Introduction
Point-of-care testing (POCT) has various names, including point of service testing, near-patient testing, bedside testing, and home monitoring. POCT refers primarily to testing that is performed close to the patient. POCT can occur in outpatient or inpatient settings and can be performed by the patient or the health care provider.
POCT is a burgeoning market that comprises 25% of all in vitro laboratory testing; it is growing at an annual rate of 12%. Blood glucose monitoring is the most widely adopted form of POCT; however, use of other diabetes-specific POCT measurements is growing. This presentation discusses the benefits of A1c and blood ketone POCT in diabetes care.
A1c
Clinical Utility
A1c is the traditional “gold standard” for assessing glycemic control, guiding diabetes therapy, and identifying patients' risk of complications. It has been shown that provider awareness of A1c facilitates improvement in metabolic outcomes.
Use of A1c POCT offers additional utility; immediate A1c values help verify the accuracy of the self-monitoring of blood glucose (SMBG) results that patients present in the clinic. This is particularly important when patients do not bring their log books or meters into the clinic. The availability of immediate A1c testing allows clinicians to expedite results to patients and readily change treatments as needed to improve glycemic control. It is important to note that validation studies have shown A1c POCT to be equivalent to laboratory reference measurements.1,2
A1c POCT Systems
Two point-of-care A1c measuring systems of particular interest are the Bayer DCA 2000 and Metrika A1c Now analyzers. The DCA 2000 uses a 6-minute test while the A1c Now gives an 8-minute result. Both systems require only finger stick samples rather than vena punctures.
Data strongly support the importance of obtaining immediate A1c results. As early as 1990, Larsen reported that physician knowledge of A1c results improved glycemic control in 222 patients with Type 1 diabetes.3 Cagliero and colleagues assessed the impact of immediately available A1c results on glycemic control at an academic diabetes center.4 They reported that immediate feedback of A1c levels while seeing patients resulted in significant improvement of glycemic control at 6 and 12 month follow-up visits (Figure 1).
Figure 1.
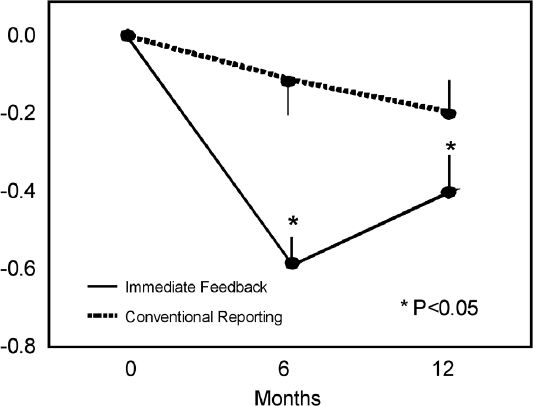
Changes in A1c levels (%) from baseline at 6- and 12-month follow-up.
A recent study by Kennedy and colleagues looked at the effects of active versus usual titration of insulin on glycemic control in primary care settings when coupled with POCT or laboratory A1c measurements.5 The 24-week, randomized, open-label study recruited 7,893 subjects from 2,164 primary care sites in the U.S. Patients were randomized to insulin glargine with either: 1) usual insulin titration using a simple algorithm with laboratory A1c testing (no unsolicited contact between visits); 2) usual titration with POCT A1c; 3) active (monitored weekly) titration with laboratory A1c testing; or 4) active titration with POCT A1c. The “active titration” groups showed significantly greater reductions in A1c than subjects in the “usual titration” groups. This was not unexpected, as the additional support provided with active titration provides a “study effect”, or the Hawthorne effect. Plus, more intensive care tends to promote patient adherence and better outcomes. Significantly, point-of-care A1c testing was associated with an increase in the proportion of study participants who achieved A1c < 7%. Although this shift appears modest, it is important to remember that, given the large population of patients with diabetes, even a small downward shift in the distribution of A1c levels makes a tremendous impact because of the reduction or postponement of complications associated with lower A1c levels.
Blood Ketones
Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) remains a significant problem. Approximately 8 out of every 1,000 patients with diabetes develop diabetic ketoacidosis per year. The U.S. sees over 110,000 cases of DKA each year at an annual cost of almost $3 billion. Despite significant improvements in diabetes therapy over the years, the rate of DKA remains relatively flat.
Contrary to what many believe, only about 1 in 10 cases of DKA result from undiagnosed diabetes; the majority of cases occur in established patients. Clearly, point-of-care ketone testing can reduce DKA in established patients.
