Abstract
Background
Hyperglycemia is prevalent in critical care and tight control can save lives. Current ad-hoc clinical protocols require significant clinical effort and produce highly variable results. Model-based methods can provide tight, patient specific control, while addressing practical clinical difficulties and dynamic patient evolution. However, tight control remains elusive as there is not enough understanding of the relationship between control performance and clinical outcome.
Methods
The general problem and performance criteria are defined. The clinical studies performed to date using both ad-hoctitration and model-based methods are reviewed. Studies reporting mortality outcome are analysed in terms of standardized mortality ratio (SMR) and a 95th percentile (±2σ) standard error (SE95%) to enable better comparison across cohorts.
Results
Model-based control trials lower blood glucose into a 72-110 mg/dL band within 10 hours, have target accuracy over 90%, produce fewer hypoglycemic episodes, and require no additional clinical intervention. Plotting SMR versus SE95% shows potentially high correlation (r=0.84) between ICU mortality and tightness of control.
Summary
Model-based methods provide tighter, more adaptable one method fits all solutions, using methods that enable patient-specific modeling and control. Correlation between tightness of control and clinical outcome suggests that performance metrics, such as time in a relevant glycemic band, may provide better guidelines. Overall, compared to the current one size fits all sliding scale and ad-hoc regimens, patient-specific pharmacodynamic and pharmacokinetic model-based, or one method fits all control, utilizing computational and emerging sensor technologies, offers improved treatment and better potential outcomes when treating hyperglycemia in the highly dynamic critically ill patient.
Keywords: clinical control, results, glucose variability, hyperglycemia, metabolism, model-based, mortality
Introduction
Hyperglycemia is prevalent in critical care.1,4 Increased counter-regulatory hormone secretion stimulates endogenous glucose production and increases effective insulin resistance,3,4 elevating equilibrium glucose levels and reducing the amount of glucose that the body can utilize with a given amount of insulin. Nutritional regimes with high glucose content further exacerbate hyperglycemia.5–10
Hyperglycemia worsens outcomes, increasing the risk of severe infection,11 myocardial infarction,1 and polyneuropathy and multiple-organ failure.2,12 Evidence also exists of significant reductions in other therapies with aggressive glycemic control.2,13–17 Van den Berghe et al 2,16,17 and Krinsley14,15 reduced ICU patient mortality 18% to 45% for patients with length of stay greater than three days. Both studies also showed significant cost savings per patient.18,19
Thomas et al20 achieved an average glucose reduction of approximately 15% using the protocol from van den Berghe et al,2 but saw no change in mortality. However, their results and report focused primarily on the implementation of a web-based glycemic control protocol, rather than on tight control per se. Other studies that focus primarily on glucose control are limited in duration or patient numbers, and do not extend to mortality endpoints.20–22
All of these studies used ad-hoc sliding scale or titration-based protocols developed primarily by clinical experience, (i.e. expert-based control) a typical one size fits all solution. Therefore, they are less optimal when faced with the dynamic patient variation typical of critical care. This issue has been illustrated in simulation23,24 and clinical analysis.7,24–30 In contrast, model-based tight glycemic control protocols have been successful in producing consistent control.25,31–36
Next, the more critically ill the cohort, the likelier the occurrence of severe stress-induced hyperglycemia stemming from higher insulin resistance.16,37–40This effect is illustrated in van den Berghe et al's two studies. Both used limits of 110 mg/dL,2,17 and achieved average glycemia of 102 ± 18 and 108 ± 26 mg/dL with median APACHE II scores of 9.0 and 24, respectively. However, the relative ICU mortality reduction was a significantly smaller in the more critically ill cohort. Thus, cohort severity of illness is a significant confounding factor in comparing clinical results.
All protocols are challenged by the significantly elevated insulin resistance encountered in broad critical care cohorts. In addition, insulin effect saturates at high concentrations.32,41,42 Hence, glycemic reductions using insulin alone can be limited depending on the patients level of stress-induced insulin resistance,43 leading to larger variability in performance. This effect might be particularly true for those protocols commanding up to limits of 20-50 U/hour of insulin.
However, glycemic control is also possible by controlling the nutritional inputs exacerbating the problem.5–10,23,24 Research that specifically lowered caloric intake of carbohydrates significantly reduced blood glucose levels.5,8,10,45–47 In particular, feeding only sb 33% to 66% of the ACCP guidelines48 minimized mortality and hyperglycemia versus the other two tertiles.10 Hence, this approach provides an additional effective pathway for glycemic control.
Finally, some studies find intensive insulin therapy “taxing”, 49–51 noting that van den Berghe et al2,16 used additional staff. Hence, despite the potential, many intensive care units do not use fixed protocols.4,13,50–52There is also little agreement on what constitutes desirable glycemic performance,50,52,53 particularly with regard to how tight control affects outcome.
Overall, any glycemic control protocol must reduce elevated blood glucose levels, while accounting for inter-patient variability, conflicting therapies and dynamically evolving physiological condition. Hence, it must be adaptive and/or able to identify changes in patient metabolic status, particularly with respect to insulin sensitivity. More specifically, it must accurately match therapeutic insulin and nutrition inputs, or demand, with the ability to utilize these inputs, a very difficult task in the highly dynamic critical care patient.
This paper reviews current clinical studies of tight glycemic control in critical care. A series of basic performance criteria is presented. Analysis focuses on how glycemic performance may be linked to clinical outcome.
