Abstract
Background
Clinical trials are increasingly being designed to collect data directly from patients through the use of paper diaries or electronic diaries (e-diaries). E-diaries can be advantageous over paper diaries, but actual benefits may depend on the particular features of a given e-diary. The objective of this study was to determine which e-diary system features are most important to consider when selecting such a tool.
Methods
A 42-question survey was distributed to 295 site coordinators participating in four diabetes clinical trials, using e-diary systems provided by three different vendors. The survey gathered information about the site coordinators' experience with the e-diary system used. Analyses included a comparison of global satisfaction rating scores and individual survey item responses among the different e-diaries utilized and correlation of individual survey items with the global satisfaction rating for each system.
Results
The survey was completed by 131 site coordinators (44% response rate). Mean global satisfaction rating scores varied from 3.4 to 7.2 (p < 0.0001). Individual survey items such as technical problems that cannot be resolved easily, the ability of the help line to fully address problems, and organization and user-friendliness of the e-diary's data management Web site were most closely correlated with the global satisfaction rating. The site coordinators' prior e-diary experience and years of job experience did not significantly correlate with the global satisfaction rating.
Conclusions
This survey highlights features of e-diaries for sponsors to consider in clinical trials, including the importance of minimizing technical problems, assessing vendors' help line capabilities, and choosing an e-diary system with an efficient and user-friendly data-management Web site.
Keywords: clinical trials, diabetes, electronic diary, patient diary
Introduction
Clinical trials in the field of diabetes management (as well as other therapeutic areas) are increasingly being designed to include data collected directly from patients and require the use of some form of diary, either paper or electronic (e-diaries). These diaries are designed to capture various aspects of the patient's therapeutic experience, including frequency and severity of disease symptoms, treatment outcomes, and impact of the disease and/or treatment on everyday activities and overall quality of life.1 In particular, patients with diabetes are often asked to track numerous parameters of disease management, such as blood glucose levels, dietary intake, and activity levels.2
Possible advantages of e-diaries versus traditional paper diaries include the potential for improved patient compliance and reductions in practices such as parking-lot compliance(filling in the entire diary immediately prior to the study visit) or forward filling (entering data prior to the scheduled time).1,3–5 In a study of 84 adults with chronic pain, Stone and colleagues4 used a paper diary system equipped with a mechanism to electronically record the time and date that the diary binder was actually opened and closed. The authors observed that patients submitted paper entries reported as occurring within 15 minutes of assigned times with 90% compliance, whereas the actual (electronically recorded) compliance was only 11%. Overall, 75 to 80% of the times and dates of paper diary entries in this study were shown to be falsified.4 E-diaries have built-in mechanisms for addressing these types of behaviors, such as time stamping of all entries and automatic alarm reminders.2 In the study by Stone and colleagues, actual compliance with an e-diary system was 94%.4
The use of e-diaries has also been shown to reduce missing data values in questionnaires. For example, in a study of 60 children aged 8 to 16 years with recurrent pain, e-diaries were significantly more accurate than paper diaries (100% versus 51% of diaries were returned with no errors or omissions, p < 0.001).3 In a 1-year e-diary validation study (N = 36 patients with chronic low back pain), patients using an e-diary demonstrated nearly 90% compliance with daily monitoring, entering data on average 6.75 times per week. Correlations with paper diary entries, phone reports, and other measures indicated that data from e-diaries were both reliable and valid.5 In addition, e-diaries may allow speedier and more accurate handling of data, including faster time to analysis and reduced data management workload.2,5
Some of these benefits, however, may depend on the particular type of e-diary selected for a clinical trial. The e-diary systems offered by different vendors can vary in a number of ways: the type of palm device provided to patients, the form and quality of technical support available, the frequency of technical problems that arise during routine use, the Web site for storing and retrieving uploaded patient data, and the overall user-friendliness of the e-diary tools provided by the vendor. Because of these important differences, a survey was conducted involving clinical site coordinators who had participated in several different diabetes clinical trials utilizing different e-diary systems for data collection. The objectives of this study were the following: to identify features of e-diary systems that are important to consider in the design of future diabetes clinical trials, particularly those that simplify use; to understand how to improve clinical trial management with e-diaries; and to assess possible effects of e-diary utilization on clinical trial factors such as patient enrollment and patient training.
