Abstract
Background
Insulin treated diabetic patients often do not adjust their insulin doses. We developed a method to provide a quantitative and qualitative assessment of this behavior.
Methods
Fourteen patients provided logbook pages of their self-monitoring of blood glucose (SMBG) data and insulin doses. We compared the actual decisions of patients in real-life to what they would decide on the same SMBG, as an a posteriori exercise. We also compared these decisions and those proposed by 6 diabetologists on the same sets of data to the recommendations made by HumaLink, an automated insulin dosage system.
Results
1) Patients in real-life modified their insulin doses least often. However, given a chance to make these decisions a posteriori, they modified their insulin doses more often. HumaLink proposed changes even more often, and diabetologists were the most aggressive in changing insulin doses. 2) The decisions proposed by the patients in real-life or a posteriori and by the diabetologists were compared to the recommendations made by HumaLink, using a decisions analysis grid (DAG). For these three groups, full disagreement with HumaLink (patient or physician increases while HumaLink decreases and the opposite) was observed for less than 5% of the cases. 3) By comparison to HumaLink, patient decisions seemed guided by the desire to avoid hypoglycemia. By contrast, decisions by diabetologists seemed often to be guided by the desire to avoid hyperglycemia.
Conclusion
These methods provide an objective evaluation of insulin dose adjustments by patients with diabetes and may be useful to assess the effectiveness of educational programs.
Keywords: adherence, decision analysis grid, HumaLink, hypoglycemia, insulin adjustment
Introduction
Independent insulin dose adjustment by diabetic patients on the basis of self-monitoring of blood glucose (SMBG) could represent an important tool in insulin therapy. To these ends, in the DCCT study, patients in the intensive group were asked to have monthly contact with the medical team and weekly phone contact with the nurse to discuss glycemic results and receive decision support in regards to insulin dose adjustment.1 However, these ideal conditions are not currently met in the daily life of insulin treated diabetic patients. Even if they do monitor blood glucose (BG) at the recommended frequency, they often do not adjust their insulin doses.2,3 This may explain in part the etiology of the poor diabetic control prevailing in a number of patients. Avoiding dose adjustment while SMBG is high may be due to a lack of technical knowledge concerning insulin dose adjustment. However, this is unlikely, since clinical experience indicates that pregnant diabetic women start adjusting their insulin doses, often without any need for additional instruction. In a recent study, the lack of insulin adjustment was shown due to two main factors, a fear of hypoglycemia and a fear of weight gain.4
The aim of this study was to design a method to objectively evaluate insulin dose adjustments by insulin treated, diabetic patients. First, we compared their actual decisions in real-life to what they would decide if they were given the chance, on the same SMBG data, to decide merely as a theoretical exercise. We also assessed proposals made by diabetologists on the same sets of data. Secondly, in order to provide a qualitative assessment of these decisions, we compared these decisions to the recommendations which would be proposed by an automated system, HumaLink5,6,7 designed to follow standard, clinical algorithms for insulin dose adjustment.
Subjects and Methods
Subjects
To be eligible, patients had to be treated with a basal-bolus insulin regimen using glargine (Lantus®) and either Lispro (Humalog®) or Aspart (Novorapid®) ultrarapid insulin, had to measure blood glucose at least four times per day, and had to have a diabetes duration of more than one year. They were treated by the same diabetologist (GR), and had received a standard educational program. More specifically, when glargine was introduced in their treatment in 2003, they were provided with a one-page form describing rules for insulin dose adjustment in a basal-bolus insulin regimen, indicating that the glargine dose had to be determined on the basis of the fasting blood glucose value, and that the ultrarapid insulin dose had to be decided on the basis of both the pre-prandial and postprandial blood glucose values. Since the study consisted only in a retrospective analysis of logbooks, without any potential consequence on the patient health, this study was not submitted for Institutional Review Board (IRB) approval. Furthermore, the patients were not informed before the study that their logbooks would be investigated, ruling out an effect of the study on their behavior.
Fourteen type 1 diabetic patients, 6 female and 8 male, participated in this study. The mean ± SD age was 32.8 ± 7.9 years and the duration of diabetes was 19.4 ± 6.7 years. The HbA1c (glycated hemoglobin) was 7.4 ± 0.9%. No one patient had experienced severe hypoglycemia within the year preceding the study and no one presented with severe diabetes complications.
