Abstract
Blood lactate concentration ([La−]b) is one of the most often measured parameters during clinical exercise testing as well as during performance testing of athletes. While an elevated [La−]b may be indicative of ischemia or hypoxemia, it may also be a “normal” physiological response to exertion. In response to “all-out” maximal exertion lasting 30-120 seconds, peak [La−]b values of ≈15–25 mM may be observed 3–8 minutes postexercise. In response to progressive, incremental exercise, [La−]b increases gradually at first and then more rapidly as the exercise becomes more intense. The work rate beyond which [La−]b increases exponentially [the lactate threshold (LT)] is a better predictor of performance than and is a better indicator of exercise intensity than heart rate; thus LT (and other valid methods of describing this curvilinear [La−]b response with a single point) is useful in prescribing exercise intensities for most diseased and nondiseased patients alike. H+-monocarboxylate cotransporters provide the primary of three routes by which La− transport proceeds across the sarcolemma and red blood cell membrane. At rest and during most exercise conditions, whole blood [La−] values are on average 70% of the corresponding plasma [La−] values; thus when analyzing [La−]b', care should be taken to both (1) validate the [La−]b-measuring instrument with the criterion/reference enzymatic method and (2) interpret the results correctly based on what is being measured (plasma or whole blood). Overall, it is advantageous for clinicians to have a thorough understanding of [La−]b responses, blood La− transport and distribution, and [La−]b analysis.
Keywords: lactate analyzers, lactate threshold, maximal lactate, onset of blood lactate accumulation, plasma, whole blood
Introduction
Blood lactate concentration ([La−]b) is one of the most often measured parameters during clinical exercise testing as well as during performance testing of athletes. Because measurements of an elevated [La−]b may be used to evaluate an underlying pathology during a routine stress test (coronary artery disease, chronic airway obstruction, chronic renal failure, metabolic impairment),1–7 and also to prescribe appropriate exercise intensities for different patient populations,8–12 it has been recommended that [La−]b analysis be added to normal stress testing measurements.13 However, interpretation of [La−]b is complicated because (1) dramatic increases in [La−]b characterize a normal response to exercise if a patient exceeds the work rate at which La− can be removed from the blood as quickly as it enters the blood14,15 and (2) elevations of [La−]b are not necessarily indicative of either ischemia or hypoxemia.16
The current paradigm for La− metabolism is embodied in the cell-to-cell lactate shuttle, which was originated in 1984 by George Brooks.17 Since its introduction, this hypothesis has been repeatedly supported by studies using a wide variety of experimental approaches. It posits that La− formation, and its subsequent distribution throughout the body, is a major mechanism whereby the coordination of intermediary metabolism in different tissues, and different cells within those tissues, can be accomplished.16 The importance of La− as a carbohydrate fuel source is underscored by the fact that during moderate intensity exercise, blood La− flux may exceed blood glucose flux.18
Because clinicians may rely on [La−]b measurements in order to investigate underlying pathologies present in chronically fatigued, diseased, or otherwise compromised patients, it is imperative that clinicians understand the normal changes of both plasma and whole blood [La−] in response to various levels of exertion. Furthermore, care should be taken to validate the method of analyzing [La−]bwith the criterion/reference enzymatic assay.19 Therefore, the purpose of this article is to provide physicians and other clinicians with a concise yet thorough review of normal [La−]b changes and measurements in response to varying degrees of exertion.
Response to Progressive, Incremental Exercise
Perhaps most often studied during exercise testing is the response to progressive, incremental exercise in which the work rate is increased at regular intervals (typically, 1–4 minutes) until the subject reaches volitional exhaustion. In this type of exercise, [La−]b increases gradually at first and then more rapidly as the exercise becomes more intense (Figure 1). In 1930, Owles20 was the first to report this “threshold” effect when he measured his own [La−]b in response to walking at varying intensities.
Figure 1.
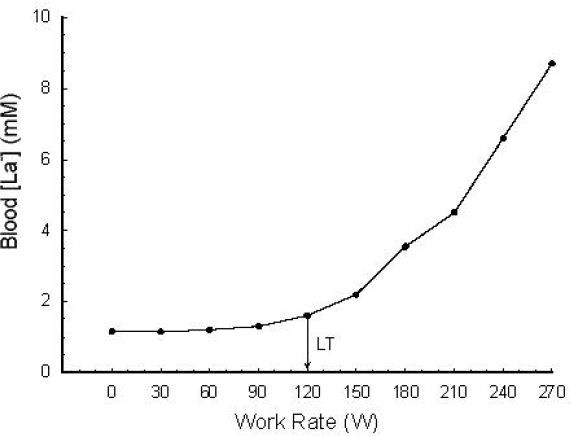
A typical [La−]b response to a progressive, incremental exercise test. LT represents the “lactate threshold.” These are whole blood measurements corrected for water content. Used (redrawn) with permission from Smith EW, Skelton MS, Kremer DE, Pascoe DD, Gladden LB. Lactate distribution in the blood during progressive exercise. Med Sci Sports Exerc. 1997 May;29(5):654-60.
Figure 1 illustrates the [La−]b response to a typical progressive, incremental exercise test protocol. Subjects performed unloaded pedaling at 60 rpm for 4 minutes on a standard laboratory cycle ergometer. Each subsequent minute, the work rate was increased by 30 W. Blood samples were taken via a forearm venous catheter during the last 30 seconds of each work rate and whole blood samples were analyzed using the criterion/reference enzymatic method.21 Warming each subject's hand with warm air and wrapping a heating pad around the subject's arm permitted sampling of “arterialized” blood (PO2 ≥70 mm Hg).22 Because venous blood drains from different tissue beds (some being net La− consumers; others being net La− producers), [La−]b differs depending on the site of venous sampling. Thus, an arterial sample is preferred. Due to difficulty with obtaining an arterial sample, “arterialized” blood is often used to approximate arterial [La−]b. “Arterialization” involves heating the hand in order to increase blood flow sufficiently to decrease the transit time of blood through the capillaries so that the venous concentration of La− is hardly changed from the arterial concentration. These “arterialized” samples provide [La−]b values that are representative of arterial values.22
A primary issue concerning data of this nature is determination of a single value that represents an attempt to describe the curvilinear [La−]b profile.23 Among the best known of these are the lactate threshold (LT), the Dmax method of determining the LT (LTD), and the onset of blood lactate accumulation (OBLA),23 although numerous other approaches exist.
The traditional determination of LT is shown in Figure 1. The LT is defined as the work rate or oxygen uptake beyond which the [La−]b increases more rapidly.24 With the “visual” inspection method, two or more investigators evaluate the graphical relationship between [La−]b and (or time or work rate) to determine the “breakpoint” for [La−]b. Several other methods are also widely used in the determination of LT. For example, the visual LT method may incorporate the use of a log-log transformation.25 The visual approach as applied to either the untransformed La− response or the log-log approach is often assisted by using a ruler to draw two lines of best fit to the data and then selecting the intersection of the two lines as the LT. Another refinement used on either raw data or log-log data involves the use of a computer program to determine all possible combinations of two straight line fits to the data; the two-line combination that minimizes the total sum of squares for fitting all data is then used to derive the LT (from the intersection of these two lines). The visual method (Figure 2A) and a log-log transformation (Figure 2B) are illustrated in Figure 2.
Figure 2.