Methods
A total of four diabetes clinical trials were identified that used e-diary systems provided by three different vendors (henceforth referred to as systems A, B, and C). Each e-diary system was contained on a personal digital assistant (PDA) device on which all other features had been disabled. Patients could either input data manually or send information electronically from their glucose meters. Data from each PDA device were transmitted to a central database for later analysis via phone or through a docking station. A survey containing 42 questions and estimated to require 15 to 20 minutes to complete was distributed via the Internet to site coordinators participating in these four clinical trials. Survey questions covered areas such as the site coordinators' characteristics, e-diary design and features, and the experience of the site coordinators with any e-diary technical issues. All of the survey questions were close ended and of the 42 total questions, 5 pertained to background characteristics and 37 involved rating the e-diary system. For example, the individual items of the survey consisted of questions such as “How long have you been a study coordinator?” (0–2 years, 2–4 years, or 5+ years), whether “The e-diary ran out of memory so that data could not be stored on the device” (often, sometimes, seldom, or never), and if “The text on the screen of the e-diary was easy-to-read for elderly patients” (strongly agree, agree, disagree, strongly disagree, or N/A). The complete survey may be found in the Appendix.
Clinical Trials
The four clinical trials included in this survey of e-diary technology are ongoing (currently unpublished) phase 3 or phase 4 studies with differing patient populations, study designs, and treatment regimens for type 2 diabetes mellitus. The e-diary system used in each clinical trial is tailored to the parameters being evaluated in each study. For example, the e-diary used for one trial may collect data on diabetes treatment satisfaction, whereas the e-diary used for another trial may collect data on the amount of daily physical activity in diabetes patients. Thus, each e-diary is designed to capture the most relevant data for a given clinical trial. It is important to note, however, that all of these trials included adults aged 18 to 65 years with type 2 diabetes mellitus who had failed to reach glycemic goals with oral antidiabetic medications alone and were initiated on insulin therapy. So as to more easily compare among the different e-diaries, this analysis was confined to e-diaries used only in clinical trials for diabetes, and therefore have some very important parameters in common. For instance, all of the e-diaries examined in this study collected data pertaining to patient blood glucose levels, insulin dose and titration, food intake, quality of life, and adverse events, particularly the number of hypoglycemic events.
Survey Development and Distribution
Initially, interviews and focus groups were conducted with site coordinators, study managers, and e-diary vendors to determine which issues related to e-diary use were most important to address and to solicit ideas for questionnaire development. Specifically, the survey addressed the following topics: the background experience of the site coordinator; the technical capabilities of the e-diary system used (e.g., memory capacity, ease of data upload, and ease of Web site navigation); the overall design and features of the system; overall satisfaction with the system; and perceived utility of the e-diary in clinical studies. A draft survey was then circulated to study managers and data managers to elicit feedback, after which a finalized version was distributed to 295 site coordinators from four diabetes clinical trials. Thus, while study managers and data managers, who oversee the clinical trial as a whole across all study sites, provided feedback on the content of the draft survey, site coordinators, who are responsible for the conduct of the study at the local level, completed the final survey. To be eligible to participate, site coordinators must have had experience with e-diary utilization in at least one diabetes clinical trial. Site coordinators who had e-diary experience in more than one trial were asked to complete a separate questionnaire for each trial. Surveys were distributed via the Internet using Zoomerang.com and site coordinators were given one month to complete the online survey.
