Abstract
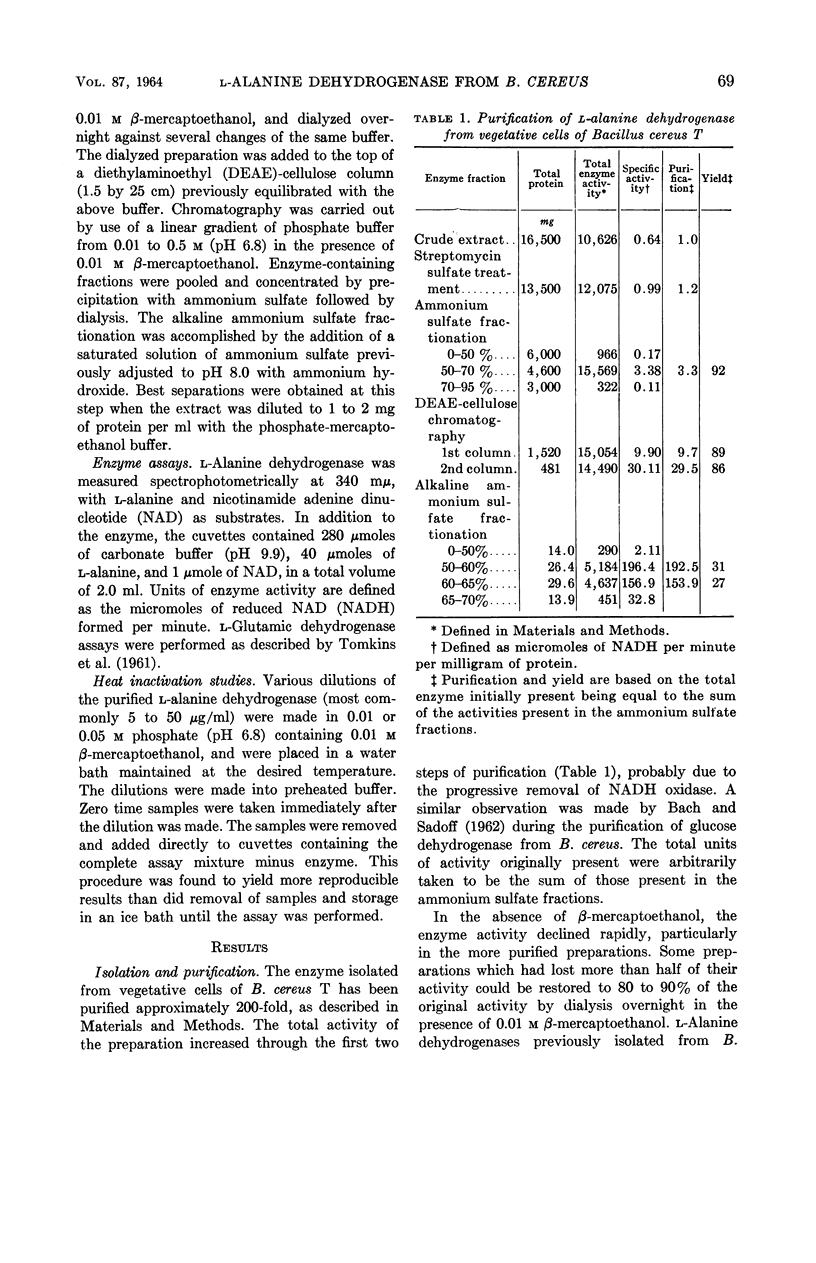
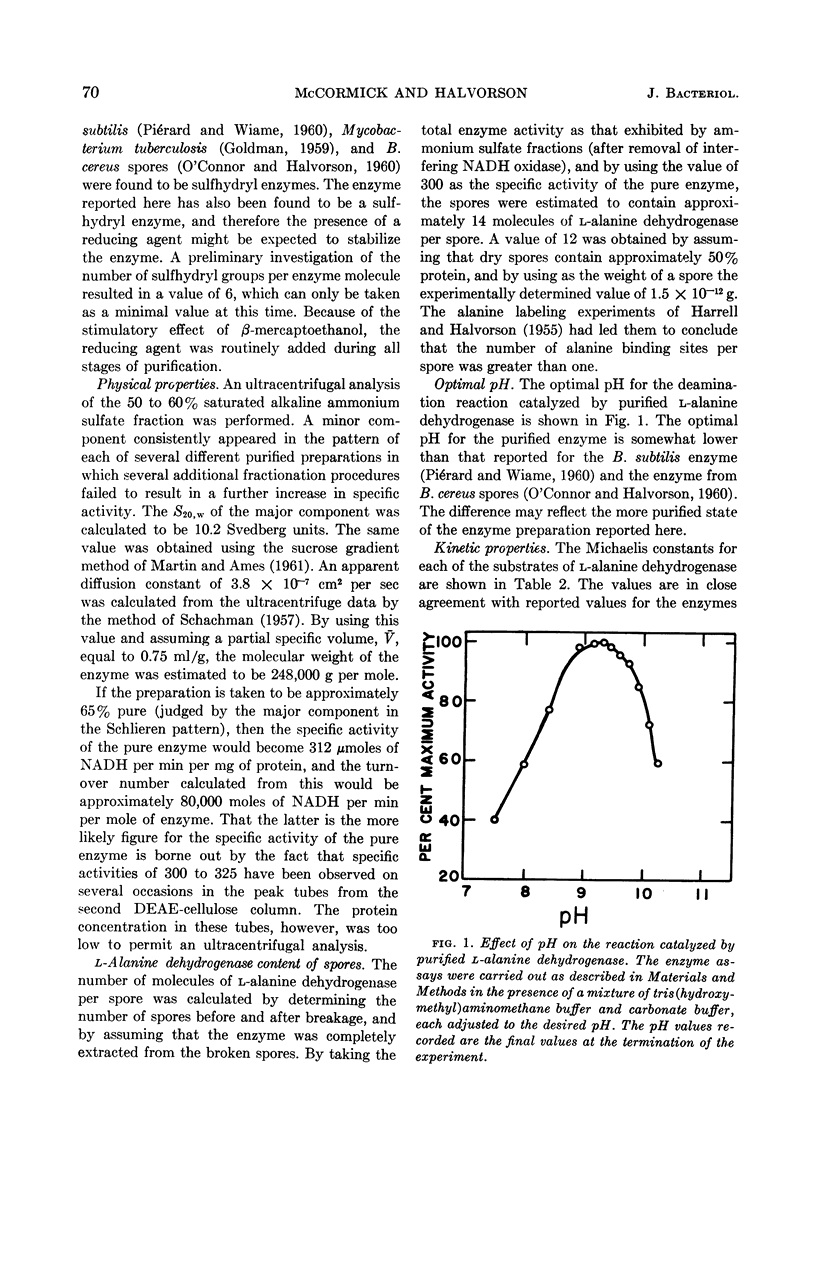
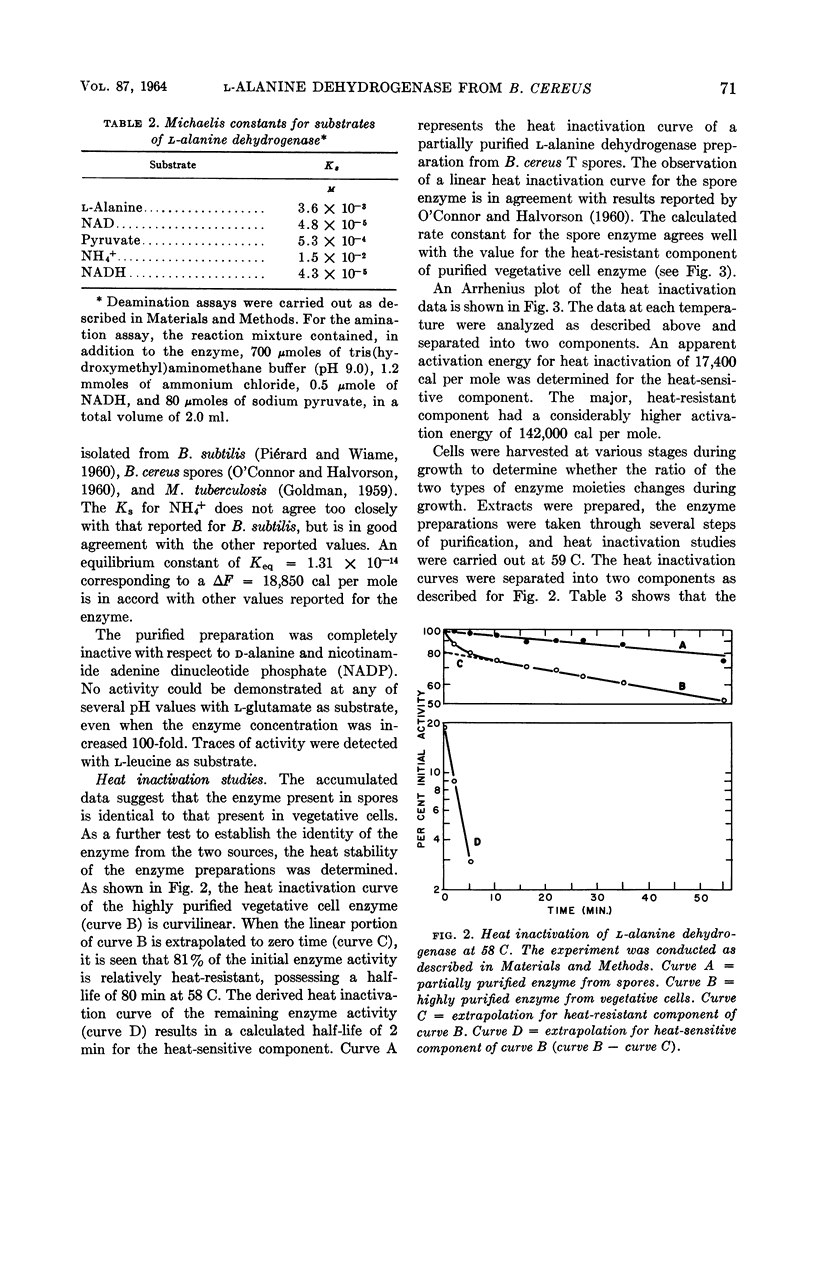
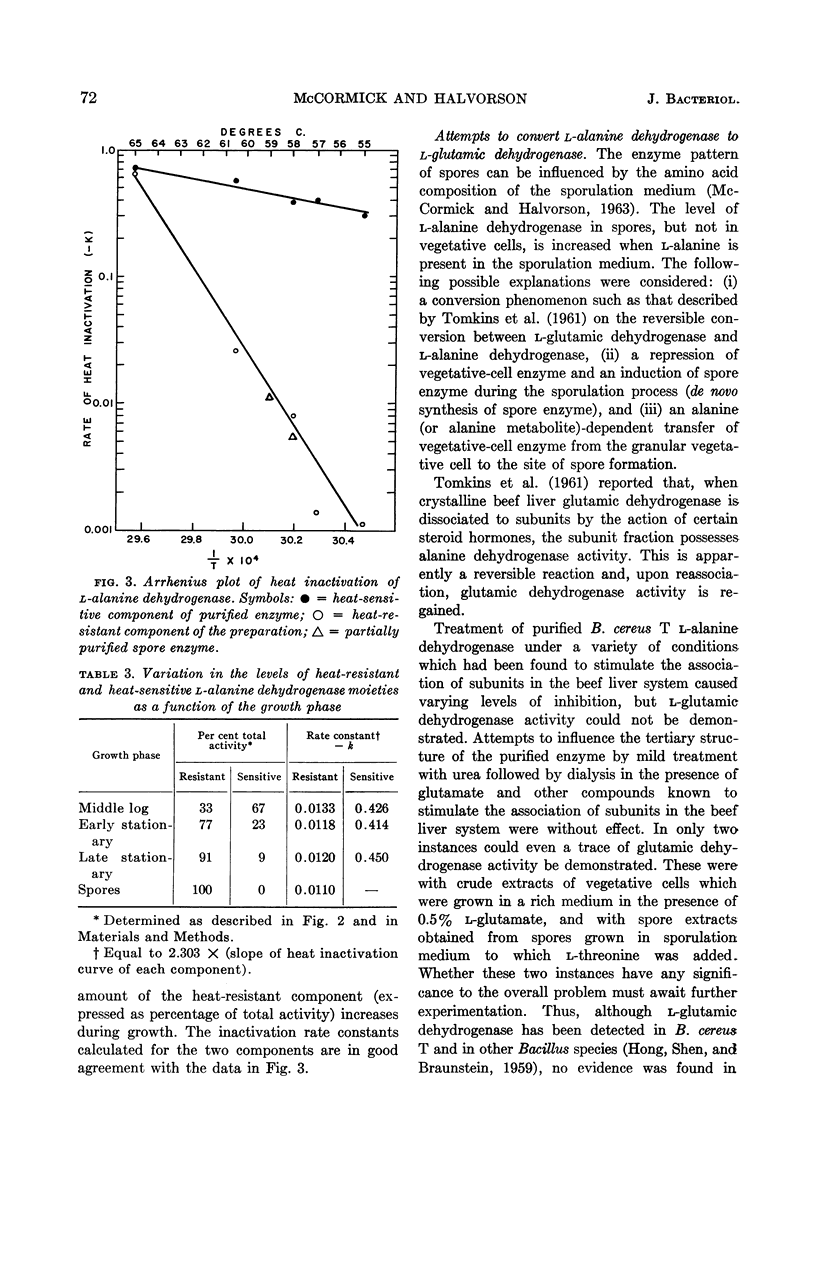
McCormick, Neil G. (University of Wisconsin, Madison), and Harlyn O. Halvorson. Purification and properties of l-alanine dehydrogenase from vegetative cells of Bacillus cereus. J. Bacteriol. 87:68–74. 1964.—The l-alanine dehydrogenase from vegetative cells of Bacillus cereus strain T has been purified approximately 200-fold. The enzyme has a molecular weight of 248,000 and a turnover number of 80,000 moles of substrate per min per mole of enzyme. The Michaelis constants for the substrates and the equilibrium constant for the reaction catalyzed by this enzyme are in close agreement with reported values for other l-alanine