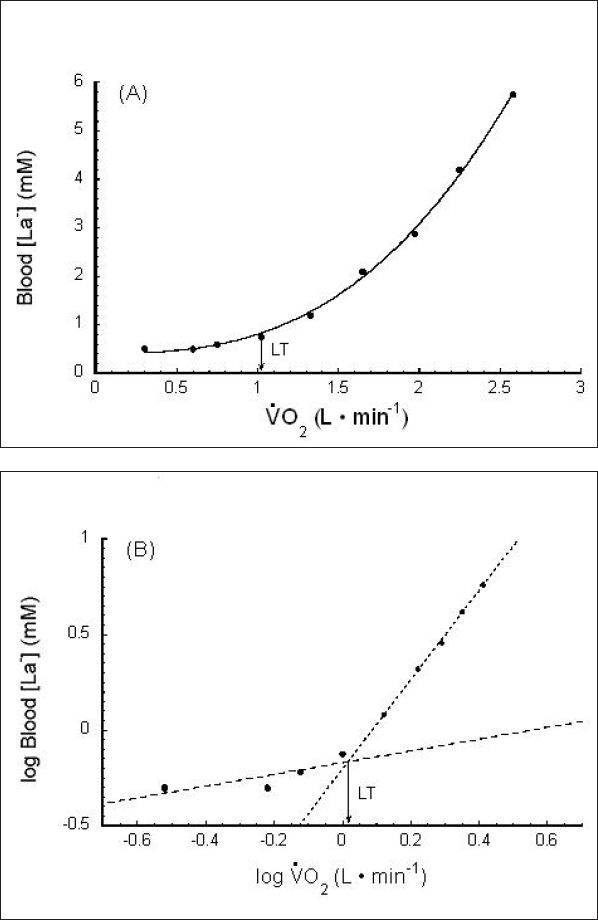
Lactate threshold (LT) measurement. (A) An example of the visual method using raw data and (B) utilizing a log-log transformation. uptake/consumption. is a linear function of work rate. Used (redrawn) with permission from Beaver WL, Wasserman K, Whipp BJ. Improved detection of lactate threshold during exercise using a log-log transformation. J Appl Physiol. 1985 Dec;59(6):1936-40.
The Dmax method was proposed in 1992 as another valid method to describe the curvilinear relationship of [La−]b and work rate during a progressive, incremental exercise test.26 In this method, a third-order curvilinear regression line of [La−]b versus is used to determine a threshold point, as shown in Figure 3. Line “A” connects the minimum point to the maximum point, and perpendicular line “B” connects line “A” to the curvilinear regression line at a point that maximizes the distance between the curvilinear regression line and line “A.” The point where line “B” intersects the curvilinear regression line is called “Dmax.” Dmax can then be used to derive a threshold work rate or (LTD) that is slightly different from the LT, although the two are highly correlated with each other.
Figure 3.
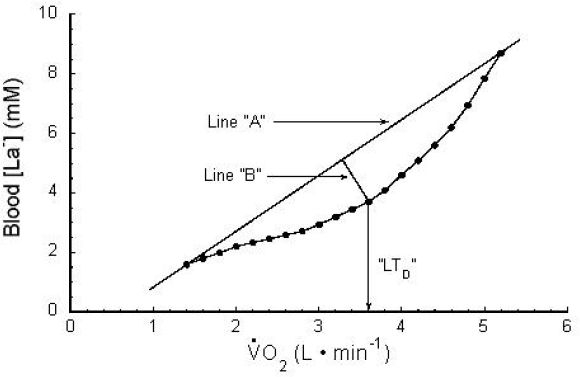
Dmax method. Refer to text for explanation. uptake/consumption. VO2 is a linear function of work rate. Used (redrawn) with permission from Cheng B, Kuipers H, Snyder AC, Keizer HA, Jeukendrup A, Hesselink M. A new approach for the determination of ventilatory and lactate thresholds. Int J Sports Med. 1992 Oct;13(7):518-22; Georg Thieme Verlag KG.
Other investigators have used a work rate or that corresponds to a fixed [La−]b value (e.g., 2.2, 2.5, 4 mM).27–32 The fixed [La−]b of 4 mM has become known as the onset of blood lactate accumulation.30,32,33 Visual LT, LTD, and OBLA are compared schematically in Figure 4.
Figure 4.
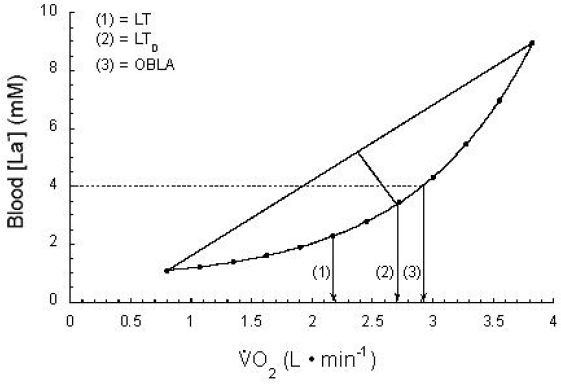
(1) Visual LT, (2) Dmax LT (LTD), and (3) OBLA. Note that each provides a different , although all three are highly correlated with each other. uptake/consumption. is a linear function of work rate. Used (redrawn) with permission from Gladden LB. Lactate metabolism during exercise In: Poortmans JR, editor. Principles of exercise biochemistry. 3rd ed. Basel: Karger; 2004. p. 152-96.
The methods using fixed [La−]b values are simpler in that they only require the clinician to match the chosen [La−]b with the corresponding work rate. However, variance among individuals is such that Stegmann and colleagues15 appropriately proposed the “individual anaerobic threshold” in 1981. These investigators stressed that using a single fixed [La−]b value as representative of all subjects can be quite misleading, as [La−]b at LT may vary between individuals from as low as 1.4 mM to as high as 7.5 mM.15
In summary, numerous methods exist to describe the [La−]b response to progressive, incremental exercise. While we have not provided an exhaustive list of methods for LT determination, our point is that several techniques offer qualitatively similar information when describing the [La−]b response to progressive, incremental exercise. Note that while each method produces a quantitatively different work rate or for describing the curve, the individual values are generally highly correlated with each other. For example, a subject having a high or low LT on the basis of the Dmax method (LTD) will also have a high or low LT on the basis of the visual technique.
More recently, the lactate response to submaximal exercise has been described by the maximal lactate steady state (MLSS). MLSS is defined as the highest intensity of exercise that can be performed while maintaining a constant [La−]b. Operationally, this is the maximal intensity for which [La−]b increases ≤1 mM during the last 20 minutes of a 30-minute constant work rate exercise test.34 Figure 5 shows a schematized view of MLSS responses. Similarly to LT, a wide range of [La−]b values at MLSS has been reported for different subjects, from as low as 1.5 mM to almost 7 mM. However, the average value is ≈3.7 mM.35 Also, while a particular individual's MLSS and LT occur at different work rates (and values), they are nevertheless correlated with each other.36
Figure 5.
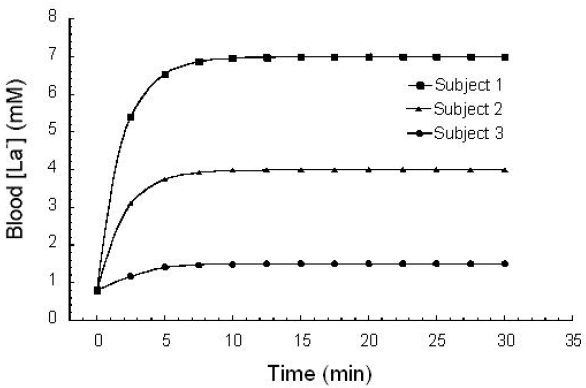
Schematic examples of [La−]b responses at MLSS for three different, hypothetical subjects. [La−]b at the MLSS may be as low as 1.5 mM or as high as 7.0 mM; the average is ≈3.7 mM.35
Response to Maximal Effort Exercise
For the subject represented in Figure 1, a normal progressive, incremental exercise test elicits a peak [La−]b on the order of 9 mM at volitional exhaustion. However, [La−]b values may approach maximum values of 15–25 mM during the first few minutes following “all-out” maximal exercise of approximately 30–120 seconds of duration.37,38 Figure 6 shows a typical [La−]b response for a subject following 60 seconds of “all-out” maximal exercise. As can be seen, peak [La−]b values typically appear at approximately 3–8 minutes postexercise, but [La−]b remains elevated for over 60 minutes. Elevated [La−]b under these circumstances is a normal physiological response to intense exercise and not necessarily symptomatic of a pathology.37–39 Furthermore, a person exhibiting a [La−]b of this magnitude would be expected to show a corresponding arterialized blood pH of about 6.95–7.1.37,40
Figure 6.