Methods
Glucose-Insulin Models and Control
Model-based control is attractive for its potential to aggregate clinical measurements into a direct assessment of glycemic status to provide patient-specific intervention; matching demand and utilization in a way that is beyond typical sliding scale protocols. Thus, they can adapt to the highly dynamic critical care patient. However, adequate models of a complex and non-linear metabolic system are required. While outside the scope of this paper, a current, comprehensive review is given in.54
There are currently three main model-based glycemic control results:
Model Predictive Control (MPC)35
The model and control methods differ significantly for each case. However, they all offer the ability to fit a patient specific model and determine a patient-specific intervention – thus determining the demand and finding the optimal intervention.
MPC uses a complex metabolic model and an adaptive filter that determines control interventions, focusing on adaptation to changing patient response.35 The PID and sliding mode control results are based on simple linear models and primarily focused on applying continuous glucose sensors.25,33,55 The Insulin+Nutrition approach uses a model of relatively moderate complexity to fit patient specific parameters and determine the optimal intervention to achieve a pre-determined target glucose level.57 SPRINT is a table-based version for easy large-scale clinical testing.23,44
Hence, while their technical approach is different, they all share a similar one method fits all approach. Specifically, each approach adapts its model to each patient using their specific fitting method. They then use that model and a control method to determine a control intervention of insulin and/or nutrition. In contrast, fixed clinical protocols and titration-based sliding scales are not this adaptable and report the need for significant modification by clinicians to get better patient-specific control,2,15 increasing clinical burden50,51 and glycemic variability,23,24 due to their one size fits all approach. Readers are directed to the review in reference 58 and other references for further details on the modeling.
Glycemic Control Problem and Performance Criteria
Glycemic control should reduce glucose levels to a safe glycemic band, minimize variability, and provide safety from hypoglycemia. Hence, basic performance requirements include:
Maintaining set maximum glycemic levels;
Minimizing variability and/or maximize time in band;
Provide safety from hypoglycemia; and
-
Reducing mortality and other outcomes.
Efficacy is thus determined by a controller's ability to provide these results across diverse cohorts in a one method fits all approach that does not increase clinical burden, yielding:
Adaptation to cohort; and
Clinically ease to use (low burden for results)
Criteria 1, 3 and 4 are reported by most studies. Criteria 2 and 5 have only been addressed in some model-based studies.
Comparing Clinical Results and Control Efficacy
Results are presented for the model-based glycemic clinical control studies. In addition, van den Berghe et al's two studies,2,17 Krinsley,14,15 Thomas,20 and Chase et al59 are presented as they reported mortality endpoints. The latter study is currently the only model-based control design to report mortality.
To account for confounding differences in cohort and glycemic control, two metrics are proposed. Standardized mortality ratios (SMR) link mortality and cohort using the ratio of ICU mortality and the average reported APACHE II score risk of death. In this analysis, reported ICU mortality for those patients in the intensive treatment arm and with length of stay greater than 3 days is used.
Similarly, glycemic control and variability can be assessed by the unit-less standard error ratio of the reported values for 95th percentile (±2σ) blood glucose range and average blood glucose, SE95%, where tighter control provides a lower value. Specifically, taking the blood glucose range that encompasses 95% of measurements in mg/dL and dividing by the mean glucose value in mg/dL yields a fractional percentage relating the independent metrics of blood glucose variation and mean value. In simple terms, it is a percentage variation of the range between the 2.5th and 97.5th percentile measurements, and the blood glucose mean value, denoted SE95% in this paper. Note that dividing by 4 would yield the traditional standard error. Finally, using the blood glucose 95th percentile range allows the use of non-parametric counted range values if the distribution is not normal, as is often the case with positive valued concentrations, as seen in the results of Krinsley.
Hence, these two metrics (SMR, SE95%) aggregate 4 criteria into 2, providing a potentially more useful insight into the interaction between control approach and clinical outcomes. The four criteria are: mortality rate achieved, APACHE II score, mean blood glucose achieved and the 95th percentile range of blood glucose values. These metrics capture performance in terms of mortality, mean glucose, and range of variability, along with differences in cohort via the APACHE II score risk of death (ROD). Thus, mortality and APACHE II ROD are combined to create a single cohort dependent metric for performance with respect to mortality. Similarly, blood glucose 95th percentile range and mean value are combined to obtain an equally unit-less measure of glycemic performance and tightness of glycemic control.
Results
Model-based Control Results
Plank et al35 used MPC in 48-hour trials for post-cardiac surgery patients (mean APACHE II=10-12), achieving average glycemic levels of 110-123 mg/dL across three centres with 42-56% of time spent in a target band of 80-110 mg/dL, indicating a standard deviation of approximately ±18 mg/dL. Chee et al25,33,55 averaged 207±(18-54) mg/dL for 5 to 9 patients in sub-48 hour trials focused primarily on full automation using CGMS. Finally, while not explicitly model-based, the GRIP decision support system,60 used a computerized estimator-predictor for 179 surgical ICU patients (average APACHE II=14), averaging 124 mg/dL with 78% in a 72-135 mg/dL target range. All of these methods used only insulin for glycemic control and left the provision of nutrition to local clinical standards.
Computerized Insulin+Nutrition control57,61 reported 8 patient trials of 10-24 hours, which reduced glucose to the 72-110 mg/dL band within 5-hours and hit 94% of pre-set target glucose values. SPRINT mimics this approach23,44 and was implemented to enable large scale trials covering 25,000 patient hours.59 The result was average glucose of 106 ± 18 mg/dL with a cohort average APACHE II score of 21. Percentage time in band was: 72-110 mg/dL = 61%; 72-126 mg/dL = 82%; 72-140 mg/dL = 90%. Finally, ICU mortality was reduced for patients with greater than 3-days of stay from 26% to 17% (p=0.04), as compared to a one-year retrospective cohort. The study is ongoing.