dehydrogenases. The kinetic properties of the enzyme purified from vegetative cells are identical to those of the enzyme isolated from spores of the same organism, but differ with respect to relative heat stability. Whereas spores contain a heat-resistant enzyme, vegetative cells contain, in addition, a heat-sensitive enzyme. No evidence was found to support the hypothesis that a molecular conversion type of phenomenon plays a role in the appearance of spore enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN D. S. Enzyme systems in the mycobacteria. VII. Purification, properties and mechanism of action of the alanine dehydrogenase. Biochim Biophys Acta. 1959 Aug;34:527–539. doi: 10.1016/0006-3002(59)90305-1. [DOI] [PubMed] [Google Scholar]
- HARRELL W. K., HALVORSON H. Studies on the role of L-alanine in the germination of spores of Bacillus terminalis. J Bacteriol. 1955 Mar;69(3):275–279. doi: 10.1128/jb.69.3.275-279.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG M. M., SHEN S. C., BRAUNSTEIN A. E. Distribution of L-alanine dehydrogenase and L-glutamate dehydrogenase in Bacilli. Biochim Biophys Acta. 1959 Nov;36:288–289. doi: 10.1016/0006-3002(59)90111-8. [DOI] [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- O'CONNOR R. J., HALVORSON H. O. Intermediate metabolism of aerobic spores. V. The purification and properties of L-alanine dehydrogenase. Arch Biochem Biophys. 1960 Dec;91:290–299. doi: 10.1016/0003-9861(60)90503-8. [DOI] [PubMed] [Google Scholar]
- PIERARD A., WIAME J. M. [Properties of L(d)-alanine dehydrogenase]. Biochim Biophys Acta. 1960 Jan 29;37:490–502. doi: 10.1016/0006-3002(60)90506-0. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Concurrent regulation of glutamic acid dehydrogenases of Neurospora. Arch Biochem Biophys. 1962 Jun;97:582–588. doi: 10.1016/0003-9861(62)90127-3. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., YIELDING K. L., CURRAN J. Steroid hormone activation of L-alanine oxidation catalyzed by a subunit of crystalline glutamic dehydrogenase. Proc Natl Acad Sci U S A. 1961 Mar 15;47:270–278. doi: 10.1073/pnas.47.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIELDING K. L., TOMKINS G. M. An effect of L-leucine and other essential amino acids on the structure and activity of glutamic dehydrogenase. Proc Natl Acad Sci U S A. 1961 Jul 15;47:983–989. doi: 10.1073/pnas.47.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]