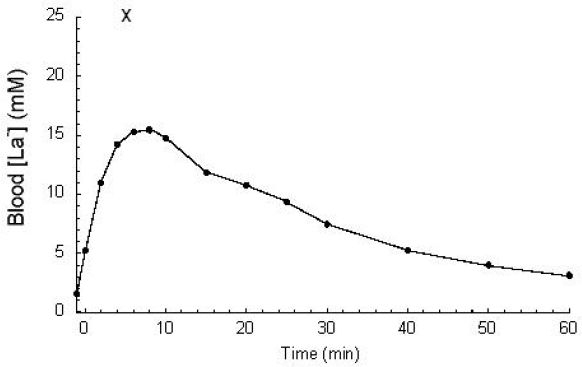
[La−]b response to 60 seconds of “all-out” maximal exercise. Peak values are typically observed 3–8 minutes postexercise. Unpublished data from Smith, Skelton, Kremer, Pascoe, and Gladden. Plotted point “X” demonstrates a very high [La−]b (26.0 mM) observed for a subject in a similar study.38
While taking muscle biopsies during a clinical exercise test may be of relatively little value to a physician when blood samples are readily available, studies of muscle homogenates have led to an increased understanding of La− influx to and efflux from muscles. Muscle biopsies have shown that active muscle [La−] displays very similar patterns to those of [La−]b.23,41
It should be noted that the muscle to blood [La−] relationship will vary depending on whether the muscle sample was taken from a muscle that was a source of La− production or a muscle that was a site of La− removal. It is critical to understand that [La−]b represents a balance between La− production and release into the blood by various tissues versus simultaneous La− uptake and removal from the blood by other tissues.42,43 Thus, when a person exhibits an increase in [La−]b during exertion, it could represent (1) an increase in La− production and release from muscle, (2) a decrease in La− uptake and removal, or (3) a relatively greater increase in production and release in comparison to uptake and removal. Thus [La−]b is not necessarily a good indicator of glycolytic rate or La− production. During light to moderate exercise, [La−]b may differ only slightly from the resting level, yet the flux of La− may be many times greater than at rest. Figure 7 shows a schematic representation of La− appearance, La− disappearance, and overall net [La−]b in response to a progressive incremental exercise test.44
Figure 7.
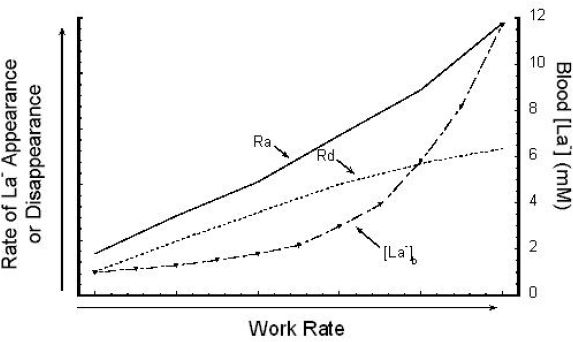
Schematic view of La− rate of appearance (Ra), La− rate of disappearance (Rd), and resulting [La−]b during progressive, incremental exercise. Used (redrawn) with permission from Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985 Feb;17(1):22-34.
It must be considered that a person exhibiting physiological or pathological increases in [La−]b may have impaired La− removal/uptake, increased La− production/release, or some combination of the two. The cell-to-cell lactate shuttle has greatly increased understanding of these processes, as La− is no longer considered simply a dead-end waste metabolite, but is instead recognized as a useful metabolic intermediate because it can be exchanged rapidly among tissue compartments.23 Furthermore, because of the large metabolic capacity of skeletal muscle, it is now considered not only the foremost source of La−, but also the primary consumer of La−.45 Oxidation is the most likely route of La− removal by skeletal muscle, although La− may also be used to synthesize glycogen.23 As the metabolic rate increases during exercise, La− oxidation by skeletal muscles and the heart also increases.45–47 The fact that the heart is an active consumer of La− has been frequently overlooked.48,49 Several different experimental approaches have shown that as [La−]b increases, La− becomes the preferred fuel of the heart, accounting for as much as 60% of oxidized substrate.49,50–53
Practical Relevance of the Lactate Threshold and Maximal Lactate Steady State
Lactate threshold is perhaps the best single predictor of endurance performance.10,31,32,54,55–59 Figure 8 shows the relationship between LT and long-distance running performance in young endurance athletes.57 Similar results have been observed by other investigators.10,31,32,54–56,58,59
Figure 8.
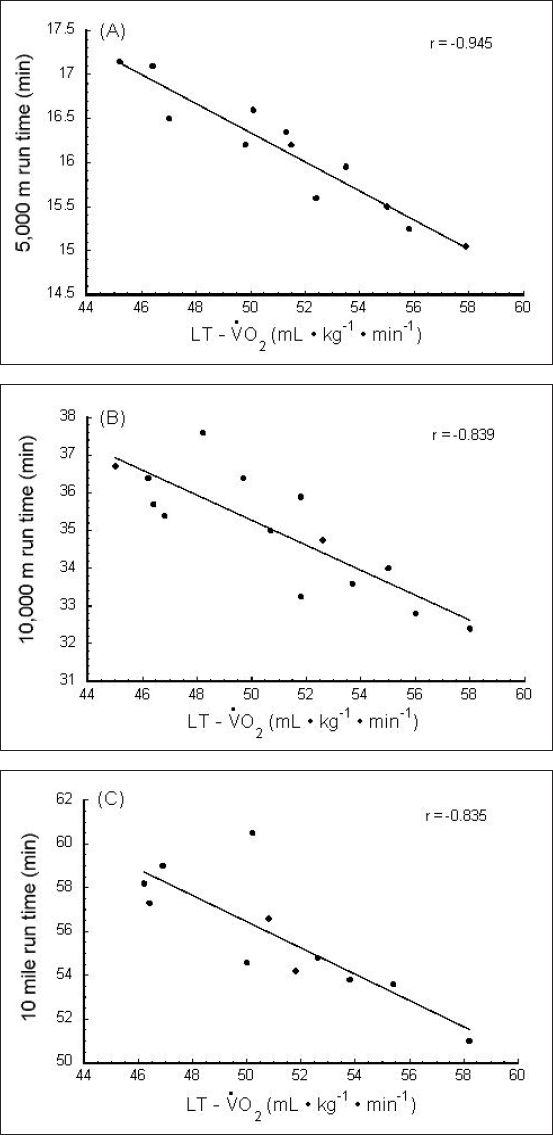
Correlation of endurance performance with LT. (A) 5,000-m run time vs LT, (B) 10,000-m run time vs LT, and (C) 10-mile run time vs LT. LT is presented as the (O2 uptake/consumption) at which the LT occurred. Used (redrawn) with permission from Kumagai S, Tanaka K, Matsuura Y, Matsuzaka A, Hirakoba K, Asano K. Relationships of the anaerobic threshold with the 5 km, 10 km, and 10 mile races. Eur J Appl Physiol. 1982;49(1):13-23 (Figure 4).