Clinical Glycemic Control Studies with Mortality Endpoint
Table 1 summarizes the results of all reviewed studies with a mortality endpoint, where, for example, SMR for vdB 2001 is simply the 4.6% final ICU mortality rate divided by the average APACHE II ROD of 8%, yielding SMR = 4.6/8 = 0.58 in column eight. Figure 1 plots SMR versus SE95% where the correlation line (r = 0.84) excludes the outlying result of,20 as it focused primarily on a web-based protocol implementation and failed to achieve a significant (>18mg/dL) change in average glucose levels over 500 patients. Potential variation due to errors in determining APACHE II score,62or unreported glucose measurements leading to different glucose variation or average, are shown by ±15% error bars for mortality and additional reported points connected by dashed lines for ±5% glucose variation. These additional points and error bars are included primarily to visually illustrate the impact of potential variations on the overall trend seen. Finally, SMR < 0.85-1.0, as shown in the shaded area, represents an improvement over predicted values given the over-estimation of ROD reported in some studies for the APACHE II score.63,64
Table 1.
Clinical results for clinical studies that report ICU mortality for patients with length of stay greater than 3 days, including standardized mortality ratio (SMR) using end of study mortality and tightness metric SE95%. Where vdb stands for van den Berghe et al and Thomas for Thomas et al.
| Average APACHE II (ROD%) | ICU Mortality Change (%) | Blood Glucose (mg/dL) | 95th Percentile ±2s Range (mg/dL) | Hypo Rate (%) | SMR | Tightness | |
|---|---|---|---|---|---|---|---|
| vdB, 2001 [2] | 9 (8%) | 8 → 4.6 | 102 ± 20 | 80 | 5.2 | 0.58 | 0.77 |
| vdB, 2006 [17] | 24 (40%) | 38.1 → 31.3 | 108 ± 26 | 104 | 25.0 | 0.78 | 0.97 |
| Thomas [20] | 14.5 (20%)a | 26 → 26b,d | 112 ± 23.5 | 94 | 4.0 | 1.30 | 0.84 |
| Krinsley [14] | 16 (25%) | 20.9 → 14.8b | 131 ± 56c | 128c | ∼0 | 0.6 | 0.96 |
| SPRINT [59] | 21 (40%) | 26 → 17b | 106 ± 18 | 72 | 1.5 | 0.43 | 0.68 |
= Average ROD as APACHE II score sits on boundary of two levels in original definition.78
= Mortality change from a retrospective cohort rather than randomised trial
= Calculated directly from data in Figure 2 of Krinsley14 as it was not normally distributed and the normal standard deviation significantly over estimates the true 95% range of data.
= Mortality was not necessarily a main focus of the Thomas et al20 study.
Figure 1.
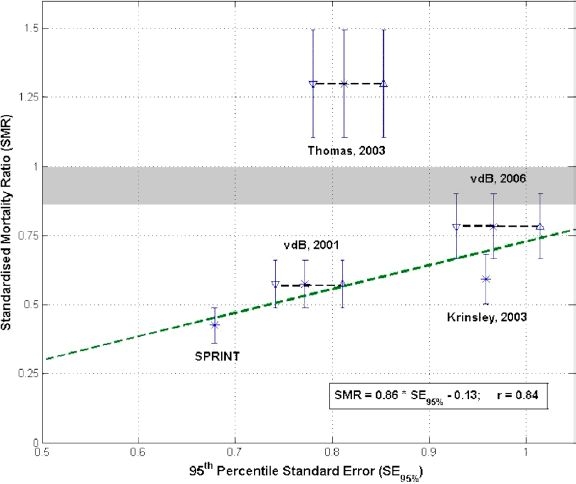
Standardised mortality ratio (SMR) versus tightness of control represented as SE95%. All values are based on published results2,14,17,20,59 where vdb stands for van den Berghe et al and Thomas for Thomas et al. The line is fit excluding the outlying data from Thomas et al. The vertical error bars show 15% potential variation in reporting or determining average APACHE II scores.62 The average glucose variation due to not including all measurements is estimated at 5% and additional error bars are generated for van den Berghe et al and Thomas et al at these values with their center points connected by a dashed line, thus accounting for potential variation in the average glucose or glucose variation determined.
Discussion
There is a great deal of glycemic variability in all the clinical results due to a combination of differences in: glycemic limit, cohort, control method, and implementation effectiveness. For example, van den Berghe et al and Thomas et al use the same protocol and similar glycemic limits, yet still obtained significantly different results in mortality and glycemic variability. In particular, the more critically ill the cohort, the more dynamic the patient evolution may be, and thus the more dynamic the changes in stress-induced insulin resistance and control. This issue can be further exacerbated in ad-hoc titration-based protocols by significant patient-specific clinical modification to improve control.2,14,17Hence, direct performance comparisons for optimising treatment strategy are, at best, qualitative.
In particular, Table 1 shows van den Berghe et al achieved average morning glycemia of 103-108 mg/dL using insulin alone, with cohorts ranging from 9 to 24 in APACHE II score. However, the higher APACHE II score, the potentially more dynamic the cohort, as seen in the larger variation in reported glucose values in both,17,20 as well as the 25% incidence of hypoglycemia17compared to 5% for the less ill cohort.2,20 It might be said that the two results of van den Berghe et al show the impact of cohort, while the outlying result of Thomas et al indicates a lack of effective control implementation, as their study only achieved an insignificant, <18 mg/dL change in average glycemia compared to their retrospective cohort.