Survey Analysis
The final survey consisted of 42 individual items organized into six different sections entitled background information, e-diary technical issues, e-diary design and features, study parameters, global rating, and general considerations. Site coordinators were asked to provide a global satisfaction rating of the e-diary technology: “On a scale from 1 (lowest) to 10 (highest), how would you rate your overall experience with this e-diary?” Survey analysis consisted of calculating correlations between individual items and the global satisfaction rating to determine which e-diary features were most closely associated with site coordinators' overall e-diary experience. An analysis was also performed to assess differences among the three e-diaries in global satisfaction rating, background experience of site coordinators with each system, responses to technical issues with the e-diary, and questions regarding the user-friendliness of the e-diary data-management Web site. Differences were then compared among the different e-diary systems for the individual survey items, particularly items pertaining to the usefulness and accuracy of the e-diary—in the opinion of site coordinators—as a tool to capture specific types of data (i.e., quality of life, food intake, blood glucose levels, dose titration of insulin, and drug utilization). These comparisons were conducted using analysis of variance and the Kruskal–Wallis (nonparametric) test.
Results
The survey was distributed to 295 site coordinators and completed by 131, for an overall response rate of 44%. There were no meaningful differences in study coordinator background characteristics. Individual survey items with the highest correlation with the e-diary global satisfaction rating are listed in Table 1. In general, the highest correlations were related to (1) technical problems not easily resolved (i.e., those requiring a call to a help line, requiring a new device, or resulting in loss of data that the patient had input), (2) the organization and ease of navigating through the e-diary Web site, and (3) the ability of the help line service to answer questions and fully resolve any problems. In addition, the site coordinator's belief that the e-diary was a good tool to capture quality-of-life and food intake data also correlated highly with the global satisfaction rating. In contrast, items that were not significantly correlated with the global satisfaction rating were the site coordinators' experience with e-diary use in previous studies, the number of years of job experience as a site coordinator, and different age groups willing to participate in a clinical trial utilizing an e-diary (i.e., use of an e-diary had no impact on recruitment of elderly patients).
Table 1.
Questions with the Highest Correlation with the Global Satisfaction Ratinga
| Questiona | Correlation value (R) |
|---|---|
| Lack of problems that required a call to help line | 0.665 |
| Well-organized e-diary Web site (i.e. tables, text, and language) | 0.639 |
| Lack of technical problems requiring patient to receive a replacement e-diary | 0.624 |
| Site coordinators belief that “beaming” technology increased patient technology | 0.606 |
| Frequency that e-diary help line answered all questions and/or resolved all concerns for a particular problem for the SC | 0.605 |
| Site coordinators belief that e-diary is a good tool to capture QOL data | 0.598 |
| Site coordinators belief that e-diary collects QOL data accurately | 0.596 |
| Avoidance of loss of patient-inputted data due to memory problems on the device | 0.595 |
| Site coordinators belief that e-diary is a good tool to capture data on food intake | 0.582 |
| Easiness of printing uploaded data from the e-diary Web site for the SC | 0.575 |
SC, site coordinator; QOL, quality of life.
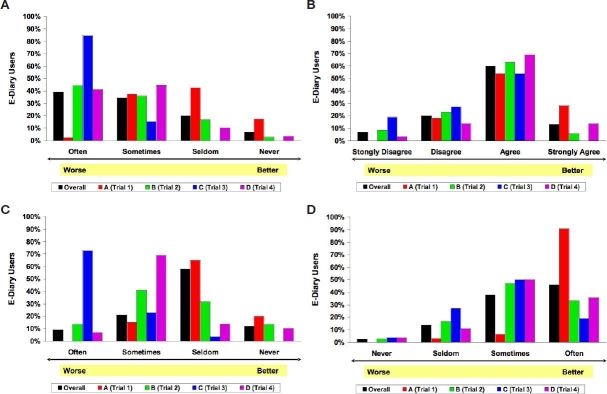
Figure 1 shows responses to several individual e-diary survey questions that were most highly correlated with e-diary global satisfaction rating. These responses are shown for overall survey respondents (N = 131) and were broken down according to each specific e-diary system (system A, trial 1, n = 40; system B, trial 2, n = 36; system C, trial 3, n = 26; system C, trial 4, n = 29). There were considerable differences among responses for the various e-diary systems. Therefore, the three e-diary systems were compared using the Kruskal–Wallis test to identify significant differences in mean global satisfaction rating and responses to individual survey items (Table 2). Mean global satisfaction rating varied from a high score of 7.2 (1–10 scale) for system A to a low score of 3.4 for system C, trial 3 (p < 0.0001). As expected, given the correlations to global satisfaction rating, system A also received the best responses regarding frequency of technical problems, ability of the help line to address those problems, and user-friendliness of the Web site. Furthermore, system C as used in trial 3, which received the lowest global satisfaction rating, also had the lowest scores on individual survey items pertaining to the frequency with which there were problems that required a call to the help line, the number of technical problems that required a replacement e-diary, and the ease with which data uploaded to the central data management Web site could be printed.