Furthermore, aerobic training has been shown to improve LT, with a concomitant improvement in endurance performance or even middle-distance performance.57 A “good” or “trained” runner will exhibit a higher LT and a lower [La−]b than a “poor” or “untrained” runner at any absolute intensity above resting. Figure 9 depicts differences that may be seen between a “good runner” and a “poor runner” in terms of the [La−]b response to progressive incremental exercise.57
Figure 9.
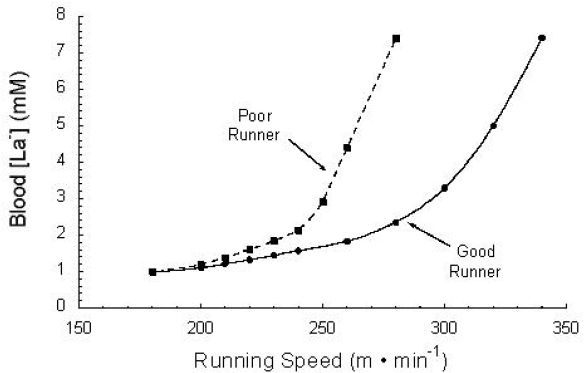
Schematic showing differences between a “good runner” and a “poor runner” in terms of the [La−]b response to progressive incremental exercise. Used (redrawn) with permission from Kumagai S, Tanaka K, Matsuura Y, Matsuzaka A, Hirakoba K, Asano K. Relationships of the anaerobic threshold with the 5 km, 10 km, and 10 mile races. Eur J Appl Physiol. 1982;49(1):13-23 (Figure 3).
Measuring a patient's [La−]b has clinical value not only because it can be used to evaluate an underlying pathology,1,2,5 but also because it circumvents the use of heart rate or expired gases as indices of training intensity. Patients with a chronic airway obstruction60 or a cardiac pathology10 may present with expired gases and/or heart rates that are not necessarily indicative of exercise intensity; however, an individual's LT can be used to accurately evaluate and prescribe exercise intensity for most diseased and nondiseased patients alike. Frequently, LT is used to prescribe exercise intensity for diabetic patients, as they often have cardiac limitations and/or microcirculatory pathologies that hasten the transition to significant La− accumulation.8,11,12
In 1983, Coyle and co-workers10 found that patients with ischemic heart disease had disproportionately high endurance capacities when compared to their values, yet their performance was well correlated with their LT values.10 Figure 10 shows that these patients had LT values that were equal to 100% of their values. This evidence supports the idea that LT is a stronger predictor of endurance performance than 10,31,32,54,55,57–59,61 and further illustrates the utility of using LT when prescribing exercise intensities.
Figure 10.
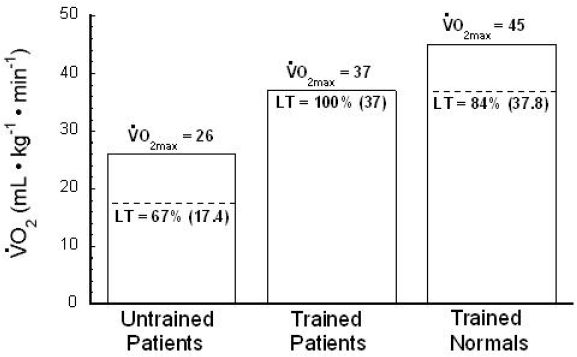
Well-trained ischemic heart disease patients exhibit an LT that is 100% of . In this study,9 “Trained Normals”' and “Trained Patients” exhibit similar performance ability because their absolute LT values are similar. Percentages inside of histograms indicate LT as a percentage of O2max; numbers in parentheses indicate the absolute (mL. kg-1. min-1) at LT. Used (redrawn) with permission from Coyle EF, Martin WH, Ehsani AA, Hagberg JM, Bloomfield SA, Sinacore DR, Holloszy JO. Blood lactate threshold in some well-trained ischemic heart disease patients. J Appl Physiol. 1983 Jan;54(1):18-23.
Lactate Transport across the Sarcolemma
Shuttling of La− between adjacent muscle fibers and between muscles and blood (and among other tissues) raises important questions concerning how and at what rates La− is able to cross membranes. Functioning of the La− shuttle is dependent on the rapid exchange of La− across cell membranes. It is now firmly established that La− transport across the sarcolemma membrane occurs by three pathways: (1) a H+-monocarboxylate cotransporter (MCT) that accounts for ≈75–90% of La− transport within the physiological range of [La−], (2) an inorganic antiporter (La− exchanged for either Cl− or HCO3− via the “band 3” protein), and (3) diffusion of undissociated HLa, a pathway that makes greater contributions at higher [La−] and lower pH conditions.62
Lactate Transport across the Red Blood Cell (RBC) Membrane
Because red blood cells rely on anaerobic metabolism that results in La− formation, an efficient system of La− efflux out of these cells is essential. On the one hand, the small amount of La− produced by RBCs can probably be handled by diffusion and does not necessarily require a La− carrier. On the other hand, intense exercise results in an accumulation of La− and H+ within the muscle, which may inhibit contractile function.63 In this case, a system designed to cotransport La− and H+ from the plasma into the RBCs could aid in establishing a gradient between the plasma and the interstitial fluid and enhance the efflux of La− and H+ from the exercising muscles.62,64 Indeed the transport of La− across the RBC membrane proceeds by the same three distinct pathways that are employed in transport across the sarcolemma, all shown schematically in Figure 11: (1) nonionic diffusion of undissociated HLa (≈5–12%); (2) the inorganic anion exchange system utilizing the “band 3” protein, often referred to as the “band 3 system” (≈5–15%); and (3) MCT1, the primary pathway of La− transport across the human RBC membrane (≈75–90%).65–71 At physiological pH levels, less than 0.5% of the total HLa + La− is in the undissociated HLa form. Accordingly, ≈5–12% may appear quite large for the contribution of undissociated HLa transport. However, it should be noted that HLa diffuses across plasma membranes readily so that even a small concentration gradient can contribute significantly to overall transport. At higher La− concentrations, the MCT carriers become saturated, so the diffusion component becomes a greater percentage of total transport.
Figure 11.
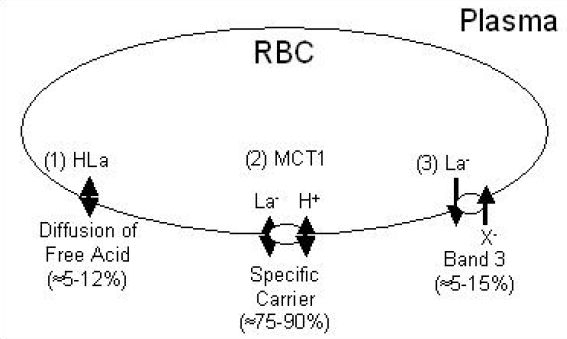
Schematic representing the transport of La− across RBC membranes. Used (redrawn) with permission from Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993 Apr;264(4 Pt 1):C761-82.
As blood circulates throughout the body, the [La−]b gradient is typically reversed, causing La− influx from plasma through the interstitial fluid and into the various tissues, especially the liver, heart, and inactive and moderately active skeletal muscles. As plasma [La−] declines, La− will leave the RBCs.