Similarly, Thomas et al achieved similar average glycemic results to van den Berghe et al with a cohort similar to that of Krinsley.14 The standard deviation or variability of their results were also similar after accounting for Krinsley's lognormal (rather than normal, as reported) glucose distribution. However, as noted, the mortality outcome was very different, indicating unknown differences in either the method used or the efficacy of its application.
More specifically, the differences behind the outlying mortality results of Thomas et al are hard to diagnose from the data presented. However, the protocol of van den Berghe et al relies on some level of clinical customization and intervention for some patients. Thus, their results might be very different given different choices. Secondly, reporting only morning glucose values may have less meaning if tight control is not maintained for any reason throughout the day. Finally, the initial average glycemic levels in the Thomas et al study were quite low at 131 ± 32 mg/dL and thus already within the tight control range of Krinsley, which extended to 140 mg/dL indicating already good glycemic control relative to this study. In addition, the decreases to 119 ± 23 mg/dL and then 112 ± 22 mg/dL were relatively quite small and are all contained within one standard deviation. Hence, and perhaps most likely, it is possible that such relatively small glycemic reductions in this already relatively low glycemic range might not be expected to impact mortality. Therefore, for all these reasons, the Thomas et al study was excluded from the trend line calculation, as the lower changes in mortality for the tightness of control could not be accounted for from the published data. However, given more data, the more complete proof of the metrics presented will be determined. Thus, this approach to comparison is primarily put forward as hypothesis generating rather than conclusive.
In contrast, model-based methods show much tighter control on average in the limited studies to date, when compared to the clinically derived protocols of van den Berghe et al and Krinsley, as shown in Table 1. Specifically, the MPC and Insulin+Nutrition control approaches have effectively clamped blood glucose (∼108 ± 18 mg/dL) with minimal variability. Similarly, the SPRINT protocol delivered a standard deviation of ±18 mg/dL, similar to the shorter MPC trial, yet with a cohort that was much more critically ill than for those studies reporting similar variability. This reduced variability using model-based control is also seen in the 0-2% incidence of hypoglycemia for model-based methods. Hence, model-based methods appear better able to match patient-specific demand and utilization over highly dynamic, critically ill patient cohorts, without large glycemic variation.
Hence, the question arises: What is the best measure of blood glucose control? Van den Berghe et al2,16,17 used mean morning glucose, but did not include several daily measurements. This limitation hinders assessment of the actual glycemic variability and control performance. In contrast, several studies14,15,21,22,35,60 reported average glucose over all treatment, even if the actual distribution was lognormal, thus skewing the standard deviation results.
It is therefore proposed that time in a glucose band, such as 72 to 110 mg/dL, provides more complete information. In particular, maximizing time in a band tightens control, while still allowing variation within the band as patient condition evolves. In addition, using multiple bands, such as 72 to110 and 72 to140 or 72 to126 mg/dL, will also delineate the tightness as well as the fundamental distribution in terms of clinically relevant glycemic values. Time in band also equally weights any values within the band, rather than penalizing values small distances away from a specific target, thus allowing flexibility in control to within clinically acceptable ranges rather than to specific target values, as in previous studies. In support of this fundamental proposal, Figure 1 indicates that the tighter the control (smaller SE95%), the lower SMR obtained.
Further support for limiting glycemic variability to get better outcomes is found in studies linking excessive pro-inflammatory immune response to observed insulin resistance and hyperglycemia, as well as reduced bactericidal and immune system effectiveness.12,65,66 In particular, this inflammatory cascade has been linked to decreased insulin sensitivity, which is a primary cause of hyperglycemia in critical care.12,67,68 Other studies indicate that augmenting certain proteins can have a positive effect on carbohydrate metabolism and insulin sensitivity.69 There are also emerging results linking repeated exposure to elevated blood glucose to increased cellular level damage.70–73 Note that there are some recent studies that indicate that glycemic variability is not necessarily associated with increased inflammation and potentially related micro-vasculature damage,74 however these results are based on the DCCT study data, which may not have had frequently enough sampled data to capture glycemic variability as it would affect critical care patients. However, overall, there is a growing body of evidence to support minimizing exposure to elevated blood glucose in critical care by minimizing variability under glycemic control – at any reasonable average blood glucose level.
However, given the limited data available to create Figure 1 this result should be taken as hypothesis generating. While the metrics in Figure 1 aggregate clinical results and control criteria into a more readily visualised format, normalizing out differences in cohort or glycemic limit, they do have limitations. A particular limitation is the implicit assumption of a relatively low, clinically relevant average glucose level in analysing the trend. For example, very tight control to a high glycemic average of 150 to180 mg/dL would yield a low SE95% value, but (potentially) high SMR due to poor control not reducing the higher average values and thus not affecting mortality. This case would result in a point outside the trend of tighter control producing reduced mortality, as shown in Figure 1. Hence, given that hyperglycemia is strongly correlated with mortality in a variety of studies,2,16,38 the tightness metric presumes that the clinical control protocol being evaluated achieved a clinically relevant average glycemic value based on this current evidence.
Other limitations include the use of average APACHE II scores, although adjusting for reported distribution did not affect the plot. The APACHE II risk of death can also be overstated by approximately 15%,62,63 as shown by the grey area in Figure 1, where a value below the grey area covering the SMR range of 0.85 to 1.0 would indicate an improvement over predicted values for that cohort. Additionally, not all studies include every glucose measurement, potentially understating the glucose variability, particularly if longer measurement intervals are used.23,26 For example, these same protocols were simulated23,24 with good mean value correlation to published results, but significantly larger variability, showing the potential impact of reporting all the measurements. Hence, significant data is missing for a complete picture at this time, as approximated schematically by the additional error bars and points in Figure 1 for studies that don't report all glucose measurements, and the single error bars in SMR for those that do.
