Introduction
Glucose monitoring technology has evolved significantly since the early years of urine glucose testing. Although today's continuous glucose monitoring (CGM) devices show great promise for improving diabetes management, we are only beginning to understand how to use this technology. This presentation discusses some proposed clinician strategies for utilizing CGM data with patients.
Impact of Glycemic Variability
There are two goals in effective diabetes therapy: 1) to safely achieve an A1C level that is as close to normal as possible; and 2) to reduce glycemic variability. Clearly, A1C is an important and widely recognized measure of diabetes control. However, a growing body of evidence strongly suggests that glucose variability, independent of A1C, may also play a significant role in the risk for complications.
During euglycemia, glucose inside the mitochondria reacts to generate adenosine triphosphate (ATP). However, when glucose concentrations become elevated, reactive oxygen species (ROS) are also generated, causing oxidative stress to cells. Superoxide, the most influential ROS, appears to be a key molecule responsible for activating several pathways (polyol, hexosamine, protein kinase C, and advanced glycation endproduct [AGE]) which have been linked to the development of diabetes complications.
One marker for oxidative stress that has recently become commonly used in the literature is 8-isoprostane PGF2 alpha, an indicator of free radical production derived from esterified aracadonic acid. In a recent study, Monnier and colleagues used urinary excretion rates of 8-isoprostane PGF2 alpha to assess the relative contributions of sustained hyperglycemia and acute glucose fluctuations to levels of oxidative stress in subjects with Type 2 diabetes.1 Results showed that glucose fluctuations, particularly during postprandial periods, exhibited a more specific activating effect on oxidative stress than sustained hyperglycemia.
Getting Started with CGM
Insulin-on-Board
Before initiating CGM, it is important that both clinicians and patients understand the concept of insulin-on-board; the difference between the pharmacokinetics and pharmacodynamics of insulin. Today's smart pumps can aid in calculating appropriate correction dosages and avoid insulin stacking.
Timing
Patients on insulin therapy must understand the timing issues in insulin treatment, such as lag times between bolusing and meals. Appropriate use of pramlintide must also be factored into a successful therapy. Another aspect that must be addressed is finding an appropriate method for matching food to insulin in order to minimize glycemic variability. CGM data should make it easier for clinicians and patients to handle these and other issues by providing detailed glycemic feedback about the efficacy of various treatment strategies.
Optimal Use of Insulin Pumps
Patients on insulin pumps must master several key points in the optimal use of pumps, such as the appropriate setting of basal rates. It is not uncommon for patients new to insulin pump therapy to receive 80% of their daily insulin as basal infusion. Just as important is learning how and when to use temporary and extended basal rates. CGM will help patients and clinicians better acquire these skills.
Monitoring
The current literature is sparse (at best) regarding glucose monitoring. However, the data which are available show a strong correlation between higher frequency of glucose testing and lower A1C.2,3 What we do not yet have are data relating frequency of testing to glycemic variability, mainly because the concept of glycemic variability is relatively new. Larger studies on this relationship must be done.
Lag Time
It is important for clinicians to make sure that their patients understand that data from CGM sensors will not always match up directly with blood glucose meter results, especially when glucose levels are changing rapidly. There is a lag time whose length will fluctuate depending on how rapidly and how frequently glucose levels are changing.
Trend vs Point-in-Time Data
The glucose trending data from CGM devices will become a new and important factor upon which insulin dosing is based. In the past, only static “point-in-time” glucose values were available to guide therapy decisions; there was no way of knowing whether glucose levels were rising, falling, or remaining stable.
Downloading the Data
Clinicians can only evaluate blood glucose profiles effectively by downloading CGM data. Even with traditional blood glucose meters, it is impossible to evaluate patients without knowing the time-specific averages and standard deviations of daily glucose values. The need to download data from the CGM sensors is even more critical given the amount of data generated. This puts the responsibility on sensor manufacturers to develop evaluation software that is comprehensive, yet simple and flexible enough to accommodate the needs of a variety of clinicians. Everyone has a different way of looking at data.
However, in addition to downloading the data themselves, clinicians also need to ask patients to keep written records of their glucose levels, insulin doses and adjustments, and other relevant information during the first week of sensor use.
It is important to review this information with patients to see how they are doing with the technology.
Pearls
Upward Trends
When glucose is trending upward, patients should not eat a meal when taking prandial insulin; eating a meal when the trend is greater than +1 mg/dL per minute will result in a significant postprandial spike. Therefore, if the glucose level is rising by 1-2 mg/dL per minute, patients should take their insulin and then wait until their glucose level stabilizes before eating their meal. Patient also taking pramlintide are excepted from this rule.
Another key aspect to consider in upward trending glucose is the importance of timing insulin in relation to food. One challenge for pump patients is learning how to effectively use today's insulin pump software, which does not take into account glucose trending and velocity. A good rule for patients to follow is that trending glucose trumps insulin-on-board. Clinicians need to teach patients how to override the software in order to address glucose trending as well as insulin-on-board.
Patients are using a variety of strategies to address upward trends, such as reducing their carbohydrate intake, using greater lag times between bolusing and eating, and using pramlintide with their insulin. Again, it is important to remember that the correction doses required for uptrending glucose must be determined by trial and error; insulin pump software cannot calculate these doses. Figure 1 presents examples of insulin additions based on insulin-sensitivity factor and upward trending glucose velocity. However, these examples are simply a starting point; patients must be consistent with their own needed correction doses. Again, the process requires trial and error.