Study design
Patients agreed to send us copies of at least 6 pages of their logbook, each page recording 5 consecutive days of data with at least 4 SMBG measurements per day and the doses of insulin injected, before each meal (ultrarapid insulin) and at bedtime (glargine). From each of these pages, we generated a spreadsheet file including SMBG target, which was 100 mg/dl for all patients before meals, and 120 mg/dl at bedtime for all patients; the insulin doses considered by the patient as optimal to reach these targets; the real SMBG values recorded in the logbook; and hypoglycemic episodes, if any. The resulting 86 sheets, which were anonymized, were actually identical to the pages of the logbook, except that the doses of insulin actually administered had been masked.
We sent back these 86 masked pages to each of the diabetic patients, asking them, as an exercise, to propose insulin doses for all 86 files. The pages were also presented to 6 diabetologists working in the same department, who proposed an insulin dose for each of the 1690 BG measurements. Finally, the data were processed by HumaLink, which similarly proposed recommendations.
HumaLink™
HumaLink is a central, shared resource (Diabetes Data Center hosted by BCMC Better Control Medical Computers, Inc., Hollywood, FL). It can offer computer assisted decision support, including algorithms for insulin dose adjustment. The HumaLink algorithms8,9 are customizable. These can be individually programmed by the physician for each particular patient. Programming requires physicians to specify (1) four pre-meal targets, (2) a glucose sensitivity factor and, (3) maxima for each prescribed type of insulin dose. Instructions in the event of diabetes glucose extremes are standardized (hyperglycemia or hypoglycemia). HumaLink is accessible via the Web or the keypad of any touch-tone telephone. In the present study, SMBG values were entered retrospectively by direct access to the computer program.
Data analysis
In order to provide a quantitative description, insulin adjustments were simply categorized into “no change” [0], “increase” [+] or “decrease” [-], vs. the amount of insulin that had been chosen by the patient to be optimal to reach the target. This first part of the analysis made it possible to determine the respective percentages of decisions corresponding to these three categories (made by the patients in real-life, the patients a posteriori as a theoretical exercise, and the diabetologists), and by HumaLink. Comparisons of the respective percentages of decisions were done using the chi-squared test, and pairwise comparisons were also performed in order to assess the difference between pairs of groups. To take into account the multiplicity of the tests, we applied the Bonferonni correction to the test levels.
In addition, we used the recommendations made by HumaLink in order to evaluate in a normative way the decisions made by the patients (in real-life or a posteriori) and by the diabetologists. For that, we designed a simple DAG comparing these decisions to those made, for the same situations, by HumaLink (Figure 1). The diagonal of the DAG indicates full agreement between the patient or the diabetologist and HumaLink (A1, A2, A3), the top right (C1), and bottom left corners (C2) indicate full disagreement, and the other cells can be referred to as neutral (B1, B2, B3, B4). Two-sided Student's tests were used to analyse the data of the DAG observed in the diabetic patients (in real-life and a posteriori) and in the diabetologists. To take into account the heterogeneity of the variances induced by the difference of the size of the populations, we used the Satterthwaite approximation to compute the degrees of freedom. HbA1c testing corresponding to the period of the study, was determined by a HPLC method certified to be conform to the DCCT standards.
Figure 1.
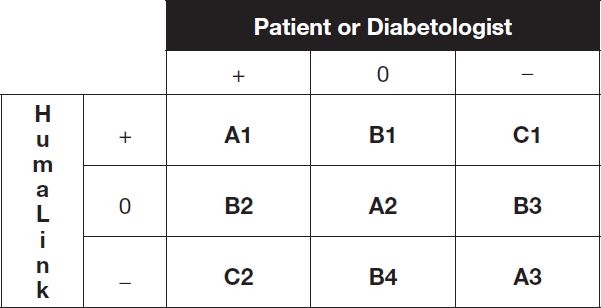
Decision Analysis Grid : [+]=increase, [0]=no change and [-]=decrease insulin dose vs. the insulin dose considered by the patient to be optimal to reach the target. Cells A1, A2, A3 indicate full agreement ([++], [00], [–]); cells C1, C2 indicate full disagreement ([+-], [-+]); cells B1, B2, B3, B4 are referred as neutral ([+0], [0+], [0-], [-0]).