The use of the 95th percentile metric, using either standard deviation or non-parametric counted range, is a useful measure of the tightness of glycemic control, although not necessarily unique. Several other potential measures exist already, such as MAGE (mean average glycemic excursion)75 or the peak/range of blood glucose.76 However, both of these metrics require all glucose values to be reported, rather than just morning averages as in the van den Berghe et al studies or the study by Thomas et al. In addition, the MAGE metric is effectively equivalent to the standard deviation metric, and the peak and range of blood glucose are highly correlated to the mean glucose value,76 adding no additional information to the metric used. In addition, reporting multiple time in band percentages can compactly illustrate both the average glucose levels and fundamental distribution of glycemic values. Finally, more advanced metrics, such as the risk and Markov-based high and low blood glucose indices presented by Kovatchev et al,77 introduce significant added information. However, this metric is based on continuous sensing not used in these studies and requires the full blood glucose data to be reported and made available, which was not possible for all studies used in this paper.
Of note in this discussion is that there was no correlation between the average blood glucose achieved and mortality (r = .04) or the 95th percentile range (r = .68). In addition, similar results are obtained comparing these values to SMR (r = .06 and r = .54) respectively. It is only in their combination in defining the tightness metric, SE95%, that the there is a significant correlation with mortality or SMR for the blood glucose mean and variability achieved across these independent studies. The specific reason for the lack of correlation is that outlying values for at least one study, excluding in all cases the results of Thomas et al, result in poor correlation. This result further supports the use of these two metrics for this type of analysis across very different clinical studies.
Finally, one other potential metric which is not commonly used is readily available from the reported data. Specifically, the rate of hypoglycemic events, shown as a percentage of patients in Table 1, is an independent variable, as no significant correlation was seen between this metric and the 95th percentile metric used (r < 0.5) to illustrate variability. This result suggests that it could be used as an added metric. This value would indicate variability of glucose levels, but only accounts for one side of the equation, specifically low levels. When plotted versus SMR, this one-sided value is correlated to SMR with r = 0.87. If one then assumes that more hypoglycemic events might lead to increased mortality, and thus reduced SMR, a further derived metric can be created. Specifically, correlating the following revised metric to SMR:
where Hypo is the fractional representation of the rate of hypoglycemic events per patient, gives a correlation coefficient of r = 0.98, as shown in Figure 2. This high value, excluding the result of Thomas et al, further illustrates the hypothesized trend between tightness of control and mortality. However, while the fit is tight, the assumption of increased hypoglycemia and mortality is not proven and more data is required to validate this metric. Overall, Figure 2 further illustrates the potential trends and possible metrics that might arise on further examination and the need to begin developing consensus on this topic.
Figure 2.
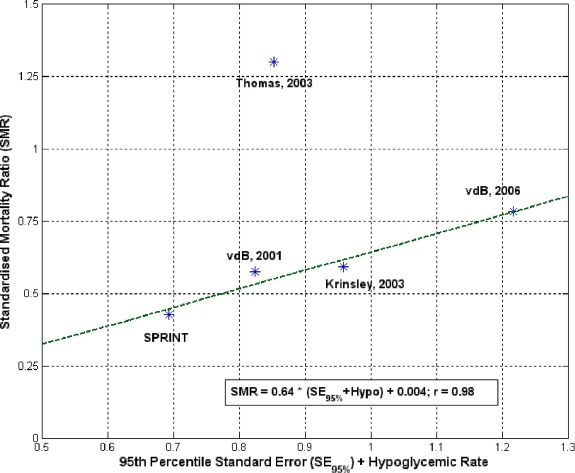
Correlation of SMR and modified 95th percentile standard error metric, where all abbreviations are the same as in Figure 1.
Overall, the results argue for more examination of metrics for assessing control effectiveness and allowing useful comparison across studies. The metrics presented here are only proposed for hypothesis generation and discussion, and likely do not represent a conclusive performance metric. However, these results do begin to suggest that tighter, more perfectly clamped control will provide better mortality results, particularly for more critically ill cohorts. Hence, metrics such as time in band, using all control measurements should be considered in future studies.
Conclusions
Hyperglycemia in critical care has a significant impact on patient mortality, outcome, and cost. Tight regulation can significantly reduce these negative outcomes, but consistently achieving it, as an expected outcome of care, remains clinically elusive. In particular, there is no standard definition of tight control and what level is necessary for optimal outcomes. Therefore, it is very difficult to determine the best protocol in terms of results and clinical effort. This overview has examined the current performance of clinical glycemic control studies in critical care focusing on the differences in emerging model-based approaches that utilize a variety of computational and emerging sensor technologies, and current ad-hoc clinical methods. With limited published studies it is very much an emerging field rather than a mature area of research.
Specific examination of clinical results shows that model-based control can provide tighter, more adaptive control than existing clinical protocols – a one method fits all approach. A hypothesis generating approach to evaluating glycemic control in critical care is proposed utilizing standardized mortality ratios and 95th percentile standard error to enable better comparison across cohorts and control methods. Analysis indicates that tighter control may lead to better outcome. Given that tight control can be obtained consistently using frequent measurement and model-based methods argues for the greater application of these technologies in clinical research and practice. More specifically, these results all support the eventual use of adaptive model-based methods, instead of ad-hoc one size fits all approaches, to provide the customized, patient-specific and eventually automated intervention required to treat the highly dynamic, hyperglycemic patients found in broad intensive care cohorts.