DKA is caused by unrestrained ketogenesis resulting from significant insulin insufficiency. In the setting of acidosis, acetoacetate is converted into ß-hydroxybutyrate. With resolution of the ketoacidosis, ß-hydroxybutyrate levels fall, causing an increase in the conversion of ß-hydroxybutyrate to acetoacetate that remains detectable in the urine. This often results in persistent urine ketonuria despite resolution of blood ketonemia.
Limitations of Urine Ketone Testing
Figure 2 presents data from Schade and Eaton who look at how ketone levels evolve in the treatment of patients with DKA.6 As shown, a steady decline in ß-hydroxybutyrate occurs over time with successful insulin and rehydration therapy. The decline in the plasma concentration of ß-hydroxybutyrate corresponds well to the overall ketone concentration trend, whereas there is a slight rise followed by decline in acetoacetate levels due to the conversion of ß-hydroxybutyrate into acetoacetate during DKA resolution. This means that urine tests for acetoacetate remain positive and their resolution lags behind and is thus a false indicator of ongoing ketosis. Since urine ketone tests only detect acetoacetate and acetone, not ß-hydroxybutyrate, it is possible to misinterpret the effects and success of treatment, which can lead to overtreating the condition.
Figure 2.
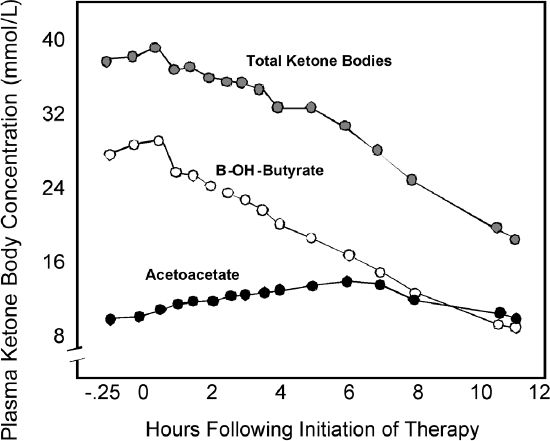
ß-hydroxybutyrate is a better indictor of metabolic status than acetoacetate when detecting and treating DKA: ß-hydroxybutyrate levels compared to acetoacetate levels during the treatment of ketoacidosis.
There is also significant inconvenience associated with urine ketone testing; most patients find it unpleasant. Additionally, it is hydration dependent – patients cannot perform the test if they do not have to urinate.
Another limitation of point-of-care urine ketone testing is that it is qualitative only; color changes on the test strip indicate high, medium or low levels of ketones. These color changes may be difficult to distinguish and therefore subject to misinterpretation.
There are significant false positives and false negatives associated with urine ketone testing due to drug interferences and environmental factors. For example, medications that contain sulfhydryl groups (such as captopril) cause false positives whereas high doses of vitamin C cause false negatives. Additionally, most people store their urine ketone test strips in the bathroom where exposure to excess humidity can significantly impair the strips' accuracy.
Blood Ketone Monitoring
Individuals who are at risk for ketosis and therefore DKA may benefit from using a blood ketone monitoring system. Potential candidates for blood ketone monitoring include insulin pump patients and patients at risk for insulin omission due to eating disorders, emotional turmoil, or educational deficiency.
Today's blood ketone testing systems require a very small amount of blood; test results are obtained in 30 seconds or less, with a linear response range from 0.0-6.0 mmol/L for ß-hydroxybutyrate. A key advantage of these systems is that the results can be quantified, which increases accuracy. Table 1 presents ranges for ß-hydroxybutyrate levels for clinical interpretation and provides a comparison with traditional urine ketone analysis.
Table 1.
Interpretation and Comparison of Blood Ketone Test Results
| ß-hydroxybutyrate (mmol/L) | Interpretation | Urine Ketones |
|---|---|---|
| 0.0 – 0.5 | Normal | Negative to Trace |
| 0.6 – 1.0 | Mildly elevated, recheck | Small |
| >1.0 | Moderately elevated, action | Moderate to Large |
Discordance of Urine Ketone versus Blood ß-Hydroxybutyrate
We collected data at Joslin and several other sites in the U.S. showing discordance between urine ketone measurements and blood ß-hydroxybutyrate. We measured blood and urine simultaneously in 86 children for a number of months in the setting of everyday life, not in the setting of an illness. We found that 2% of patients displayed significant urine ketonuria when their blood ß-hydroxybutyrate was actually negative (0.0–0.5 mmol/L). In other words, based on urine testing alone 2% of these patients could have received supplemental insulin unnecessarily, which would have led to hypoglycemia. Conversely, when blood ketones were elevated (≥0.6 mmol/L), more than half of urine ketone tests remained trace or negative, potentially leading to delays in treatment with supplemental insulin. Similar findings were seen in adults.