Results
Figure 2 represents the distributions of the three kinds of decisions (no change [0], increase [+], or decrease [-]) made by patients in real-life, patients a posteriori, HumaLink and diabetologists, respectively. Patients in real-life were found to modify their insulin doses least often (only 30% of decisions to [+] or [-] changes). However, given a chance to make these decisions a posteriori, they changed doses more often (45%). HumaLink proposed changes even more often (49%), but diabetologists were most aggressive in the rate of changing insulin doses (62%). The chi-squared test comparing the distributions of decisions in the four groups was significant (test=1081, df=6, p<0.00001). Paired comparisons were also performed in order to assess the difference between patients in real-life, patients a posteriori, HumaLink and diabetologist. These comparisons were all significant (p<0.00001).
Figure 2.
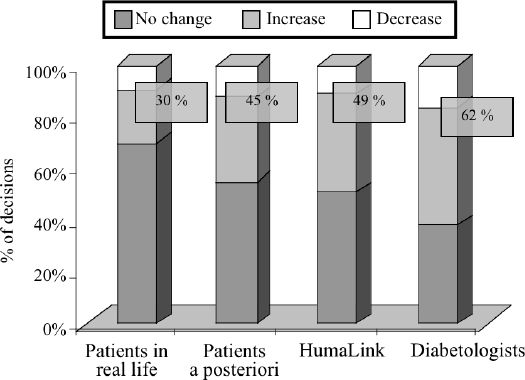
Distribution of the decisions made by the 14 patients in real-life, the 14 patients a posteriori, HumaLink and the 6 diabetologists, as a no-change, an increase or a decrease of the insulin doses. All paired comparisons are highly significant (p<0.00001). Data are presented as means. Numbers inside the boxes refer to the percentages of change.
Figure 3 compares the decisions proposed by the diabetologists, the patients a posteriori, and the patients in real-life to the recommendations made by HumaLink, using the DAG shown on Figure 1. Both for diabetologists, patients a posteriori and in real-life, full disagreement with HumaLink was observed for less than 5% of the decisions. Agreement was observed for approximately 60% of the cases, the lowest case being for the patients in real-life. More precisely, for decisions made by the 6 diabetologists, the range of disagreement was 2.4% to 6.2% of the decisions, with only one diabetologist having a score of full disagreement higher than 5%. The range of full agreement was 56.9% to 70.2%. For the 14 patients a posteriori, the range of full disagreement was 1.4% to 4.6% of the decisions, and the range of full agreement was 48.9% to 72.1% with only one patient having a score below 60%. For the 14 patients in real-life, the range of full disagreement and full agreement were larger, being 0 to 7.3%, and 41.0% to 77.5%, respectively.
Figure 3.
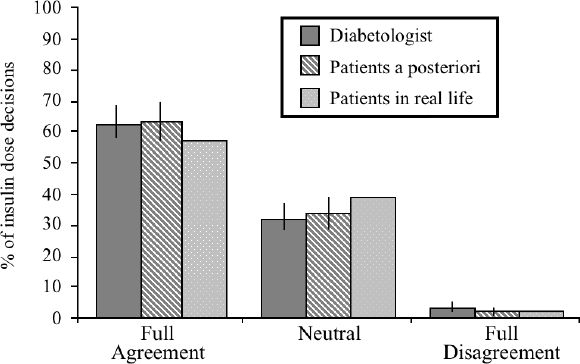
Categorization of the percentages of insulin dose decisions into full agreement, neutral, full disagreement, according to the Decision Analysis Grid. Data are presented as mean and SD. For the comparison between the decisions made by HumaLink on one hand, and the diabetologists or the patients a posteriori on the other hand, the SD bar represents the variability of the data observed when considering the 6 diabetologists or the 14 patients, respectively. For the comparison between HumaLink® and the patients in real-life, we present the percentages, among the 1690 decisions, of full agreement, full disagreement, or neutral comparisons, explaining the absence of SD bar.