Abbreviations
- MAGE
mean average glycemic excursion
- MPC
model predictive control
- ROD
risk of death
- SMR
standardized mortality ratio
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 2.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 3.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 4.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 5.Patino JF, de Pimiento SE, Vergara A, Savino P, Rodriguez M, Escallon J. Hypocaloric support in the critically ill. World J Surg. 1999;23:553–559. doi: 10.1007/pl00012346. [DOI] [PubMed] [Google Scholar]
- 6.Weissman C. Nutrition in the intensive care unit. Crit Care. 1999;3:R67–R75. doi: 10.1186/cc360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolfson AM. Control of blood glucose during nutritional support in ill patients. Intensive Care Med. 1980;7:11–14. doi: 10.1007/BF01692915. [DOI] [PubMed] [Google Scholar]
- 8.Ahrens CL, Barletta JF, Kanji S, Tyburski JG, Wilson RF, Janisse JJ, Devlin JW. Effect of low-calorie parenteral nutrition on the incidence and severity of hyperglycemia in surgical patients: a randomized, controlled trial. Crit Care Med. 2005;33:2507–2512. doi: 10.1097/01.ccm.0000186746.64572.8a. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Son E, Kim J, Choi K, Kim C, Shin W, Suh O. Association of hyperglycemia and markers of hepatic dysfunction with dextrose infusion rates in Korean patients receiving total parenteral nutrition. Am J Health Syst Pharm. 2003;60:1760–1766. doi: 10.1093/ajhp/60.17.1760. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124:297–305. doi: 10.1378/chest.124.1.297. [DOI] [PubMed] [Google Scholar]
- 11.Bistrian BR. Hyperglycemia and infection: which is the chicken and which is the egg? JPEN J Parenter Enteral Nutr. 2001;25:180–181. doi: 10.1177/0148607101025004180. [DOI] [PubMed] [Google Scholar]
- 12.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–756. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 13.Diringer MN. Improved outcome with aggressive treatment of Hyperglycemia: hype or hope? Neurology. 2005;64:1330–1331. doi: 10.1212/01.WNL.0000162348.81440.BD. [DOI] [PubMed] [Google Scholar]
- 14.Krinsley JS. Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol. Crit Care Med. 2003;31:A19. [Google Scholar]
- 15.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 16.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31:359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 18.Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest. 2006;129:644–650. doi: 10.1378/chest.129.3.644. [DOI] [PubMed] [Google Scholar]
- 19.Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis Of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med. 2006;34:612–616. doi: 10.1097/01.ccm.0000201408.15502.24. [DOI] [PubMed] [Google Scholar]
- 20.Thomas AN, Marchant AE, Ogden MC, Collin S. Implementation of a tight glycemic control protocol using a web-based insulin dose calculator. Anaesthesia. 2005;60:1093–1100. doi: 10.1111/j.1365-2044.2005.04375.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27:461–467. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 22.Laver S, Preston S, Turner D, McKinstry C, Padkin A. Implementing intensive insulin therapy: development and audit of the Bath insulin protocol. Anaesth Intensive Care. 2004;32:311–316. doi: 10.1177/0310057X0403200302. [DOI] [PubMed] [Google Scholar]
- 23.Lonergan T, Le Compte A, Willacy M, Chase JG, Shaw GM, Wong XW, Lotz T, Lin J, Hann CE. A simple insulin-nutrition protocol for tight glycemic control in critical illness: development and protocol comparison. Diabetes Technol Ther. 2006;8:191–206. doi: 10.1089/dia.2006.8.191. [DOI] [PubMed] [Google Scholar]
- 24.Wong XW, Chase JG, Shaw GM, Hann CE, Lin J, Lotz T. Comparison of adaptive and sliding-scale glycemic control in critical care and the impact of nutritional inputs. Proc of the 12th International Conf on Biomedical Engineering (ICBME 2005); Singapore. 2005. p. 4. [Google Scholar]
- 25.Chee F, Fernando T, van Heerden PV. Closed-loop control of blood glucose levels in critically ill patients. Anaesth Intensive Care. 2002;30:295–307. doi: 10.1177/0310057X0203000306. [DOI] [PubMed] [Google Scholar]
- 26.Chase JG, Lonergan T, LeCompte A, et al. Tight glucose control in critically ill patients using a specialized insulin-nutrition table. Proc of the 12th International Conf on Biomedical Engineering (ICBME 2005); Singapore. 2005. p. 4. [Google Scholar]
- 27.Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical in patients with diabetes mellitus. Arch Intern Med. 1997;157:545–552. [PubMed] [Google Scholar]
- 28.Kletter GG. Sliding scale fallacy. Arch Intern Med. 1998;158:1472. doi: 10.1001/archinte.158.13.1472. [DOI] [PubMed] [Google Scholar]
- 29.Radack HB. Sliding scale insulin use. Arch Intern Med. 1997;157:1776. [PubMed] [Google Scholar]
- 30.Sawin CT. Action without benefit. The sliding scale of insulin use. Arch Intern Med. 1997;157:489. doi: 10.1001/archinte.157.5.489. [DOI] [PubMed] [Google Scholar]