Figure 1.
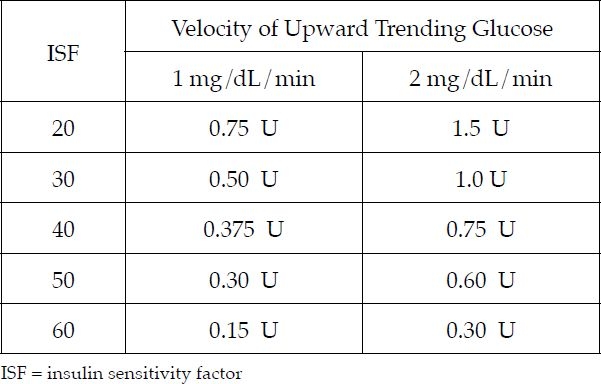
Addressing uptrending glucose
Although there has been little or no clinical discussion of velocities of glucose movement, all of the CGM systems will eventually provide this information. It is important that various manufacturers standardize the way this information is displayed and reported.
Downward Trends
If the glucose trend is downward, patients should be advised not to snack until their glucose levels approach the low end of the target value. An exception to this is if the trend is going down fast, >2 mg/dL/min. In this case, a snack can be consumed in the upper end of the target phase; sophisticated patients learn how to do this on their own.
As presented in Figure 2, if the glucose is <70 mg/dL before a meal, but not trending down sharply, we recommend that our patients decrease their prandial bolus insulin by 25-50% and perhaps add some carbohydrate. We also recommend this if the glucose level is 70-90 mg/dL but trending down at >1 to <2 mg/dL/min or when glucose is 91-110 mg/dL but trending down sharply at >2mg/dL/min. Dose adjusting is really a matter of matching up the current glucose level with the velocity of downward change; the greater the velocity, the earlier or more aggressively patients need to act.
Figure 2.
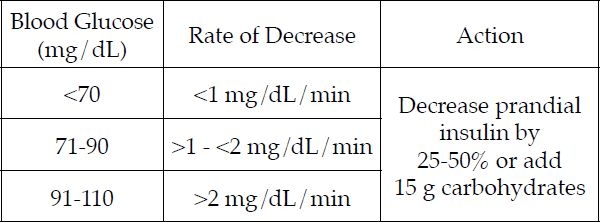
Addressing downtrending glucose
Recommendations
The most important predictor of success using CGM is the frequency of sensor checking. Patients cannot optimize their control if they only look at their sensors when the alarm sounds. Once the alarm has sounded they have already missed their opportunity to stop the glycemic excursion that they are experiencing.
In addition, we recommend that the sensor be put in place in the morning. This allows adequate time during the day for calibration and troubleshooting before bedtime; patients are not very happy when the sensor alarm is sounding throughout the night.
The following are some additional recommendations for using CGM effectively:
It is important to select appropriate patients for CGM; not all patients are good candidates. Although there are no defined criteria for patient selection, a key factor is a good understanding of how to use insulin effectively.
Clinicians need to pay particular attention to patients using CGM; they require more time and attention than other patients.
Patients and clinicians alike must stay calm when watching CGM trends. Learning to identify and respond to trends is a process of trial and error that will take time to master.
Prepare patients for “sticker shock”. The cost of CGM technology is currently quite high because there is no reimbursement in place. However, given our current technology for managing Type 1 diabetes, I believe the cost-benefit for CGM will be positive when considering the benefits of improving glycemic variability.
It is important to remember that there is a lag time between interstitial glucose and blood glucose during periods of steep up trends and down trends. This is an issue with all CGM systems. Although we are getting close, CGM cannot yet replace SMBG; we still need both.
Remember that even for patients using CGM not all days are good; surprises occur frequently, resulting in bad results. Patients need to understand this. CGM is not yet a stand-alone technology; patients still require SMBG. Although today's CGM devices are better than what we had before, they are not perfect. The key is to accept this at the beginning and continue to look at the big picture: how much better patients can do
Conclusions
A growing body of evidence strongly supports glycemic variability as an important measure of diabetes control. CGM offers tremendous potential to positively impact glycemic variability. However, several issues will become clear immediately after CGM is introduced. First, one cannot utilize this tool effectively without a good understanding of insulin therapy. Next, with the incorporation of CGM it will no longer be useful to consider blood glucose readings as only stagnant numbers; we must develop algorithms that include “glycemic trending”. These algorithms will vary based on insulin dose, Type of food recently eaten, insulin-on-board, and exercise. The challenge will be to make these algorithms comprehensive, yet simple enough that a majority of patients can use CGM technology.
Disclosures
Dr. Hirsch is a recipient of a research grant from Medtronic MiniMed and is a consultant with Abbott Diabetes Care.
Abbreviations
- AGE
advanced glycation endproduct
- ATP
adenosine triphosphate
- CGM
continuous glucose monitoring
- ROS
reactive oxygen species
References
- 1.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 2.Davidson P, Hebblewhite H, Bode B, Steed RD. Increased frequency of self blood glucose monitoring improves A1C in non-insulin-using persons with diabetes. Diabetes. 2004;53(Suppl 2):A101. [Google Scholar]
- 3.Karter AJ, Ackerson LM, Darbinian JA, D'Agostino RB, Jr, Ferrara A, Liu J, Selby JV. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]