What Is Being Measured
Plasma versus Whole Blood
Thus far we have only mentioned whole blood [La−] values. However, confusion often occurs because La− equilibration between RBCs and plasma does not exist in a 1:1 ratio. Instead, there is a marked plasma to RBC [La−] gradient during exercise as well as at rest. When corrections are made for water content, the ratio between the RBC [La−] and the concomitant plasma [La−] is about 0.5.21,72–75 For example, a person exhibiting a water-corrected plasma [La−] of 1.0 mM would have a RBC [La−] of ≈0.5 mM. This uneven distribution of La− under equilibrium conditions is consistent with a distribution based on a Donnan relationship. According to the Donnan theory of equilibrium, permeable ions such as La− will be distributed based on the net charge of impermeable molecules inside the cell. Hemoglobin, the main impermeable constituent of the RBC, has an associated negative charge. Therefore, RBCs normally have a lower concentration of negatively charged La− ions in comparison to plasma, as noted earlier.
The plasma to RBC [La−] gradient increases with increasing exercise intensity, and therefore increasing plasma [La−]. However, despite this increasing [La−] gradient, the ratio of RBC:plasma [La−] remains at the resting value of ≈0.5.21 The exception occurs immediately after intense, “all-out” exercise, when the rate of La− release into the blood from the exercising muscles is apparently too rapid for the La− transport systems of the RBC membrane to achieve equilibrium.
As a result of this plasma to RBC [La−] gradient, it is of paramount importance for the clinician to understand what is being measured (i.e., whole blood or plasma). If a clinician is measuring plasma [La−] and interpreting the results based on “whole blood” parameters, then he or she will arrive at erroneously high [La−] values. Our laboratory has observed that whole blood [La−] values are on average only 70% of the corresponding plasma [La−] values (see Figure 12). This issue is complicated by the fact that different La− analyzers measure either plasma or whole blood [La−] values and it is sometimes difficult to know which is being measured.
Figure 12.
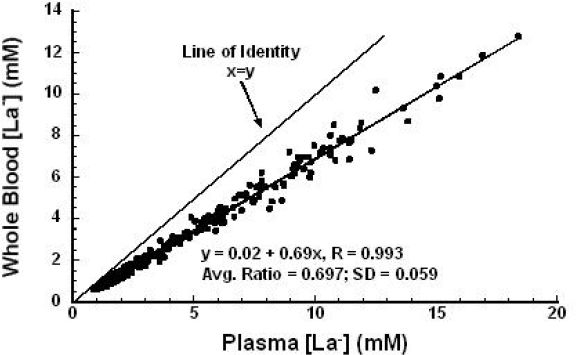
Whole blood [La−] versus corresponding plasma [La−]; SD, standard deviation of the ratio of whole blood [La−] to plasma [La−]. N = 324 blood samples. Unpublished data from Dobson, Smith, and Gladden.
It is worth noting that the aforementioned relationship would be altered for patients with anemia or polycythemia due to the differing contributions of RBCs versus plasma in such samples. As an approximation, the ratio of whole blood [La−] to plasma [La−] might be expected to vary from about 63% at a hematocrit of 55% to about 81% at a hematocrit of 25%.
Analyzers and Analysis
Once we are aware of the differences between plasma [La−] and whole blood [La−], how does this affect analysis? First, and most obvious, is the simple point that it is imperative to know whether plasma or whole blood is being analyzed. When the criterion/reference enzymatic method is used (as is typical during laboratory experiments), this is obvious. For example, after drawing a blood sample, an aliquot of the whole blood would be added to ice-cold, 4.2% perchloric acid to lyse the RBCs and deproteinize the sample. Following centrifugation, an aliquot of the supernatant would be analyzed via the criterion/reference enzymatic method.19 However, if plasma [La−] was being measured, the blood sample would be centrifuged first and then an aliquot of the plasma would be added to the ice-cold, 4.2% perchloric acid. From this brief description, it should be clear that the sample type being analyzed (either whole blood or plasma) with this method is unequivocal. (Typically water content corrections are not performed in either case.) However, as described later, the blood sample type being analyzed (whole blood or plasma) is often not so clear when an automatic analyzer is used.
Several studies since the early 1990s have compared various analyzers to each other.76–78 These studies are inherently weak in comparison to studies that include a criterion/reference enzymatic method. Other studies do not clearly indicate that some of the discrepancy between analyzers is the result of one analyzer measuring whole blood [La−] while another is measuring plasma [La−]. Perhaps the best paper to date regarding different La− analyzers is that by Medbø and colleagues.19 In their experiments, 800 blood or plasma samples were measured with at least two different methods or instruments. Altogether, almost 3000 single measurements were performed.
As an example, consider comparisons between Yellow Springs Instruments, Inc. (YSI) (Yellow Springs, Ohio) analyzers and the criterion/reference enzymatic method. Because YSI analyzers assume that a known and constant amount of blood is used for each measurement, the analyzers are equipped with pipettes for inserting a known volume of sample. According to YSI analyzer manuals, only La− in plasma is measured unless the blood samples are hemolyzed.
There was a strong linear correlation (r = 0.995) between the YSI 23L and the criterion/reference enzymatic method, but on average the values obtained with the YSI 23L were 22% lower. This result may be due to the YSI instrument analyzing nonhemolyzed blood, thus measuring only the La− in the plasma, but reporting the La− based on the total volume of blood injected. When hemolyzed blood was analyzed with the YSI 1500, there was a strong linear correlation with the criterion/reference enzymatic method. However, values obtained with this instrument were 20% higher than those obtained via the criterion/reference enzymatic method. Because the same sample type is being compared in this case, the error must be in the measurement by the instrument.19 A second YSI 1500 was also compared to the criterion/reference enzymatic method. This yielded another strong linear correlation with values averaging 5% higher than the criterion/reference values, but with no significant difference from the line of identity. Overall, five YSI analyzers were tested and there was variability among the group.19
A comparison of the Dr. Lange miniphotometer LP8+ (Dr. Bruno Lange GmbH; Berlin, Germany) versus the criterion/reference enzymatic method is illustrated in Figure 13A. For this analyzer, blood is hemolyzed in a reagent solution, processed, and read from the miniphotometer. Values for the LP8+ were on average 26% higher than those of the criterion/reference enzymatic method.19
Figure 13.
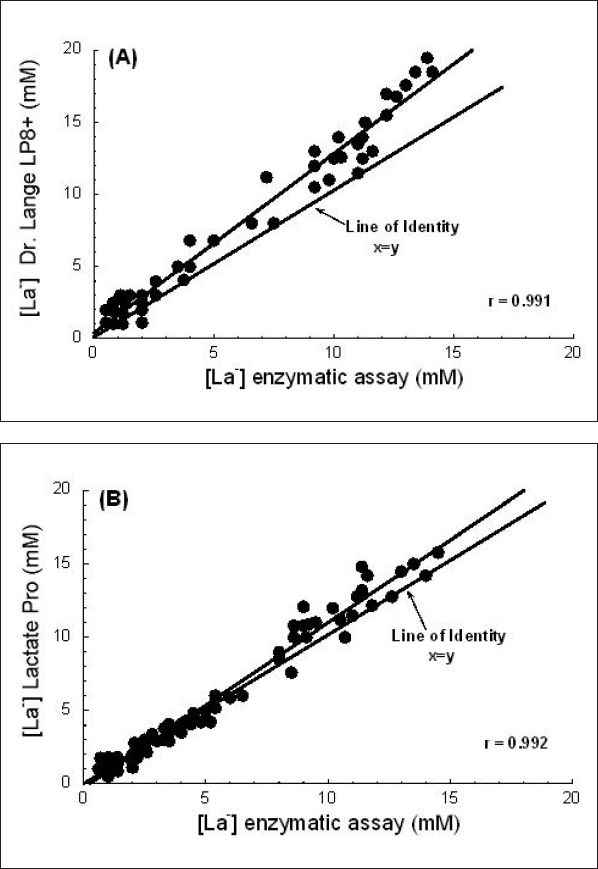
(A) Comparison of the Dr. Lange LP8+ [La−] analyzer with the criterion/reference enzymatic photofluorometric method. (B) Comparison of the Lactate Pro [La−] analyzer with the criterion/reference enzymatic photofluorometric method. See text for full explanation. Used (redrawn) with permission from Medbø JL, Mamen A, Holt Olsen O, Evertsen F. Examination of four different instruments for measuring blood lactate concentration. Scand J Clin Lab Invest. 2000 Aug;60(5):367-80.