Figure 1.
Responses to individual e-diary survey questions that were correlated most highly with e-diary global satisfaction rating, shown for overall survey respondents (N = 131) and broken down according to specific e-diary vendors (system A, trial 1, n = 40; system B, trial 2, n = 36; system C, trial 3, n = 26; system C, trial 4, n = 29). Questions for each panel were as follow. (A) How often were there problems with the e-diary that would require a call to the e-diary help line? (B) Were tables, text, and language well organized on the e-diary Web site? (C) How often did technical problems occur that required the patient to receive a replacement e-diary? (D) How often did the help line answer all your questions for a particular problem?
Table 2.
Site Coordinators' Comparison of Responses to Survey Items by Systems (A, B, and C) and Trials (1, 2, 3, and 4) (Kruskal-Wallis Test) Questiona
| Questiona | System A, trial (mean) | System B, trial 2 (mean) | System C, trial 3 (mean) | System C, trial 4 (mean) | P |
|---|---|---|---|---|---|
| Global score for e-diary | 7.2 | 5.5 | 3.4 | 5.3 | <0.0001 |
| 1=worst, 10=best | |||||
| Problems required a call to the help line | 2.8 | 1.8 | 1.2 | 1.8 | <0.0001 |
| 1=often; 2=sometimes; 3=seldom; 4=never | |||||
| Tables, text, and language on Website were well organized | 3.1 | 2.7 | 2.3 | 2.9 | 0.0011 |
| 1=strongly disagree; 2=disagree; 3=agree; 4=strongly agree | |||||
| Technical problems required a replacement e-diary | 3.5 | 2.9 | 1.3 | 2.3 | <0.0001 |
| 1=often; 2=sometimes; 3=seldom; 4=never | |||||
| Help line answered all questions/resolved all concerns for a particular problem | 3.9 | 3.1 | 2.8 | 3.2 | <0.0001 |
| 1=never; 2=seldom; 3=sometimes; 4=often | |||||
| Loss of inputted data due to memory problems | 3.5 | 3.2 | 2.6 | 3.1 | <0.0001 |
| 1=often; 2=sometimes; 3=seldom; 4=never | |||||
| Help line responded in a timely manner | 3.8 | 3.4 | 2.9 | 3.3 | <0.0001 |
| 1=never; 2=seldom; 3=sometimes; 4=often | |||||
| Difficulty navigating Web site | 3.4 | 2.9 | 3.0 | 3.0 | 0.0542 |
| 1=often; 2=sometimes; 3=seldom; 4=never | |||||
| Printing uploaded data from Web site was easy | 3.3 | 3.1 | 2.2 | 2.9 | <0.0001 |
| 1=strongly disagree; 2=disagree; 3=agree; 4=strongly agree |
Higher mean values translate into better outcomes for each question.
Conclusions
These results have a number of implications for the use of e-diary technology in collecting patient-reported data as part of clinical trials. First, a PDA device that works properly and is accompanied by high-quality technical support may be the most important feature of an e-diary system. Technical issues, such as problems with data transfer or with viewing data on the Web site, could potentially limit the utility of an e-diary system by making e-diaries appear to be more cumbersome than helpful. Accordingly, the results of this survey suggest that some key features of an appropriate system for use in a clinical trial include reducing the need for help line calls (e.g., through a more comprehensive user's manual), low potential for loss of patient data input, reduced number of technical problems that are difficult to resolve (such as those requiring the patient to receive a replacement PDA device), and reliable help line support able to fully address problems. In addition, the e-diary Web site is an essential tool for site coordinators in data management of a large clinical trial. Thus, any e-diary system under consideration should provide a well-organized Web site layout that enables easy navigation and patient data management.