In a further analysis of non-agreement between the patients and the diabetologists on one hand, and HumaLink on the other hand (cells B and C of the DAG), we evaluated the percentage of decisions present in cells B1, C1 and B3, i.e. the right-top corner of the grid. This corresponds to the decisions where patients or diabetologists avoid an increase in the insulin dose, or even to decrease it, while HumaLink would have proposed an “increase” or a “no-change.” Figure 4 represents the percentage (B1+C1+B3) / [(B1+C1+B3) + (B2+C2+B4)] determined for the 14 patients in real-life, the 14 patients a posteriori and for the 6 diabetologists. The index was significantly higher than 50% for the patients, especially in real-life (p<0.005). By contrast, this index was significantly lower than 50% for the diabetologists (p<0.05). Moreover, the index obtained for the diabetologists was significantly different by comparison to patients in real-life (p<0.0001) and to the patients a posteriori (p=0.002). There was no significant difference between patients in real-life and patients a posteriori.
Figure 4.
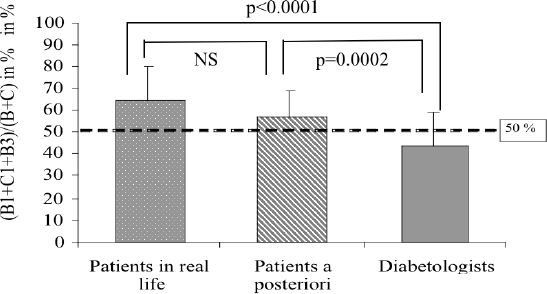
Index defined as the ratio (B1+C1+B3) / (B+C) for patients in real-life, patients a posteriori and diabetologists. For patients, this index is significantly higher than 50% (p<0.005 and p<0.0001, respectively). For the diabetologists, this index is significantly lower than 50% (p<0.05). Data are presented as mean and SD.
Discussion
Many patients do not adjust their doses of insulin while their SMBG levels are high, explaining in part their high level of glycated hemoglobin. This avoidance of adjustment of the insulin doses is often noticed while the other tasks of their treatment, for example insulin injections, SMBG, and even the recording of the data in a diabetes logbook, are accomplished correctly.2,3 First, this study confirmed that in the real-life, patients indeed modify their insulin doses less often than if they were given a chance to make these decisions a posteriori, suggesting that this behavior is not due to a lack of knowledge. Deciding on the same sets of data, diabetologists were even more aggressive in the rate of changing insulin doses (Figure 2).
In order to make it possible to evaluate in a normative way decisions made by patients or diabetologists, we used as an objective reference the recommendations made by HumaLink; a computer program designed to propose insulin adjustment on the basis of standard algorithms. We devised a simple 9-cell DAG, making it readily possible to categorize the decisions made by the patients or the diabetologists versus the recommendations made by HumaLink into “full agreement,” “full disagreement,” and “neutral.” It is important to keep in mind that this trichotomization was qualitative, and not quantitative, and did not take into account the magnitude of the proposed changes. A quantitative assessment would be difficult to set up, as the magnitude of the changes would have to take into account the absolute insulin dose.
First, we demonstrated that the rate of full disagreement between HumaLink on one hand, and the patients and the diabetologists on the other hand was almost always lower than 5% (Figure 3); on the basis of this rather low rate of full disagreement, computed on a very high number of decisions, and for 14 different patients and 6 diabetologists, we felt that it was appropriate to use proposals made by HumaLink as a standard in the second part of this analysis.
Focusing on discordance between HumaLink and the patients on the one hand and the diabetologists on the other, we realized that the specific cells of DAG are not symmetrical when comparing the decisions made by the patients or the diabetologists to the recommendations proposed by HumaLink. Indeed, the 3 cells placed at the right top corner represent behaviors where patients avoid increasing the insulin dose, or decrease it, while HumaLink would have proposed an increase or a “no-change.” This may actually represent a trend to cautious behavior vis-à-vis the risk of hypoglycemia. Data shown on Figure 4 indicate that the percentage of the top right corner cells of the grid was higher than 50% for the patients, both concerning their behavior both in real-life and when they had to find the solution as a theoretical exercise. This may suggest that the fear of hypoglycemia may represent a significant deterrent of patient decisions. In this regard, hypoglycemia has been described as the complication that patients fear most,10,11 and the risk of severe hypoglycemia represents a major limit to diabetes self management.12,13 The opposite figure was observed for the diabetologists, who may be more concerned by the risks related to hyperglycemia. Interestingly, this phenomenon was observed in patients who did not have a history either of frequent severe hypoglycemia, or of severe diabetic complications, ie a context often observed during the first decades of the disease.