- 31.Chase JG, Shaw GM, Lin J, Doran CV, Hann C, Robertson MB, Browne PM, Lotz T, Wake GC, Broughton B. Adaptive bolus-based targeted glucose regulation of hyperglycemia in critical care. Med Eng Phys. 2005;27:1–11. doi: 10.1016/j.medengphy.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Chase JG, Shaw GM, Lin J, Doran CV, Hann C, Lotz T, Wake GC, Broughton B. Targeted glycemic reduction in critical care using closed loop control. Diabetes Technol Ther. 2005;7:274–282. doi: 10.1089/dia.2005.7.274. [DOI] [PubMed] [Google Scholar]
- 33.Chee F, Fernando TL, Savkin AV, van Heeden V. Expert PID control system for blood glucose control in critically ill patients. IEEE Trans Inf Technol Biomed. 2003;7:419–425. doi: 10.1109/titb.2003.821326. [DOI] [PubMed] [Google Scholar]
- 34.Blaha J, Hovorka R, Matias M, et al. Intensive insulin therapy in critically ill patients: comparison of standard and MPC protocols. Intensive Care Med. 2005;31:S203. [Google Scholar]
- 35.Plank J, Blaha J, Cordingley J, Wilinska ME, Chassin LJ, Morgan C, Squire S, Haluzik M, Kremen J, Svacina S, Toller W, Plasnik A, Ellmerer M, Hovorka R, Pieber TR. Multicentric, randomized, controlled trial to evaluate blood glucose control by the model predictive control algorithm versus routine glucose management protocols in intensive care unit patients. Diabetes Care. 2006;29:1987–1988. doi: 10.2337/dc06-0838. [DOI] [PubMed] [Google Scholar]
- 36.Doran CV. Mechanical Engineering. Christchurch, New Zealand: University of Canterbury; 2004. Modelling and control of hyperglycemia in critical care patients. [Google Scholar]
- 37.Basi S, Pupim LB, Simmons EM, Sezer MT, Shyr Y, Freedman S, Chertow GM, Mehta RL, Paganini E, Himmelfarb J, Ikizler TA. Insulin resistance in critically ill patients with acute renal failure. Am J Physiol Renal Physiol. 2005;289:F259–F264. doi: 10.1152/ajprenal.00002.2005. [DOI] [PubMed] [Google Scholar]
- 38.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 39.Lind L, Lithell H. Impaired glucose and lipid metabolism seen in intensive care patients is related to severity of illness and survival. Clin Intensive Care. 1994;5:100–115. [PubMed] [Google Scholar]
- 40.Christiansen C, Toft P, Jorgensen HS, Andersen SK, Tonnesen E. Hyperglycemia and mortality in critically ill patients. A prospective study. Intensive Care Med. 2004;30:1685–1688. doi: 10.1007/s00134-004-2325-2. [DOI] [PubMed] [Google Scholar]
- 41.Prigeon RL, Roder ME, Porte D, Jr, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest. 1996;97:501–507. doi: 10.1172/JCI118441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natali A, Gastaldelli A, Camastra S, Sironi AM, Toschi E, Masoni A, Ferrannini E, Mari A. Dose-response characteristics of insulin action on glucose metabolism: a non-steady-state approach. Am J Physiol Endocrinol Metab. 2000;278:E794–E801. doi: 10.1152/ajpendo.2000.278.5.E794. [DOI] [PubMed] [Google Scholar]
- 43.Chase JG, Shaw GM, Lin J, Doran CV, Hann C, Robertson MB, Browne PM, Lotz T, Wake GC, Broughton B. Impact of insulin-stimulated glucose removal saturation on dynamic modelling and control of hyperglycemia. Int J Intell Systems Technol Applications. (IJISTA) 2005;1:79–94. [Google Scholar]
- 44.Lonergan T, Compte AL, Willacy M, Chase JG, Shaw GM, Hann CE, Lotz T, Lin J, Wong XW. A pilot study of theSPRINT protocol for tight glycemic control in critically ill patients. Diabetes Technol Ther. 2006;8(4):449–462. doi: 10.1089/dia.2006.8.449. [DOI] [PubMed] [Google Scholar]
- 45.Dickerson RN. Hypocaloric feeding of obese patients in the intensive care unit. Curr Opin Clin Nutr Me Care. 2005;8:189–196. doi: 10.1097/00075197-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Dickerson RN, Boschert KJ, Kudsk KA, Brown RO. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition. 2002;18:241–246. doi: 10.1016/s0899-9007(01)00793-6. [DOI] [PubMed] [Google Scholar]
- 47.McCowen KC, Friel C, Sternberg J, Chan S, Forse RA, Burke PA, Bistrian BR. Hypocaloric total parenteral nutrition: effectiveness in prevention of hyperglycemia and infectious complications–a randomized clinical trial. Crit Care Med. 2000;28:3606–3611. doi: 10.1097/00003246-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Cerra FB, Benitez MR, Blackburn GL, Irwin RS, Jeejeebhoy K, Katz DP, Pingleton SK, Pomposelli J, Rombeau JL, Shronts E, Wolfe RR, Zaloga GP. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest. 1997;111:769–778. doi: 10.1378/chest.111.3.769. [DOI] [PubMed] [Google Scholar]
- 49.Waeschle R, Moerer O, Wahaha D, Neumann P, Quintel M. Intensive insulin therapy on ICU: comparison of two algorithms to control the blood glucose level. Intensive Care Med. 2005;31:S203. [Google Scholar]
- 50.Mackenzie I, Ingle S, Zaidi S, Buczaski S. Tight glycemic control: a survey of intensive care practice in large English hospitals. Intensive Care Med. 2005;31:1136. doi: 10.1007/s00134-005-2677-2. [DOI] [PubMed] [Google Scholar]
- 51.Bland DK, Fankhanel Y, Langford E, Lee M, Lee SW, Maloney C, Rogers M, Zimmerman G. Intensive versus modified conventional control of blood glucose level in medical intensive care patients: a pilot study. Am J Crit Care. 2005;14:370–376. [PubMed] [Google Scholar]