A finding from the current survey was that the global satisfaction ratings differed significantly among the three e-diary systems. Furthermore, differences among the systems were observed for a number of the issues discussed earlier. Another finding was that the global satisfaction rating did not significantly correlate with the patient age group participating in the trial, suggesting that young and elderly patients alike are willing to use the e-diary system. Importantly, because survey items such as previous e-diary experience or years of job experience did not correlate with the global satisfaction rating, differences among the three systems were not because of site coordinator background characteristics. Thus, the e-diary system chosen for a clinical trial should be selected with care.
While this survey provided valuable insight, it also had inherent limitations. One major limitation of this survey was the fact that the different e-diary systems under investigation were utilized differently in the four clinical trials employing them (i.e., not all parameters were assessed across studies). This difference in utilization may have influenced the differences seen in individual ratings. For example, although the same e-diary was used in trials 3 and 4 (system C), mean global satisfaction ratings (3.4 and 5.3, respectively) and individual survey item ratings were substantially lower in trial 3 than in trial 4. Therefore, it is possible that the manner in which the e-diary was utilized in trial 3, rather than the e-diary itself, may account for the low ratings. This limitation was taken into account during the design of the current analysis and, anticipating that the variation in the type of data collected would increase substantially if surveys were sent to site coordinators in clinical trials in other therapeutic areas (pain, asthma, etc.), the analysis was limited to diabetes studies.
Another potential limitation of this survey is the relatively low response rate of 44%. This was not entirely unexpected, given the length of the survey (requiring 15 to 20 minutes to complete), for very busy site coordinators. However, because no information is available on the background characteristics of the full sample versus those who responded, responder bias cannot be ruled out.
Despite these limitations, this survey of site coordinators clearly highlights issues that need to be considered in selecting an e-diary for use in clinical trials, including the importance of minimizing technical issues with devices, assessing a vendor's help line capabilities, and choosing an e-diary system with an efficient and user-friendly Web site. Additional research will help to further clarify which types of data are best suited for e-diary collection in diabetes trials (e.g., blood glucose, hypoglycemic events, food intake, activity levels, and quality of life) or any other specific therapeutic area.
Acknowledgements
The authors acknowledge Louise Traylor and Carol Havens for their contributions to the preparation and review of this manuscript. Editorial support was provided by the sanofi-aventis U.S. Group.
Abbreviations
- e-diary
electronic diary
- PDA
personal digital assistant
Appendix: E-diaries Survey
We have used several e-diaries in different metabolism clinical trials. We would like to ask for your cooperation at this time to recall your knowledge and experience regarding the most recent e-diary you have worked with. Please take a few moments to share your experiences with us as well as your thoughts on the patients' perspectives. Your feedback will be highly valuable to us in evaluating the uses of e-diaries for future clinical studies.
In answering each question, please put a check mark (√) in the box alongside the answer that best describes your experience.
1.0 Background Information
-
1. Please choose one sanofi-aventis e-diary for which you are completing this survey. Please check off the Protocol(s) Numbers(2) for the e-diary that you are basing your evaluation on.
[ ] System A [ ] System B [ ] System C
__ Trial 1 __ Trial 2 __ Trial 3 __ Trial 4
-
2. Have you had any experience with e-diaries in previous studies (prior to the study you are basing this evaluation on)?
[ ] Yes [ ] No
If yes, please specify: ____________________
-
3. How long have you been a study coordinator?
[ ] 0-2 years [ ] 2-4 years [ ] 5+ years
-
4. How much e-diary training does the average patient require for this particular e-diary?
[ ] 0-15 minutes [ ] 15-30 minutes
[ ] 30-45 minutes [ ] >45 minutes
-
5. Which of the following patient age was the most willing to sign onto the clinical trial knowing that the e-diary will be used as a method for data collection?
