Introduction
Intensive diabetes therapy is complicated by an increased rate of hypoglycemia and the development of hypoglycemia unawareness. This presentation focuses on the potential role of real-time continuous glucose monitoring (CGM) in preventing hypoglycemia, and in overcoming this barrier to the implementation of intensive therapy. Issues to be discussed include the differences between capillary blood and interstitial glucose levels, the impact of lag times on sensor calibration and accuracy, and the importance of patient education in minimizing the risk for hypoglycemia from uncontrolled postprandial bolusing.
Hypoglycemia: The barrier tointensive insulin therapy
Impact of DCCT/EDIC
Findings from the Diabetes Control and Complications Trial (DCCT) showed that patients in the intensively-treated group achieved A1c levels approximately 2 points below those achieved in the conventionally-treated group. The Epidemiology of Diabetes Interventions and Complications (EDIC) study, an epidemiologic follow-up of the DCCT subjects, showed a convergence of A1c levels at 4 years post-DCCT; patients in the conventional group improved their A1c somewhat, whereas glycemic control deteriorated in subjects from the experimental group. There have been no systematic evaluations to tease out the factors underlying this lapse in self-care and deterioration in control; however, published data indicate that this deterioration is not related to lack of healthcare insurance.
A study by Thompson and colleagues, which looked at how patients have reacted to the findings reported by the DCCT, revealed that approximately 70% of those who wanted to improve their glycemic control cited fears of hypoglycemia as a significant barrier to intensifying their diabetes management.1
In essence, there is a trade-off between reducing complications and increasing the risk for hypoglycemia. Hypoglycemia is the clinical risk barrier one faces when trying to initiate more intensive diabetes management.
Hypoglycemia Unawareness
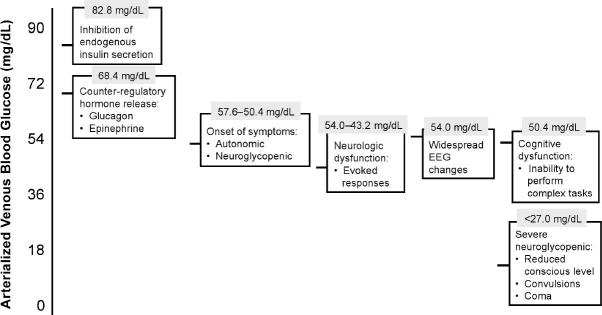
As shown in Figure 1, adapted from work by Zammitt and Frier, declining glucose levels initiate a number of responses: insulin secretion is inhibited, followed by the release of counterregulatory hormones (glucagon and epinephrine) when glucose levels reach approximately 70 mg/dL; this is the basis for the consensus statement that proposes 70 mg/dL as the diagnostic criteria for hypoglycemia.2 Continued decline in glucose eventually leads to neurologic dysfunction, widespread electroencephalogram (EEG) changes, cognitive dysfunction and severe neuroglycopenia. Counterregulatory abnormalities such as absent suppression of insulin secretion, blunted or absent glucagon response, and blunted adrenaline response further exacerbate the hypoglycemic condition in Type 1 and advanced Type 2 diabetes. Patients who experience frequent episodes of hypoglycemia often develop these abnormalities and lose their ability to detect hypoglycemia; this is referred to as “hypoglycemia unawareness” and it perpetrates a vicious cycle of recurrent hypoglycemia.
Figure 1.
Hierarchy of Responses to Hypoglycemia
There is strong evidence, however, that minimizing hypoglycemic episodes through more meticulous glucose control can actually reverse many of these counterregulatory defects. An early study by Fanelli and colleagues looked at patients with Type 1 diabetes who were being treated with multiple daily insulin injections.3 Patients were converted to a more intensive regimen and closely followed for three months. The goal was to tighten glucose controls in order to minimize hypoglycemic episodes. Subjects underwent a standard hypoglycemic clamp at baseline, 2 weeks, and 3 months duration to measure threshold levels for hypoglycemic symptoms and neuroendocrine responses. As shown in Figure 2, the glycemic thresholds for symptoms and responses normalized after 3 months of meticulous glucose control and hypoglycemia prevention; even some of the glucagon responses recovered. The researchers concluded that hypoglycemia unawareness in Type 1 diabetes is largely reversible.
Figure 2.
