental Setup
The experimental setup is shown in Figure 5. PE10 tubing (Intramedic polyethylene tubing, i.d.: 0.28 mm, o.d.: 0.61 mm) was used to connect the device to the syringes. Three syringe pumps were employed for time domain microdialysis. Two syringe pumps were required for changing the solution concentration within the reservoir PDMS channel by alternating the relative flow rates from two syringes, where one was filled with a phosphate-buffered saline (PBS) solution and the other was filled with a glucose solution (398 mg/dl). The micromixer was used to generate a well-mixed flow without concentration gradients introduced into the reservoir channel. The third syringe pump was used for producing a steady perfusion flow within the SU-8 lower channel. The perfusate contained glucose oxidase at a concentration of 1000 unit/ml mixed within 5 ml of PBS solution. A CHI electrochemical station (model type 750B, (CHI Instruments, Austin, TX) was used to collect the oxidation current for determining the glucose concentration within the sensing area.
Figure 5.
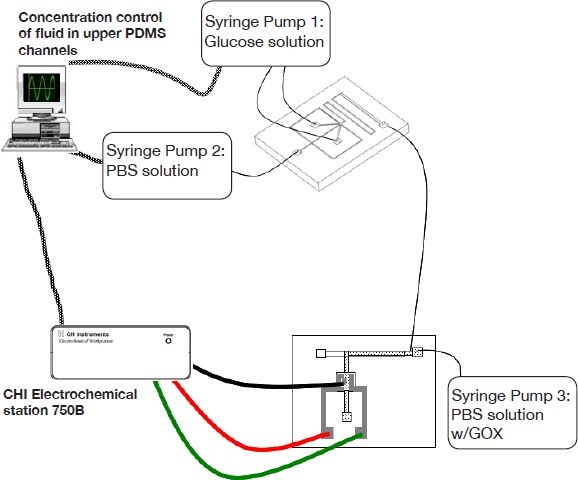
Experimental setup for glucose sensing.
Results and Discussion
Thin film platinum electrodes were used here for glucose sensing based on the detection of hydrogen peroxide. Pt has the advantage that it is less reactive toward the Cl− ions in the electrolyte under a positive potential bias, whereas Au and Ag electrodes will chlorinate under these conditions.23
The relationship between the anodic oxidation current and the glucose concentration was determined using a Therasense FreeStyle glucose monitor as a reference and was obtained over the experimental operating range (Figure 6). The calibration curve is divided into two segments. The slope is larger in the more dilute (hypoglycemic) region and then decreases as the glucose solution becomes more concentrated (hyperglycemic region). The overall sensing process is influenced by the kinetics of the reaction, by diffusion of the involved elements toward the electrode, and by stoichiometric limitations. In a kinetically controlled process, the enzymatic reaction is not rapid enough to oxidize all the glucose in the solution. Kinetic control usually takes place at low enzyme concentrations. The volume between the microdialysis region of the device and the sensing area was about 0.15 μl. It took less than 20 seconds for the perfusion flow to reach the sensing area at the experimental flow rate of 0.5 μl/min. Despite the high enzyme concentration used in this work, it is still possible that the enzymatic reaction was not sufficiently fast enough to convert all the glucose into hydrogen peroxide prior to reaching the sensing area. Diffusional control also occurs when the electrodes are covered with a low permeability membrane. Because the electrodes were not covered, diffusional control should not play a role here. The reaction may also be limited by stoichiometric restrictions. If all the oxygen in the flow is consumed, the oxidation current does not reflect the true glucose concentration and is governed by the oxygen concentration. Oxygen deficiency often results in saturation of the sensor at high glucose concentrations.
Figure 6.