[ ] 18-30 [ ] 31-45
[ ] 46-60 [ ] 60 and older
2.0 E-Diary Technical Issues
-
6. The E-diaries ran out of memory so that data could not be stored on the device.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never
-
7. E-diaries lost patient-inputted data due to memory problems on the device.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never
-
8. There were problems with the e-diary that would require a call to the E-Diary Helpline.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never
-
9. The E-Diary Helpline answered all your questions and/or resolved all your concerns for your particular problem.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never [ ] N/A
-
10. Patients could not upload data due to lack of required equipment in order to perform the upload (i.e. LAN-line, phone, wires, etc.).
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never
-
11. You had a difficult time getting access to the E-diary Website when trying to view uploaded patient data.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never
-
12. The E-Diary User Manuals (either paper or online training courses) were helpful in answering your questions and/or resolved your concerns.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never [ ] N/A
-
13. The E-diary software gave error messages that required a call to the Helpline.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never [ ] N/A
-
14. The E-diary Helpline responded to your call in a timely manner.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never [ ] N/A
-
15. Technical problems that occurred in the E-diary required the patient to receive a replacement E-diary.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never [ ] N/A
-
16. From your PC, you found it difficult to navigate through the E-diary Website.
[ ] Often [ ] Sometimes
[ ] Seldom [ ] Never [ ] N/A
-
17. Tables, text, and language on the E-diary Website were well-organized.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
18. Printing uploaded data from the E-diary Website was easy.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
19. The E-diary software was easy to install for patients.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
3.0 Design and Features
-
20. The text on the screen of the E-diary was easy-to-read for elderly patients.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
21. The initial patient training was adequate for the patient to understand how to navigate through the E-diary.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
-
22. The capability of E-diary to receive data directly from the patient's glucometer through “beaming” technology increased patient compliance to the E-diary.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
23. The alarms/reminders on the E-diary increased patient compliance to the E-diary.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
24. Patients found the alarms on the E-diary a nuisance.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
4.0 Study Parameters
-
25. Patients were compliant to inputting blood glucose data from their glucometer into the E-diary.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
26. Patients were compliant to inputting data regarding food intake into the E-diary.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
27. Patients were compliant to finishing PRO/QOL surveys on the E-diaries.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
28. Patients should be prompted to input data on severe hypoglycemic events in order to improve the reporting of such events.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
5.0 Global Rating
-
29. On a scale from 1 (Lowest) to 10 (Highest) how would you rate your experience with this e-diary overall.
1 2 3 4 5 6 7 8 9 10
Lowest Highest
6.0 General Considerations
The E-diary is a good tool to use in clinical studies in order to do the following:
-
30. Assess patient drug utilization.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
31. Capture Quality of Life data.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
32. Capture severe hypoglycemic events.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
33. Assess dose titration of insulin.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
34. Capture blood glucose levels.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
35. Capture data on food intake.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A The E-diary accurately collects the following:
-
36. Patient drug utilization.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
37. Quality of Life data.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
38. Severe hypoglycemic events.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
39. Dose titration of insulin.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
40. Blood glucose levels.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
41. Data on food intake.
[ ] Strongly Agree [ ] Agree
[ ] Disagree [ ] Strongly Disagree
[ ] N/A
-
42. In addition to the areas cited above, would you recommend the use of the E-diary for anything else in additional future clinical studies?
Please specify: ____________________________________
References
- 1.Lauritsen K, Degl' Innocenti A, Hendel L, Praest J, Lytje MF, Clemmensen-Rotne K, Wiklund I. Symptom recording in a randomised clinical trial: paper diaries vs. electronic or telephone data capture. Control Clin Trials. 2004 Dec;25(6):585–597. doi: 10.1016/j.cct.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Kerkenbush NL, Lasome CE. The emerging role of electronic diaries in the management of diabetes mellitus. AACN Clin Issues. 2003 Aug;14(3):371–378. doi: 10.1097/00044067-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain. 2004 Feb;107(3):213–219. doi: 10.1016/j.pain.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003 Apr;24(2):182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 5.Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pa1-year validation study. Pain. 2001 Apr;91(3):277–285. doi: 10.1016/S0304-3959(00)00450-4. [DOI] [PubMed] [Google Scholar]