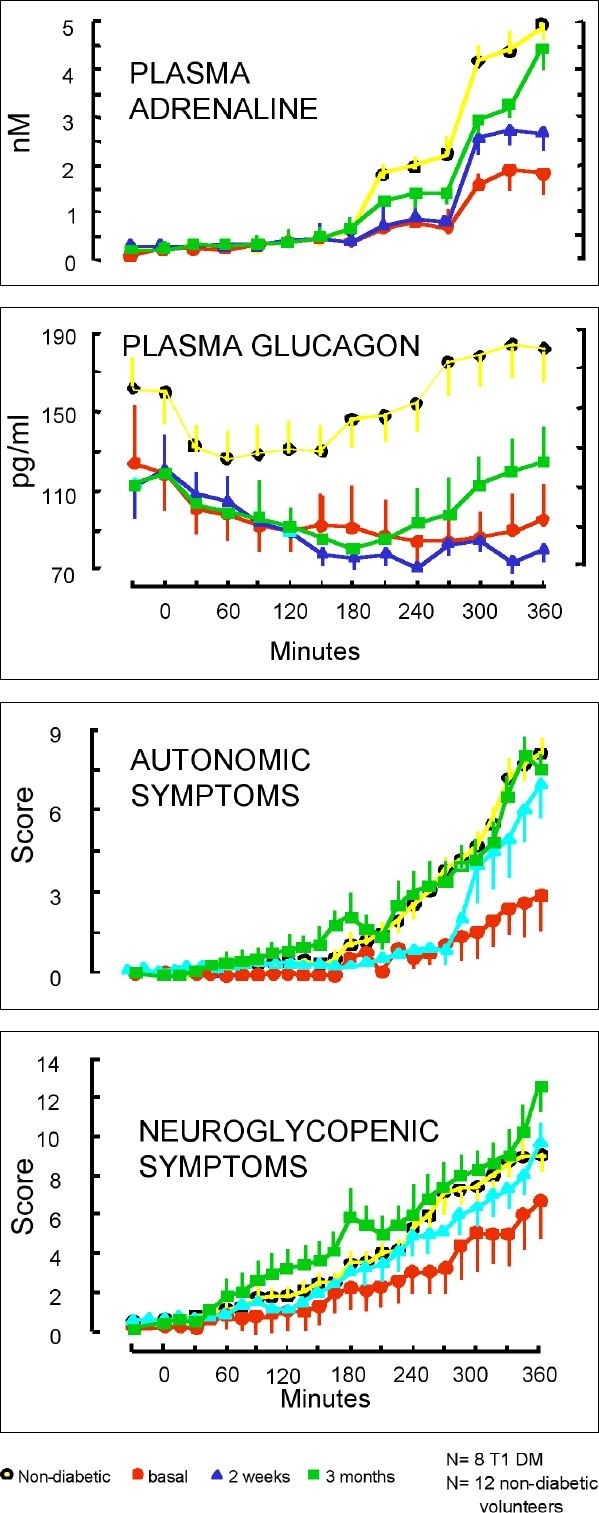
Changes in symptoms and neuroendocrine responses resulting from meticulous glycemic control.
Potential Utility of CGM to Prevent Hypoglycemia
CGM can play a significant role in preventing hypoglycemia. Unlike traditional blood glucose monitoring, which looks at only a few points in time, CGM provides comprehensive data that track glucose levels 24 hours a day. This information allows patients to optimize their glycemic control and thus minimize the frequency and severity of hypoglycemic events.
A recent study by Garg and colleagues looked at the accuracy, safety and clinical effectiveness of CGM in 91 subjects with Type 1 diabetes.4 Researchers found that subjects who wore a real-time CGM sensor spent 21% less time hypoglycemic, 23% less time hyperglycemic and 24% more time within the target range of 81-140 mg/dL than subjects who used traditional blood glucose monitoring to guide treatment.
It is important to note that there are several different sensors both on the market and awaiting approval from the Food and Drug Administration (FDA). These sensors vary in their performance characteristics and accuracy. Figure 3 presents data from a study by Clark and colleagues which compares the accuracy of the Medtronic CGMS device (FDA-approved) and Abbott FreeStyle Navigator (awaiting FDA approval) in terms of hypoglycemia detection.5
Figure 3.

Accuracy assessment of CGM versus reference blood glucose.