The Accusport (Boehringer Mannheim, East Sussex, UK) was also compared to the criterion/reference enzymatic method. According to the manufacturer, the Accusport only measures La− in the plasma, even though a drop of whole blood is applied to the reagent strip. Built-in equations convert the measured plasma values to whole blood values. The Accusport showed two types of deviation relative to the criterion/reference method. First, the Y intercept was close to 1 mM (versus an ideal intercept of 0 mM). Accordingly, the Accusport reported [La−] values around 2 mM even for resting blood samples when the true [La−] was less than 1 mM. Second, the regression slope was 0.81, well below 1.0. Overall, the Accusport showed correct values around 5 mM and was too high for lower [La−] values, but too low for higher [La−] values.
Finally, Figure 13B displays a comparison of the Lactate Pro (Arkray, Inc., Kyoto, Japan) versus the criterion/reference enzymatic method. The manufacturer of the Lactate Pro provides no information on how this instrument accounts for La− inside of RBCs. However, on the basis of its performance, it appears that it must lyse the RBCs in whole blood samples. Samples are collected by inserting a La− strip into a calculator-sized instrument and then touching the strip to a drop of blood. The sample is drawn into the strip by capillary action. Figure 13B shows that values obtained with the Lactate Pro were highly correlated with the criterion/reference method and averaged 12% higher. Furthermore, for values less than 6 mM, the Lactate Pro did not show systematic error from the criterion/reference method; this supports its use for La− threshold testing. When plasma samples were measured with the Lactate Pro (rather than whole blood directly from a patient), values below 10 mM were up to 10% higher than the criterion/reference method values. However, plasma values were too low for samples above 10 mM. When different Lactate Pro instruments were analyzed, they were found to be consistent with each other. Overall, Medbø and colleagues19 concluded that the Lactate Pro was the best of the four instruments examined.
To summarize, it is imperative to know what type of sample is being measured. RBCs, plasma, and whole blood all have different values from each other even in blood that has complete equilibrium between plasma and RBCs. Plasma and whole blood [La−] values are not interchangeable and errors will result if this is ignored. Furthermore, when using automated analyzers, the type of sample presented does not necessarily determine what is being measured (plasma or whole blood). All analyzers differ from the criterion/reference enzymatic standard by varying degrees,19 and their precision, accuracy, and bias should be taken into account when interpreting the measurements made with them.
Conclusion
Analysis of [La−]b is often used in clinical exercise testing for exercise prescription and assessments of the effects of therapy and physical training. Thus, it is important for clinicians to understand the pathological [La−]b response as well as the normal [La−]b response to exertion. In response to progressive incremental exercise, [La−]b will increase in an exponential manner once LT has been exceeded. Various methods are valid for describing this curvilinear [La−]b response with a single point, although LT is highly individualized. An individual's endurance performance is well correlated with his or her LT, and endurance training improves LT.
While [La−]b is significantly elevated (≈8–10 mM) following a progressive, incremental exercise test to volitional exhaustion, the highest [La−]b values (≈15–25 mM) are typically observed 3–8 minutes after “all-out” maximal exertion of 30–120 seconds. MCTs provide the primary of three routes by which La− transport proceeds across the sarcolemma and RBC membrane. In RBC membranes specifically, La− flux occurs mainly via MCT1. At rest and under most exercise conditions, whole blood [La−] values are on average 70% of corresponding plasma [La−] values, and thus care must be taken to ensure that the correct measurement is being made during analysis. When analyzing the validity of a [La−]b-measuring instrument, it is critical that comparisons are made with the criterion/reference enzymatic method, not simply among analyzers. The Lactate Pro has performed better than many La− analyzers when careful comparisons to the criterion/reference enzymatic method have been made. Overall, it is advantageous for clinicians to have a thorough understanding of [La−]b responses, blood La− transport and distribution, and [La−]b analysis. While an elevated [La−]b may be indicative of ischemia or hypoxemia, it may also be a “normal” physiological response to exertion.
Abbreviations
- [La−]b
blood lactate concentration
- LT
lactate threshold
- LTD
Dmax method of determining LT
- MCT
H+-monocarboxylate cotransporter
- MLSS
maximal lactate steady state
- OBLA
onset of blood lactate accumulation
- RBC
red blood cell
- YSI
Yellow Springs Instruments, Inc.
References
- 1.Cooke NT, Wilson SH, Freedman S. Blood lactate and respiratory muscle fatigue in patients with chronic airways obstruction. Thorax. 1983 Mar;38(3):184–187. doi: 10.1136/thx.38.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson J, Âstrom H, Holmgren A, Kaijser C, Orinius E. Angina pectoris and blood lactate concentration during graded exercise. Int J Sports Med. 1984 Dec;5(6):348–351. doi: 10.1055/s-2008-1025931. [DOI] [PubMed] [Google Scholar]
- 3.Marcus JH, Ingram RH Jr, McLean RL. The threshold of anaerobic metabolism in chronic obstructive pulmonary disease, a promising index of evaluation. Am Rev Respir Dis. 1971 Oct;104(4):490–498. doi: 10.1164/arrd.1971.104.4.490. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura N, Nishijima H, Kojima S, Hashimoto F, Minami M, Yasuda H. Determination of anaerobic threshold for assessment of functional state in patients with chronic heart failure. Circulation. 1983 Aug;68(2):360–367. doi: 10.1161/01.cir.68.2.360. [DOI] [PubMed] [Google Scholar]
- 5.Nakao T, Fujiwara S, Isoda K, Miyahara T. Impaired lactate production by skeletal muscle with anaerobic exercise in patients with chronic renal failure. Nephron. 1982;31(2):111–115. doi: 10.1159/000182628. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman K, McIlroy MB. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol. 1964 Dec;14:844–852. doi: 10.1016/0002-9149(64)90012-8. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT, Wilson JR, Janicki JS, Likoff MJ. Exercise testing in the evaluation of the patient with chronic cardiac failure. Am Rev Respir Dis. 1984 Feb;129(2 Pt 2):S60–62. doi: 10.1164/arrd.1984.129.2P2.S60. [DOI] [PubMed] [Google Scholar]
- 8.Belli T, Ackermann MA, Ribeiro LFP, Langeani R, Galdino da Silva R, Baldissera V. Lactate and ventilatory thresholds in type 2 diabetic women. Diabetes Res Clin Pract. 2007 Apr;76(1):18–23. doi: 10.1016/j.diabres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker PH, Berry MJ, Brozena SC, Morley DL, Walter JD, Paolone AM, Bove AA. Relationship of lactate and ventilatory thresholds in cardiac transplant patients. Med Sci Sports Exerc. 1993 Feb;25(2):191–196. [PubMed] [Google Scholar]