- 52.Schultz MJ, Spronk PE, Moeniralam HS. Tight glycemic control: a survey of intensive care practice in the Netherlands. Intensive Care Med. 2006;32(4):618–619. doi: 10.1007/s00134-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 53.Gale SC, Gracias VH. Glycemic control needs a standard reference point. Critical Care Med. 2006;34:1856–1857. doi: 10.1097/01.CCM.0000220201.72591.43. author reply 1857-8. [DOI] [PubMed] [Google Scholar]
- 54.Carson ER, Cobelli C. San Diego: Academic Press; 2001. Modeling methodology for physiology and medicine. [Google Scholar]
- 55.Chee F, Fernando T, van Heerden PV. Closed-loop glucose control in critically ill patients using continuous glucose monitoring system (CGMS) in real time. IEEE Trans Inf Technol Biomed. 2003;7:43–53. doi: 10.1109/titb.2003.808509. [DOI] [PubMed] [Google Scholar]
- 56.Wong XW, Chase JG, Shaw GM, Hann CE, Lotz T, Lin J, Singh-Levett I, Hollingsworth LJ, Wong OS, Andreassen S. Model predictive glycemic regulation in critical illness using insulin and nutrition input: a pilot study. Med Eng Physics. 2006;28(7):665–681. doi: 10.1016/j.medengphy.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Wong XW, Singh-Levett I, Hollingsworth LJ, Shaw GM, Hann CE, Lotz T, Lin J, Wong OS, Chase JG. A novel, model-based insulin and nutrition delivery controller for glycemic regulation in critically ill patients. Diabetes Technol Ther. 2006;8:174–190. doi: 10.1089/dia.2006.8.174. [DOI] [PubMed] [Google Scholar]
- 58.Chase J, Shaw GM, Wong XW, Lotz T, Lin J, Hann CE. Model-based glycemic control in critical care–a review of the state of the possible. Biomed Signal Process Control. 2006;1:3–21. [Google Scholar]
- 59.Chase J, Shaw G, Le Compte A. Tight glycemic control in critical care using insulin and nutrition–the SPRINT Protocol. In: Society DK-DT, et al., editors. Sixth Annual Diabetes Technology Meeting. Atlanta, GA: Diabetes Technology Society; 2006. [Google Scholar]
- 60.Vogelzang M, Zijlstra F, Nijsten MW. Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit. BMC Med Inform Decis Mak. 2005;5:10. doi: 10.1186/1472-6947-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chase JG, Wong XW, Shaw GM, Hann CE, Lin J, Lotz T. Clinical trials of active insulin and nutrition control in critically ill patients. Proc of the 12th International Conf on Biomedical Engineering (ICBME 2005); Singapore. 2005. p. 4. [Google Scholar]
- 62.Ledoux D, Finfer S, McKinley S. Impact of operator expertise on collection of the APACHE II score and on the derived risk of death and standardized mortality ratio. Anaesth Intensive Care. 2005;33:585–590. doi: 10.1177/0310057X0503300506. [DOI] [PubMed] [Google Scholar]
- 63.Sleigh JW, Brook RJ, Miller M. Time-dependent error in the APACHE II scoring system. Anaesth Intensive Care. 1992;20:63–65. doi: 10.1177/0310057X9202000112. [DOI] [PubMed] [Google Scholar]
- 64.Del Bufalo C, Morelli A, Bassein L, Fasano L, Quarta CC, Pacilli AM, Gunella G. Severity scores in respiratory intensive care: APACHE II predicted mortality better than SAPS II. Respir Care. 1995;40:1042–1047. [PubMed] [Google Scholar]
- 65.Gubern C, Lopez-Bermejo A, Biarnes J, Vendrell J, Ricart W, Fernandez-Real JM. Natural antibiotics and insulin sensitivity: the role of bactericidal/permeability-increasing protein. Diabetes. 2006;55:216–224. [PubMed] [Google Scholar]
- 66.Krogh-Madsen R, Moller K, Dela F, Kronborg G, Jauffred S, Pedersen BK. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2004;286:E766–E772. doi: 10.1152/ajpendo.00468.2003. [DOI] [PubMed] [Google Scholar]
- 67.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Real JM, Broch M, Richart C, Vendrell J, Lopez-Bermejo A, Ricart W. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol. Metab. 2003;88:1780–1784. doi: 10.1210/jc.2002-020173. [DOI] [PubMed] [Google Scholar]
- 69.Lin Y, Kohn FR, Kung AH, Ammons WS. Protective effect of a recombinant fragment of bactericidal/permeability increasing protein against carbohydrate dyshomeostasis and tumor necrosis factor-alpha elevation in rat endotoxemia. Biochem Pharmacol. 1994;47:1553–1559. doi: 10.1016/0006-2952(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 70.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c independent risk factor for diabetic complications. JAMA. 2006;295:1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 71.Hirsch IB. Glycemic variability: it's not just about A1C anymore! Diabetes Technol Ther. 2005;7:780–783. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 72.Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–181. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 74.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29:1486–1490. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 75.Service FJ, Hall LD, Westland RE, O'Brien PC, Go VL, Haymond MW, Rizza RA. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia. 1983;25:316–321. doi: 10.1007/BF00253193. [DOI] [PubMed] [Google Scholar]
- 76.Shaw GM, Chase JG, Lee D, Bloomfield M, Doran CV, Lin J, Lotz T. Peak and range of blood glucose are also associated with ICU mortality. Crit Care Med. 2005;32:A125. [Google Scholar]
- 77.Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7(6):849–862. doi: 10.1089/dia.2005.7.849. [DOI] [PubMed] [Google Scholar]
- 78.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]