Interstitial Fluid vs Capillary Blood Glucose
Lag Time
A key issue when comparing CGM technology with traditional blood glucose monitoring is that CGM sensors measure interstitial fluid, not capillary glucose. Glucose diffuses from capillary blood into interstitial fluid, resulting in a physiologic lag between glucose levels. The lag time can range from 5 to 15 minutes, depending on the rate of glucose change. Increasing glucose levels are reflected first in capillary blood. However, detecting decreases in glucose (after insulin injection or exercise) may be confounded by the placement of the CGM sensor. For example, if the CGM sensor is placed next to insulin-sensitive tissue, glucose level may decrease first in the interstitial fluid before it is reflected in capillary blood glucose.
Because blood glucose concentration is regarded as the reference “gold standard”, these differences are an important issue in regard to assessment of the accuracy of CGM devices. Clearly, the physiologic lag adds complexity to the development of a reliable hypoglycemic warning alarm.
Interstitial Glucose and Cognitive Performance
Accurate hypoglycemia measurements are clinically important because they are a marker for patient risk of cognitive impairment and impending severe neuroglycopenia. For CGM devices, the question is whether and/or how well interstitial glucose correlates with cognitive function.
Evans and colleagues looked at the time course for the onset of and recovery from acute hypoglycemia in healthy subjects.6 Using standard glucose clamp technique, plasma glucose was allowed to fall rapidly to approximately 48 mg/dL for a period of 90 minutes; Cognitive function was assessed through a battery of sensitive tests throughout the hypoglycemic and recovery periods. The findings showed that while cognitive function became impaired immediately at the onset of hypoglycemia, counterregulatory hormone responses and symptomatic awareness of hypoglycemia were relatively delayed. During the 20-minute recovery period, cognitive function continued to be abnormal even after resolution of symptomatic awareness. (See Figure 4) Data from a smaller study by Cheyne and colleagues, which also used a hypoglycemic clamp, showed that recovery from hypoglycemia was delayed by an average of 26 minutes as measured by interstitial glucose.7
Figure 4.
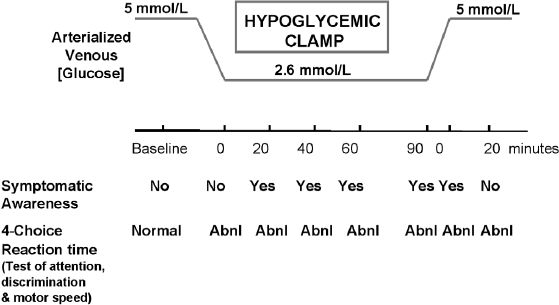
Delayed restoration of cognitive performance during recovery from hypoglycemia.
To date, there have been no studies that examined the correlation between interstitial glucose and cognitive function in individuals with diabetes. However, an animal study by Nielsen and colleagues clearly demonstrated that measurements in the subcutaneous interstitial fat corresponded very closely with measurements in the central nervous system (CNS).8 To summarize, interstitial fluid glucose concentration may be a better measure of functional status during periods of low glucose than capillary glucose concentration.
Practical considerations with use of CGM
An important consideration when using CGM technology is that sensor calibration needs to be performed when glucose is in a steady state, which relates back to the lag time issue discussed earlier. For example, if the patient performs the calibration when glucose is rising at 3 mg/dL/min and there is a 10-minute lag, then there will be a discrepancy of approximately 30 mg/dL between the capillary glucose measurement and the interstitial glucose measurement. This will shift the curve upwards and make the sensor less accurate.
A second issue rising from use of CGM technology is the tendency to “over-bolus” based on the data. Clearly, real-time data generated by CGM facilitates optimal glucose control; however, if patients overcompensate for rising glucose levels using frequent bolusing, they have an increased risk of becoming hypoglycemic. In essence, there is trade-off when using CGM with regard to hypoglycemia. On the one hand, there is an increased risk for hypoglycemia if patients are over-bolusing; on the other hand is the ability of the CGM device to detect hypoglycemic events.
Just as some patients over-bolus to control hyperglycemia, we also see patients who reduce or discontinue their basal insulin in order to avoid hyperglycemia. This often occurs because patients do not understand the lag time between capillary and interstitial glucose, thereby assessing their glucose to be much lower than it actually is. As a result, they may end up overtreating the hypoglycemia, which, in turn, will lead to a cycle of significant glycemic variability.