- 10.Coyle EF, Martin WH, Ehsani AA, Hagberg JM, Bloomfield SA, Sinacore DR, Holloszy JO. Blood lactate threshold in some well-trained ischemic heart disease patients. J Appl Physiol. 1983 Jan;54(1):18–23. doi: 10.1152/jappl.1983.54.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Kawaji K, Kanamori A, Matoba K, Yajima Y, Takeuchi A, Ishii K. Relationship between age-adjusted heart rate and anaerobic threshold in estimating exercise intensity in diabetics. Diabetes Res Clin Pract. 1990 Jan;8(1):69–74. doi: 10.1016/0168-8227(90)90098-e. [DOI] [PubMed] [Google Scholar]
- 12.Kawaji K, Fujita Y, Yajima Y, Shirataka M, Kubo H. Usefulness of anaerobic threshold in estimating intensity of exercise for diabetics. Diabetes Res Clin Pract. 1989 May 15;6(4):303–309. doi: 10.1016/0168-8227(89)90070-3. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson J, Dlin R, Kaiser P, Tesch PA, Kaijser C. Muscle metabolism, regulation of circulation and beta blockade. J Cardiac Rehabil. 1983;3:404–420. [Google Scholar]
- 14.Jones AM, Doust JH. The validity of the lactate minimum test for determination of the maximal lactate steady state. Med Sci Sports Exerc. 1998 Aug;30(8):1304–1313. doi: 10.1097/00005768-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Stegmann H, Kindermann W, Schnabel A. Lactate kinetics and individual anaerobic threshold. Int J Sports Med. 1981 Aug;2(3):160–165. doi: 10.1055/s-2008-1034604. [DOI] [PubMed] [Google Scholar]
- 16.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004 Jul 1;558(Pt 1):5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks GA. Lactate: glycolytic product and oxidative substrate during sustained exercise in mammals–the ‘lactate shuttle’. In: Gilles R, editor. Comparative physiology and biochemistry: current topics and trends. Berlin: Springer; 1985. pp. 208–218. [Google Scholar]
- 18.Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc. 2000 Apr;32(4):790–799. doi: 10.1097/00005768-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Medbø JL, Mamen A, Holt Olsen O, Evertsen F. Examination of four different instruments for measuring blood lactate concentration. Scand J Clin Lab Invest. 2000 Aug;60(5):367–380. doi: 10.1080/003655100750019279. [DOI] [PubMed] [Google Scholar]
- 20.Owles WH. Alterations in the lactic acid content of the blood as a result of light exercise, and associated changes in the CO2-combining power of the blood and in the alveolar CO2 pressure. J Physiol. 1930 Apr 14;69(2):214–237. doi: 10.1113/jphysiol.1930.sp002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith EW, Skelton MS, Kremer DE, Pascoe DD, Gladden LB. Lactate distribution in the blood during progressive exercise. Med Sci Sports Exerc. 1997 May;29(5):654–660. doi: 10.1097/00005768-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Forster HV, Dempsey JA, Thomson J, Vidruk E, DoPico GA. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972 Jan;32(1):134–137. doi: 10.1152/jappl.1972.32.1.134. [DOI] [PubMed] [Google Scholar]
- 23.Gladden LB. Lactate metabolism during exercise. In: Poortmans JR, editor. Principles of exercise biochemistry. 3rd. Basel: Karger; 2004. pp. 152–196. [Google Scholar]
- 24.Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973 Aug;35(2):236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- 25.Beaver WL, Wasserman K, Whipp BJ. Improved detection of lactate threshold during exercise using a log-log transformation. J Appl Physiol. 1985 Dec;59(6):1936–1940. doi: 10.1152/jappl.1985.59.6.1936. [DOI] [PubMed] [Google Scholar]
- 26.Cheng B, Kuipers H, Snyder AC, Keizer HA, Jeukendrup A, Hesselink M. A new approach for the determination of ventilatory and lactate thresholds. Int J Sports Med. 1992 Oct;13(7):518–522. doi: 10.1055/s-2007-1021309. [DOI] [PubMed] [Google Scholar]
- 27.Heck H, Mader A, Hess G, Mücke S, Müller R, Hollmann W. Justification of the 4-mmol/l lactate threshold. Int J Sports Med. 1985 Jun;6(3):117–130. doi: 10.1055/s-2008-1025824. [DOI] [PubMed] [Google Scholar]
- 28.Hollmann W. Historical remarks on the development of the aerobic-anaerobic threshold up to 1966. Int J Sports Med. 1985 Jun;6(3):109–116. doi: 10.1055/s-2008-1025823. [DOI] [PubMed] [Google Scholar]
- 29.Hurley BF, Hagberg JM, Allen WK, Seals DR, Young JC, Cuddihee RW, Holloszy JO. Effect of training on blood lactate levels during submaximal exercise. J Appl Physiol. 1984 May;56(5):1260–1264. doi: 10.1152/jappl.1984.56.5.1260. [DOI] [PubMed] [Google Scholar]
- 30.Kindermann W, Simon G, Keul J. The significance of the aerobic-anaerobic transition for the determination of work load intensities during endurance training. Eur J Appl Physiol. 1979 Sep;42(1):25–34. doi: 10.1007/BF00421101. [DOI] [PubMed] [Google Scholar]
- 31.LaFontaine TP, Londeree BR, Spath WK. The maximal steady state versus selected running events. Med Sci Sports Exerc. 1981;13(3):190–193. [PubMed] [Google Scholar]
- 32.Sjödin B, Jacobs I. Onset of blood lactate accumulation and marathon running performance. Int J Sports Med. 1981 Feb;2(1):23–26. doi: 10.1055/s-2008-1034579. [DOI] [PubMed] [Google Scholar]
- 33.Mader A. The contribution of physiology to the science of coaching. In: Zimri U, editor. The art and science of coaching. Netanya, Israel: The Wingate Institute; 1980. pp. 10–29. [Google Scholar]
- 34.Pringle JS, Jones AM. Maximal lactate steady state, critical power and EMG during cycling. Eur J Appl Physiol. 2002 Dec;88(3):214–226. doi: 10.1007/s00421-002-0703-4. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen PK, Sj G, Juel C. Plasma acid-base status and hyperventilation during cycling at MAXLASS in low and high lactate responders [abstract] Med Sci Sports Exerc. 2001;33:S314. [Google Scholar]
- 36.Aunola S, Rusko H. Does anaerobic threshold correlate with maximal lactate steady-state= J Sports Sci. 1992 Aug;10(4):309–323. doi: 10.1080/02640419208729931. [DOI] [PubMed] [Google Scholar]
- 37.Kindermann W, Keul J. Lactate acidosis with different forms of sports activities. Can J Appl Sport Sci. 1977;2:177–182. [Google Scholar]
- 38.Withers RT, Sherman WM, Clark DG, Esselbach PC, Nolan SR, Mackay MH, Brinkman M. Muscle metabolism during 30, 60 and 90 s of maximal cycling on air-braked ergometer. Eur J Appl Physiol Occup Physiol. 1991;63(5):354–362. doi: 10.1007/BF00364462. [DOI] [PubMed] [Google Scholar]
- 39.Fujitsuka N, Yamamoto T, Ohkuwa T, Saito M, Miyamura M. Peak blood lactate after short periods of maximal treadmill running. Eur J Appl Physiol Occup Physiol. 1982;48(3):289–296. doi: 10.1007/BF00430218. [DOI] [PubMed] [Google Scholar]
- 40.Hermansen L, Osnes J. Blood and muscle pH after maximal exercise in man. J Appl Physiol. 1972 Mar;32(3):304–308. doi: 10.1152/jappl.1972.32.3.304. [DOI] [PubMed] [Google Scholar]