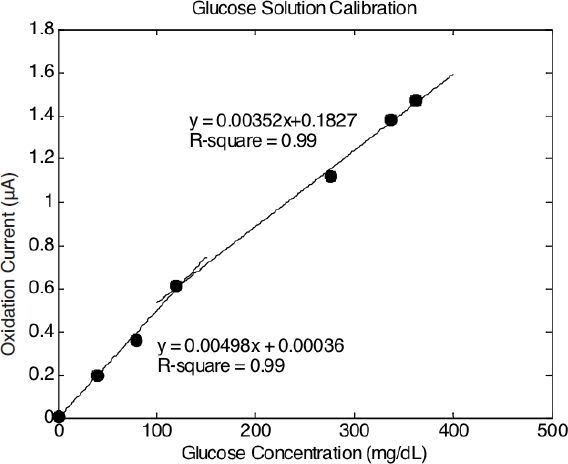
Calibration curves of glucose concentration versus oxidation current.
The system was then tested for glucose dialysis. The PDMS reservoir channel was infused with glucose solution at a concentration of 398 mg/dl at a constant flow rate. The diffusional area was 7.28 mm2. The permeability was calculated to be 5.44 ± 0.71 μm/s (Table 1). Glucose recovery as a function of perfusion flow rates was obtained in Figure 7. Recoveries at high perfusion flow rates were not performed here due to the following two reasons. First, the pressure at high perfusion flow rates might be high enough to cause the membrane to delaminate. Second, the perfusion flow rates have to be negligible compared to the flow rate of the PDMS reservoir channel in order to satisfy the assumptions used to derive Equation (3). The highest recovery was 99% at a perfusate flow rate of 0.5 μl/min.
Table 1.
Glucose Microdialysis Results
| Experiment | K0Am (μl/min) | K0Am (μm3/s) | K0 (μm/s) |
|---|---|---|---|
| Set 1 | 2.302 | 38363333.33 | 5.27 |
| Set 2 | 2.362 | 39363333.33 | 5.41 |
| Set 3 | 2.796 | 46598333.33 | 6.40 |
| Set 4 | 2.046 | 34100000 | 4.68 |
| Average | 5.44 | ||
| STD | 0.71 |
Figure 7.
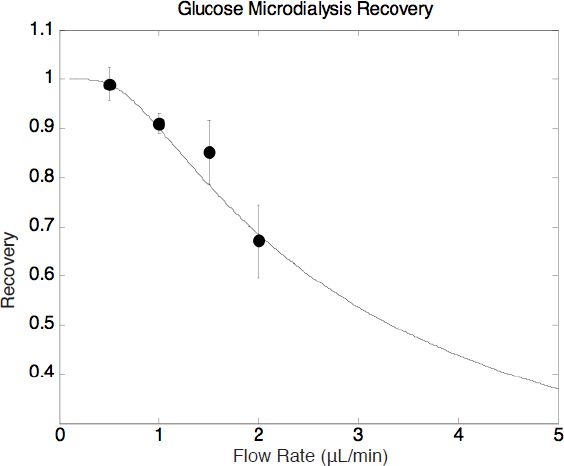
Glucose microdialysis recovery as a function of perfusion flow rate.
Subsequently, experiments monitoring glucose concentration in the time domain were conducted. The step response of the microdialysis system was first collected (Figure 8). The reservoir concentration started at a glucose concentration of 119 mg/dl, was increased to 279 mg/dl at the 10th minute, and was then reduced back to 119 mg/dl at the 20th minute. The perfusion flow rate was maintained at 0.5 μl/min throughout the experiment, which allowed a 99% recovery according to the recovery results in Figure 7.
Figure 8.
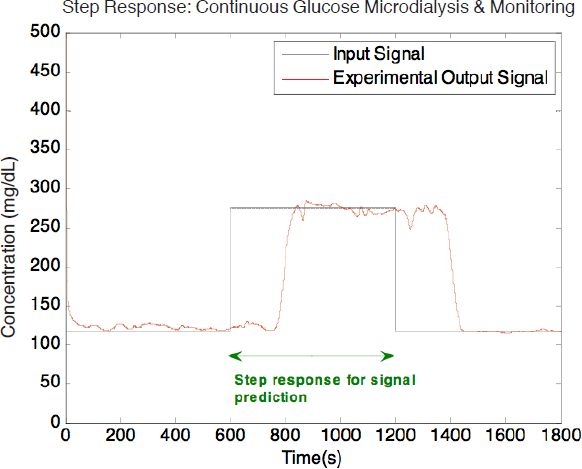
Time domain of step glucose dialysis at a perfusion flow rate of 0.5 μl/min.