This index, which may thus be an index of the cautiousness vis-à-vis the risk of hypoglycemia, determined from the data obtained in patients in real-life, was found to be significantly correlated to HbA1c (r=0.523, p<0.05, data not shown). While the number of subjects investigated was small, this may suggest that such behavior of the patient represents a risk for poor metabolic control, and thus, for the development of long term complications.
In conclusion, this study should be considered as a pilot study, aimed to demonstrate the feasibility of assessing patient behavior concerning insulin dose adjustment. Thus, this paper proposes a method for the objective assessment of this behavior, both quantitatively, determining the respective percentages of increase, decrease, and no-change in the insulin doses, and qualitatively, comparing their decisions to a reference standard. This method may prove to be useful to assess the efficacy of educational programs.
Acknowledgements
This work was presented in abstract form to meetings in 2005: European Association for the Study of Diabetes (EASD), Artificial Insulin Delivery Pancreas and Islet Transplantation (AIDPIT : a study group of the EASD), Association de Langue Française pour l'Etude du Diabète et des Maladies métaboliques (ALFEDIAM), and International Study Group on Innovative Insulin Delivery device (ISGIID).
Abbreviations
- SMBG
self-monitoring of blood glucose
- DCCT
diabetes control and complications trial; BG: blood glucose
- DAG
decisions analysis grid
- HbA1c
glycated hemoglobin
- IRB
Institutional Review Board
References
- 1.Diabetes Control and Complications Trial Research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Lombrail P, Obadia G, Thibult N, Eschwege E, Passa P. Lack of benefit of blood glucose autosurveillance in insulin-treated diabetics routinely followed up in a department specializing in diabetology. Presse Med. 1986;15:1909–1912. [PubMed] [Google Scholar]
- 3.Toljamo M, Hentinen M. Adherence to self-care and glycaemic control among people with insulin-dependent diabetes mellitus. J Adv Nurs. 2001;34:780–786. doi: 10.1046/j.1365-2648.2001.01808.x. [DOI] [PubMed] [Google Scholar]
- 4.Reach G, Zerrouki A, Leclerc D, d'Ivernois JF. Adjustment of insulin doses: from knowledge to decision. Patient Educ Counsel. 2005;56:98–103. doi: 10.1016/j.pec.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Albisser AM, Harris RI, Sakkal S, Parson ID, Chao SC. Diabetes intervention in the information age. Med Inf (Lond) 1996;21:297–316. doi: 10.3109/14639239608999291. [DOI] [PubMed] [Google Scholar]
- 6.Albisser AM, Harris RI, Albisser JB, Sperlich M. The impact of initiatives in education, self-management training, and computer-assisted self-care on outcomes in diabetes disease management. Diabetes Technol Ther. 2001;3:571–579. doi: 10.1089/15209150152811199. [DOI] [PubMed] [Google Scholar]
- 7.Meneghini LF, Albisser AM, Goldberg RB, Mintz DH. An electronic case manager for diabetes control. Diabetes Care. 1998;21:591–596. doi: 10.2337/diacare.21.4.591. [DOI] [PubMed] [Google Scholar]
- 8.Albisser AM, Schiffrin A, Schulz M, Tiran J, Leibel BS. Insulin dosage adjustment using manual methods and computer algorithms: a comparative study. Med Biol Eng Comput. 1986;24:577–584. doi: 10.1007/BF02446259. [DOI] [PubMed] [Google Scholar]
- 9.Albisser AM. Intelligent instrumentation in diabetic management. Crit Rev Biomed Eng. 1989;17:1–24. [PubMed] [Google Scholar]
- 10.McCrimmon RJ, Frier BM. Hypoglycemia, the most feared complication of insulin therapy. Diabete Metab. 1994;20:503–512. [PubMed] [Google Scholar]
- 11.Nordfeldt S, Ludvigsson J. Fear and other disturbances of severe hypoglycemia in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:83–91. doi: 10.1515/jpem.2005.18.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Cryer P. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Me Res Rev. 1999;15:42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Tupola S, Rajantie J, Akerblom HK. Experience of severe hypoglycemia may influence both patient's and physician's subsequent treatment policy of insulin-dependent diabetes mellitus. Eur J Pediatr. 1998;157:625–627. doi: 10.1007/s004310050899. [DOI] [PubMed] [Google Scholar]