- 41.Karlsson J, Diamant B, Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- 42.Brooks GA. Current concepts in lactate exchange. Med Sci Sports Exerc. 1991 Aug;23(8):895–906. [PubMed] [Google Scholar]
- 43.Stainsby WN, Brooks GA. Control of lactic acid metabolism in contracting muscles and during exercise. Exerc Sport Sci Rev. 1990;18:29–63. [PubMed] [Google Scholar]
- 44.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985 Feb;17(1):22–34. [PubMed] [Google Scholar]
- 45.Gladden LB. Muscle as a consumer of lactate. Med Sci Sports Exerc. 2000 Apr;32(4):764–771. doi: 10.1097/00005768-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Ahlborg G, Hagenfeldt L, Wahren J. Influence of lactate infusion on glucose and FFA metabolism in man. Scand J Clin Lab Invest. 1976 Mar;36(2):193–201. doi: 10.1080/00365517609055248. [DOI] [PubMed] [Google Scholar]
- 47.Ahlborg G, Hagenfeldt L, Wahren J. Substrate utilization by the inactive leg during one-leg or arm exercise. J Appl Physiol. 1975 Nov;39(5):718–723. doi: 10.1152/jappl.1975.39.5.718. [DOI] [PubMed] [Google Scholar]
- 48.Chatham JC, Gao ZP, Forder JR. Impact of 1 wk of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation. Am J Physiol. 1999 Aug;277(2 Pt 1):E342–3451. doi: 10.1152/ajpendo.1999.277.2.E342. [DOI] [PubMed] [Google Scholar]
- 49.Stanley WC. Myocardial lactate metabolism during exercise. Med Sci Sports Exerc. 1991 Aug;23(8):920–924. [PubMed] [Google Scholar]
- 50.Chatham JC. Lactate–the forgotten fuel! J Physiol. 2002 Jul 15;542(Pt 2):333. doi: 10.1113/jphysiol.2002.020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drake AJ, Haines JR, Noble MI. Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc Res. 1980 Feb;14(2):65–72. doi: 10.1093/cvr/14.2.65. [DOI] [PubMed] [Google Scholar]
- 52.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988 Dec;82(6):2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kemppainen J, Fujimoto T, Kalliokoski KK, Viljanen T, Nuutila P, Knuuti J. Myocardial and skeletal muscle glucose uptake during exercise in humans. J Physiol. 2002 Jul 15;542(Pt 2):403–412. doi: 10.1113/jphysiol.2002.018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol. 1988 Jun;64(6):2622–2630. doi: 10.1152/jappl.1988.64.6.2622. [DOI] [PubMed] [Google Scholar]
- 55.Hagberg JM, Coyle EF. Physiological determinants of endurance performance as studied in competitive racewalkers. Med Sci Sports Exerc. 1983;15(4):287–289. doi: 10.1249/00005768-198315040-00006. [DOI] [PubMed] [Google Scholar]
- 56.Karlsson J, Jacobs I. Onset of blood lactage [sic] accumulation during muscular exercise as a threshold concept. I. Theoretical considerations. Int J Sports Med. 1982 Nov;3(4):190–201. doi: 10.1055/s-2008-1026087. [DOI] [PubMed] [Google Scholar]
- 57.Kumagai S, Tanaka K, Matsuura Y, Matsuzaka A, Hirakoba K, Asano K. Relationships of the anaerobic threshold with the 5 km, 10 km, and 10 mile races. Eur J Appl Physiol Occup Physiol. 1982;49(1):13–23. doi: 10.1007/BF00428959. [DOI] [PubMed] [Google Scholar]
- 58.Rhodes EC, McKenzie DC. Predicting marathon time from anaerobic threshold measurements. Phys Sports Med. 1984;12(1):95–99. [Google Scholar]
- 59.Sjödin B, Svedenhag J. Applied physiology of marathon running. Sports Med. 1985 Mar–Apr;2(2):83–99. doi: 10.2165/00007256-198502020-00002. [DOI] [PubMed] [Google Scholar]
- 60.Nery LE, Wasserman K, Andrews JD, Huntsman DJ, Hansen JE, Whipp BJ. Ventilatory and gas exchange kinetics during exercise in chronic airways obstruction. J Appl Physiol. 1982 Dec;53(6):1594–1602. doi: 10.1152/jappl.1982.53.6.1594. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka K, Watanabe H, Konishi Y, Mitsuzono R, Sumida S, Tanaka S, Fukuda T, Nakadomo F. Longitudinal associations between anaerobic threshold and distance running performance. Eur J Appl Physiol Occup Physiol. 1986;55(3):248–252. doi: 10.1007/BF02343795. [DOI] [PubMed] [Google Scholar]
- 62.Gladden LB. Lactate transport and exchange during exercise. In: Rowell L, Shepherd J, editors. Handbook of physiology. New York: Oxford University Press; 1996. pp. 614–648. [Google Scholar]
- 63.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994 Jan;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 64.Roth DA. The sarcolemmal lactate transporter: transmembrane determinants of lactate flux. Med Sci Sports Exerc. 1991 Aug;23(8):925–934. [PubMed] [Google Scholar]
- 65.Deuticke B, Beyer E, Forst B. Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta. 1982 Jan 4;684(1):96–110. doi: 10.1016/0005-2736(82)90053-0. [DOI] [PubMed] [Google Scholar]
- 66.Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995 Jan 27;270(4):1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 67.Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994 Mar 11;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 68.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999 Oct;15(343)(Pt 2):281–299. [PMC free article] [PubMed] [Google Scholar]
- 69.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993 Apr;264(4 Pt 1):C761–782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 70.Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells from trained and untrained human subjects. Med Sci Sports Exerc. 1998 Apr;30(4):536–542. doi: 10.1097/00005768-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells of athletic and nonathletic species. Am J Physiol. 1995 May;268(5 Pt 2):R1121–1128. doi: 10.1152/ajpregu.1995.268.5.R1121. [DOI] [PubMed] [Google Scholar]
- 72.Consolazio CF, Johnson RE, Pecora LJ. New York: McGraw-Hill; 1963. Physiological measurements of metabolic functions in man; pp. 125–126. [Google Scholar]
- 73.Foxdal P, Sjödin A, Rudstam H, Ostman C, Ostman B, Hedenstierna GC. Lactate concentration differences in plasma, whole blood, capillary finger blood and erythrocytes during submaximal graded exercise in humans. Eur J Appl Physiol Occup Physiol. 1990;61(3-4):218–222. doi: 10.1007/BF00357603. [DOI] [PubMed] [Google Scholar]
- 74.Harris RT, Dudley GA. Exercise alters the distribution of ammonia and lactate in blood. J Appl Physiol. 1989 Jan;66(1):313–317. doi: 10.1152/jappl.1989.66.1.313. [DOI] [PubMed] [Google Scholar]
- 75.Juel C, Bangsbo J, Graham T, Saltin B. Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand. 1990 Oct;140(2):147–159. doi: 10.1111/j.1748-1716.1990.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 76.Fell JW, Rayfield JM, Gulbin JP, Gaffney PT. Evaluation of the Accusport lactate analyser. Int J Sports Med. 1998 Apr;19(3):199–204. doi: 10.1055/s-2007-971904. [DOI] [PubMed] [Google Scholar]
- 77.McLellan TM, Cheung KS. A comparative evaluation of the individual anaerobic threshold and the critical power. Med Sci Sports Exerc. 1992 May;24(5):543–550. [PubMed] [Google Scholar]
- 78.McNaughton LR, Thompson D, Philips G, Backx K, Crickmore L. A comparison of the Lactate Pro, Accusport, Analox GM7 and Kodak Ektachem lactate analysers in normal, hot and humid conditions. Int J Sports Med. 2002 Feb;23(2):130–135. doi: 10.1055/s-2002-20133. [DOI] [PubMed] [Google Scholar]