Utility of Blood Ketone Testing in Sick Day Management
We conducted a prospective study to evaluate the impact of blood ß-hydroxybutyrate testing versus urine ketone testing on the rate of hospitalizations.7 In the study, 123 patients were randomized to either blood ß-hydroxybutyrate or to traditional urine acetoacetate testing. The two groups were well matched for diabetes duration, age, and other factors. All patients performed glucose monitoring and were trained on sick day guidelines based on either blood ketone testing or urine ketone testing. Data were collected on episodes of illness, emergency room visits, hospitalizations, glucose monitoring frequency, ketone testing frequency, and A1c.
For six months, patients followed instructions for sick day management based on either blood or urine ketone testing, with instructions to call their healthcare team if questions arose. At the end of 3 months, patients returned to the clinic where meters were downloaded and log books collected. After 6 months, meters were again downloaded and log books collected.
At the end of the study, results showed that both groups experienced a similar number of sick days per participant; 6.3 days in the urine testing group and 5.8 in the blood testing group. However, there was a significant difference in the amount of testing that was done on those sick days. Over 90% of the blood ß-hydroxybutyrate-testing participants tested ketones during sick days whereas only 56% of the urine-testing participants tested ketones during illness. Thus, adherence to sick day management was suboptimal in the latter group.
In terms of clinical outcomes, the blood-testing group had fewer emergency room visits and hospitalizations than the urine-testing group. Extrapolating the number of emergency room visits and hospitalizations per 100 patient years – one patient followed for 100 years or 100 patients followed for one year – we found that there was almost a 50% reduction in the rate of ER visits/hospitalizations in the blood-testing group compared with the urine testing group, 38 events/100-pt-years vs. 75 events/100-pt-years (P=0.05). In short, the clinical outcomes from this study demonstrated that blood ketone testing helped to reduce the number of emergency room visits and hospitalizations compared to urine ketone testing.
Conclusions
POCT is a growing market that comprises 25% of all in vitro laboratory testing. Although blood glucose monitoring remains the most widely adopted form of POCT, use of other diabetes-specific POCT measures, such as A1c and blood ketone levels, is increasing. POCT of A1c offers the significant advantage of immediacy of information to clinicians and patients, which facilitates timely adjustments in therapy. The impact of POCT of A1c on improved clinical outcomes has been reported in the literature. POCT of blood ketones has also been shown to be effective in detecting ketosis, decreasing hospitalizations, and guiding treatment.
Abbreviations
- DKA
diabetic ketoacidosis
- POCT
point-of-care testing
- SMBG
self monitoring of blood glucose
References
- 1.Tamborlane WV, Kollman C, Steffes MW, Ruedy KJ, Dongyuan X, Beck RW, Chase P, Fox LA, Wilson DM, Tsalikian E. The Diabetes Research in Children Network (DirecNet) Study Group. Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes. 2005;6:13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy L, Herman WH. Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow point-of-care device with central laboratory testing (GOAL A1c Study) Diabetes Technol Ther. 2005;7:907–912. doi: 10.1089/dia.2005.7.907. [DOI] [PubMed] [Google Scholar]
- 3.Larsen ML, Horder M, Mogensen EF. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1021–1025. doi: 10.1056/NEJM199010113231503. [DOI] [PubMed] [Google Scholar]
- 4.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22:1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy L, Herman WH, Strange P, Harris A. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: The Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1c) trial. Diabetes Care. 2006;29:1–8. doi: 10.2337/diacare.29.01.06.dc05-1058. [DOI] [PubMed] [Google Scholar]
- 6.Schade DS, Eaton RP. Metabolic and clinical significance of ketosis. Spec Top Endocrinol Metab. 1982;4:1–27. [PubMed] [Google Scholar]
- 7.Laffel LM, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23:278–284. doi: 10.1111/j.1464-5491.2005.01771.x. [DOI] [PubMed] [Google Scholar]