The system response to any input waveform can be modeled using linear system theory by convolving the input waveform with the impulse response.24 For a given step response, g(t), the impulse response, h(t), could be obtained by taking the first derivative of the step response shown in Figure 8.
| (8) |
With the impulse response, the output signal can be predicted.
| (9) |
Next, the concentration of the reservoir channel was varied in a sinusoidal fashion from 80 to 318 mg/dl while the total PDMS reservoir flow rate remained at 10 μl/min. The period of the input signal was 800 seconds with a concentration change every 40 seconds. This period was chosen because it is a sufficient timescale for tracking physiological concentration changes, such as glucose fluctuations within a diabetic patient.
Figure 9 shows the sinusoidal response of the system. The experimental results and the output predicted by Equation (9) are in good agreement with a negligible signal amplitude reduction due to incomplete glucose recovery. At the perfusion flow rate of 0.5 μl/min, the system was able to track the concentration waveform with a 99% recovery and a phase lag of 210 seconds. Only 45 seconds out of the 210-second phase lag was contributed by the microdialysis system. The rest of this lag was because of dead volume from the tubing connection between the mixer and the microdialysis chip (∼27.5 μl tubing volume at a reservoir flow rate of 10 μl/min). Both input and output signals were converted into the frequency domain by Fourier transforms and had an obvious frequency peak at 0.00125 Hz, which represents the 800-second sinusoidal period. A Pearson's correlation coefficient (r) has been determined to be 0.99 between the experimental data and the system response predicted by linear system theory.
Figure 9.
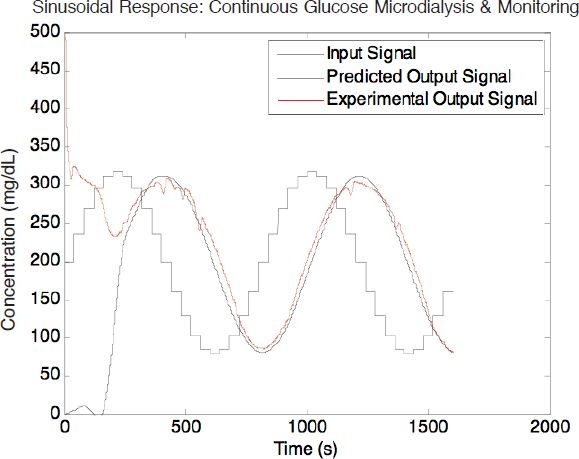
Response of sinusoidal input demonstrated with the glucose microdialysis system. The perfusion flow rate is 0.5 μl/min.
The diffusion coefficient of glucose in bulk water is 9.09 × 10−10 m2/s.25 According to the diffusion theory the mixing time in the PDMS mixer may be estimated as
| (10) |
and was calculated to be 1.76 seconds. Based on the volume of the mixer and the PDMS mixer flow rate, it would take 2.52 seconds for fluid to flow through the fluidic channel of the mixer, so the solution exiting the mixer is considered well mixed.
The pressure drop along the microchannel can also be neglected when compared with the atmospheric pressure.21 Therefore, the pressure difference across the membrane is assumed to be constant along the diffusional membrane channel. The osmotic pressure-induced flux was also found to be negligible when compared with the diffusional flux due to the concentration gradient.26
The time for the system to come into equilibrium with the surrounding fluid, tss, can be estimated as
| (11) |
where Dm is the diffusion constant through the membrane estimated as Ko*d. The equilibrium time, tss, was calculated to be 1.1 seconds for the system presented. This means that rapid perfusion channel equilibrium is possible when the microdialysis system is in contact with the interstitium for rapid continuous sensing.
One disadvantage of the membrane used is that the membrane porosity seems to vary from batch to batch. When a new batch of membranes was integrated with the device, the permeability of the membrane to the same molecules under the same conditions was not consistent with results from the previously used membranes. Technical information provided from the manufacturer indicates that the rated pore density is between 1 × 105 and 6 × 108 pores/cm2, which is an enormously wide range, and no further information about the membrane with a specific pore size could be obtained. Therefore the exact membrane permeability must be determined experimentally.