Another issue that must be considered is the trade-off between sensitivity and specificity in terms of alarm thresholds. Some patients may feel that they are getting too many alarms. They respond by turning off their alarms and thus derive no benefit from them. This can be addressed by lowering the threshold when patients start using the device.
Other patients who fear hypoglycemia, however, may be more tolerant of false alarms. These patients may benefit from starting off with higher thresholds, which lead to more false alarms but also detect more hypoglycemic episodes.
Steps Ahead to Facilitate the Adoption of CGM
Patient Education
An important component of education will be to teach patients how to minimize their risk for hypoglycemia by avoiding over-bolusing. This will require thorough instruction in insulin pharmacodynamics (versus pharmacokinetics) as well as an understanding of the factors that affect postprandial glucose patterns. Patients must also understand when additional bolusing is indicated.
At the same time, patients must learn how to minimize their risk for exaggerated rebound. As discussed earlier, this is often the result of overtreating hypoglycemia because they did not consider the lag time between interstitial and capillary glucose. Patients must be cautioned against inappropriate reduction or discontinuation of their basal rates. They must also be taught not to rely on interstitial glucose data alone to assess their response to hypoglycemia. Glucose levels should always be confirmed with a capillary glucose test before initiating treatment.
Technology Assessment and Improvement
Outcome studies are critically important for obtaining insurance coverage for CGM technology. However, if the goal is to strike a balance between accuracy and prevention of hypoglycemia, we will have to assess each device on its own merit; we cannot generalize findings from any one study or device and apply it to all CGM technology.
As far as improvements in CGM technology, a key focus should be on minimizing the risk for over-bolusing. It would be helpful to link the insulin-onboard feature on insulin pumps with the CGM screen to help temper patients taking extra boluses postprandially.
Another feature would be to have a hypothetical insulin action profile superimposed on the sensor so that patients can more easily conceptualize how much insulin they have onboard. Incorporating a locking system into the insulin pump may also be helpful as a safety measure to prevent patients who are cognitively impaired (due to hypoglycemia) from inadvertently taking additional insulin.
Conclusions
Fear of hypoglycemia poses a significant barrier to intensifying diabetes control. Frequent hypoglycemic episodes can impair normal counterregulatory responses and lead to hypoglycemia unawareness. CGM has the potential to help patients avoid hypoglycemia, improve their glycemic control and even reverse hypoglycemia unawareness. However, it is important that patients have reasonable expectations of what CGM technology can actually provide. They must receive adequate instruction to use CGM effectively.
Abbreviations
- CGM
continuous glucose monitoring
- DCCT
Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- EEG
electroencephalogram
- FDA
Food and Drug Administration
References
- 1.Thompson CJ, Cummings JF, Chalmers J, Gould C, Newton RW. How have patients reacted to the implications of the DCCT? Diabetes Care. 1996;19:876–879. doi: 10.2337/diacare.19.8.876. [DOI] [PubMed] [Google Scholar]
- 2.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 3.Fanelli CG, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Lepore M, Annibale B, Ciofetta M, Bottini P, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes. 1993;42:1683–1689. doi: 10.2337/diab.42.11.1683. [DOI] [PubMed] [Google Scholar]
- 4.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real- time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 5.Clarke WL, Anderson S, Farhy L, Breton M, Gonder-Frederick L, Cox D, Kovatchev B. Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose-error grid analysis. Diabetes Care. 2005;28:2412–2417. doi: 10.2337/diacare.28.10.2412. [DOI] [PubMed] [Google Scholar]
- 6.Evans ML, Pernet A, Lomas J, Jones J, Amiel SA. Delay in onset of awareness of acute hypoglycemia and of restoration of cognitive performance during recovery. Diabetes Care. 2000;23:893–897. doi: 10.2337/diacare.23.7.893. [DOI] [PubMed] [Google Scholar]
- 7.Cheyne EH, Cavan DA, Kerr D. Performance of a continuous glucose monitoring system during controlled hypoglycaemia in healthy volunteers. Diabetes Technol Ther. 2002;4:607–613. doi: 10.1089/152091502320798222. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen JK, Djurhuus CB, Gravholt CH, Carus AC, Granild-Jensen J, Ørskov H, Christiansen JS. Continuous glucose monitoring in interstitial subcutaneous adipose tissue and skeletal muscle reflects excursions in cerebral cortex. Diabetes. 2005;54:1635–1639. doi: 10.2337/diabetes.54.6.1635. [DOI] [PubMed] [Google Scholar]