Conclusions
A complete fabrication protocol that allows continuous glucose sensing within an on-chip microdialysis system has been developed. Even though the device was designed for direct contact between the dialysis membrane and the tissue of interest, in order to create a reservoir environment with the concentration continuously being changed for testing the time resolution of the device, a stacked system and a micromixer were designed to cooperate with the microdialysis chip.
Integration of the microdialysis chip with in-line sensing capabilities was meant to reduce the glucose sensing delay caused by the dead volume with a tubing assembly. In this system, it took less than 20 seconds for the perfusion flow to reach the sensing area at the experimental flow rate of 0.5 μl/min.
A 99% glucose recovery was obtained with the microdialysis system with a diffusional area of 7 mm2 at a flow rate of 0.5 μl/min. Compared with commercially available microdialysis probes, the on-chip microdialysis system was able to obtain high recoveries with a smaller diffusional membrane area.
Experimental data were in good agreement with analytical modeling. The electrochemical sensing component was able to continuously track concentration changes in the reservoir. This system is expected to have the proper sensitivity to track physiologically relevant concentration changes of glucose with minimal lag time and amplitude reduction for continuous glucose monitoring for diabetes treatment. Future work will focus on redesigning this system into an implantable probe and on testing the system within a diabetic animal model in order to assess the biocompatibility of the system and continuously track blood glucose concentration in real time.
Abbreviations
- GOX
glucose oxidase
- ISF
interstitial fluid
- PBS
phosphate-buffered saline
- PDMS
polydimethylsiloxane
- Pt
platinum
References
- 1.DCCT Research Group; The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Heinemann L Glucose Monitoring Study Group. Continuous glucose monitoring by means of the microdialysis technique: underlying fundamental aspects. Diabetes Technol Ther. 2003;5(4):545–561. doi: 10.1089/152091503322250578. [DOI] [PubMed] [Google Scholar]
- 4.Kubiak T, Hermanns N, Schreckling HJ, Kulzer B, Haak T. Assessment of hypoglycaemia awareness using continuous glucose monitoring. Diabet Med. 2004 May;21(5):487–490. doi: 10.1111/j.1464-5491.2004.1136.x. [DOI] [PubMed] [Google Scholar]
- 5.Lodwig V, Heinemann L Glucose Monitoring Study Group. Continuous glucose monitoring with glucose sensors: calibration and assessment criteria. Diabetes Technol Ther. 2003;5(4):572–586. doi: 10.1089/152091503322250596. [DOI] [PubMed] [Google Scholar]
- 6.Beyer U, Fleischer A, Kage A, Haueter U, Ehwald R. Calibration of the viscometric glucose sensor before its use in physiological liquids—compensation for the collid-osmotic effect. Biosens Bioelectron. 2003 Oct 1;18(11):1391–1397. doi: 10.1016/s0956-5663(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 7.Ekberg NR, Wisniewski N, Brismar K, Ungerstedt U. Measurement of glucose and metabolites in subcutaneous adipose tissue during hyperglycemia with microdialysis at various perfusion flow rates. Clin Chim Acta. 2005 Sep;359(1-2):53–64. doi: 10.1016/j.cccn.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Perdomo J, Hinkers H, Sundermeier C, Seifert W, Martinez Morell O, Knoll M. Miniaturized real-time monitoring system for L-lactate and glucose using microfabricated multi-enzyme sensors. Biosens Bioelectron. 2000;15(9-10):515–522. doi: 10.1016/s0956-5663(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 9.Petrou PS, Moser I, Jobst G. Microdevice with integrated dialysis probe and biosensor array for continuous multi-analyte monitoring. Biosens Bioelectron. 2003 May;18(5-6):613–619. doi: 10.1016/s0956-5663(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 10.Rosdahl H, Hamrin K, Ungerstedt U, Henriksson J. Metabolite levels in human skeletal muscle and adipose tissue studied with microdialysis at low perfusion flow. Am J Physiol. 1998 May;274(5 Pt 1):E936–45. doi: 10.1152/ajpendo.1998.274.5.E936. [DOI] [PubMed] [Google Scholar]
- 11.Schoonen AJ, Wientjes KJ. A model for transport of glucose in adipose tissue to a microdialysis probe. Diabetes Technol Ther. 2003;5(4):589–598. doi: 10.1089/152091503322250604. [DOI] [PubMed] [Google Scholar]
- 12.Steinkuhl R, Sundermeier C, Hinkers H, Dumschat C, Cammann K, Knoll M. Microdialysis system for continuous glucose monitoring. Sens Actuators B. 1996 Jul;33(1-3):19–24. [Google Scholar]
- 13.Rhemrev-Boom RM, Tiessen RG, Jonker AA, Venema K, Vadgama P, Korf J. A lightweight measuring device for the continuous in vivo monitoring of glucose by means of ultraslow microdialysis in combination with a miniaturised flow-through biosensor. Clin Chim Acta. 2002 Feb;316(1-2):1–10. doi: 10.1016/s0009-8981(01)00574-5. [DOI] [PubMed] [Google Scholar]
- 14.Wentholt IM, Vollebregt MA, Hart AA, Hoekstra JB, DeVries JH. Comparison of a needle-type and a microdialysis continuous glucose monitor in type 1 diabetic patients. Diabetes Care. 2005 Dec;28(12):2871–2876. doi: 10.2337/diacare.28.12.2871. [DOI] [PubMed] [Google Scholar]
- 15.Pan M, Guo X, Cai Q, Li G, Chen Y. A novel glucose sensor system with Au nanoparticles based on microdialysis and coenzymes for continuous glucose monitoring. Sens Actuators A. 2003 Nov;108(1-3):258–262. [Google Scholar]
- 16.Song S, Singh AK, Shepodd TJ, Kirby BJ. Microchip dialysis of proteins using in situ photopatterned nanoporous polymer membranes. Anal Chem. 2004 Apr 15;76(8):2367–2373. doi: 10.1021/ac035290r. [DOI] [PubMed] [Google Scholar]
- 17.Kuo TC, Cannon DM, Jr, Chen Y, Tulock JJ, Shannon MA, Sweedler JV, Bohn PW. Gateable nanofluidic interconnects for multilayered microfluidic separation systems. Anal Chem. 2003 Apr 15;75(8):1861–1867. doi: 10.1021/ac025958m. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh YC, Zahn JD. Glucose recovery in a microfluidic microdialysis biochip. Sens Actuators B Chem. 2005;107(2):649–656. [Google Scholar]
- 19.Hsieh YC, Zahn JD. On-chip microdialysis system with flow-through sensing components, Biosens Bioelectron. 2007 May;22(11):2422–2428. doi: 10.1016/j.bios.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Zahn JD, Hsieh YC, Yang M. Components of an integrated microfluidic device for continuous glucose monitoring with responsive insulin delivery. Diabetes Technol Ther. 2005 Jun;7(3):536–545. doi: 10.1089/dia.2005.7.536. [DOI] [PubMed] [Google Scholar]
- 21.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane) Anal Chem. 1998;70(23):4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen NT, Wereley S. Fundamentals and applications of microfluidics. Norwood (MA): Artech House; 2002. [Google Scholar]
- 23.Lambrechts M, Sansen W. In: Biosensors: microelectrochemical devices. Jones BE, editor. London: Institute of Physics; 1992. [Google Scholar]
- 24.Kamen EW, Heck BS. Upper Saddle River (NJ): Prentice Hall; 1997. Fundamentals of signals and systems using MATLAB. [Google Scholar]
- 25.Perry RH, Green D. Perry's chemical engineers' handbook. New York: McGraw-Hill; 1984. [Google Scholar]
- 26.Kellen M. A model for transcapillary water and solute exchange in the heart, in bioengineering [Ph.D. thesis] Seattle (WA): University of Washington; 2002. [Google Scholar]