Abstract
Background
In addition to its role in glucose metabolism, insulin has shown to exert numerous vascular effects, and an impaired vascular function of insulin is assumed to be a major contributor in the development of vascular complications. Arterial augmentation (AP) and the augmentation index (Aix) are surrogate parameters of arterial stiffness and are commonly used as predictors for cardiovascular risk. The aim of this study is to investigate the effect of insulin on arterial stiffness and parameters of endothelial function in patients with type 1 diabetes and healthy control subjects.
Methods
Fourteen patients with type 1 diabetes (six male, eight female) with a mean age of 36.6 ± 11.8 years and 14 healthy subjects (seven male, seven female) with a mean age of 27.3 ± 5.5 years were randomized to an euglygemic clamp with either a low (0.25 mU/kg/min) or a high (1.0 mU/kg/min) insulin dose on two different days. The mean HbA1c in the diabetic subjects was 7.3 ± 0.7%. In these subjects, arterial stiffness was measured by pulse wave analysis (SphygmoCor, AtCor Medical, Australia). AP was calculated as the difference between the second and the first systolic shoulders of the central pressure wave curve, and the Aix was expressed as the percentage of AP from total pulse pressure. As parameters of endothelial function, cyclic guanosine monophosphate, nitrotyrosine, and asymmetric dimethylarginine were determined at baseline and after 120 minutes.
Results
Patients with type 1 diabetes showed increased values for AP with 3.5 ± 3.1 mm Hg and Aix with 12.5 ± 12.5% compared to healthy controls with −0.7 ± 2.6 mm Hg for AP and −4.2 ± 10.6% for Aix. This difference was statistically significant (p < 0.01). During the euglycemic clamp, insulin improved, but did not normalize the increased values for AP and Aix in patients with type 1 diabetes. Concerning parameters of endothelial function, patients with type 1 diabetes showed statistically significant increased values for nitrotyrosine compared to healthy controls at baseline [low insulin: diabetes mellitus (DM) 1993.12 ± 1330.85 nmol/liter vs healthy controls 803.7 ± 726.91; high insulin DM: 2208.02 ± 1736.57 nmol/liter vs healthy controls: 750.83 ± 426.03 nmol/liter] (p < 0.05).
Conclusion
Patients with type 1 diabetes mellitus revealed an increased arterial stiffness measured as augmentation and augmentation index and increased nitrotyrosine levels as a marker of oxidative stress compared to healthy control subjects at baseline. Application of insulin improves the arterial elastic properties, but was not able to normalize the vascular function in patients with type 1 diabetes.
Keywords: ADMA, augmentation, augmentation index, endothelial function, diabetes mellitus type 1, nitrotyrosine
Introduction
Patients with type 1 diabetes are at a higher risk of cardiovascular disease, which cannot be explained by the classical risk factors.1 Morbidity and mortality in hypertension and cardiovascular disease are related to structural and functional alterations of the arterial wall.2–4 Changes in the arterial wall can lead to increased arterial stiffness, which influences cardiovascular prognosis negatively.5 Increased arterial stiffness of larger arteries in type 1 diabetes is described in several studies.6–12 Intensive insulin therapy has been shown to slow arterial stiffening in these patients.13
In addition to its role in glucose metabolism, insulin has shown to exert numerous vascular effects in recent years, and an impaired vascular function of insulin is assumed to be a major contributor in the development of vascular complications.14–16 It has been described that insulin increases nitric oxide (NO) release from endothelial cells and the cyclic guanosine monophosphate (cGMP) content in vascular smooth muscle cells and peripheral blood.17 These mechanisms have been shown to improve microvascular blood flow directly.18 Defects in cellular insulin signaling pathways impair insulin-mediated glucose uptake and insulin-induced NO generation in endothelial cells.19 Moreover, insulin decreases central pressure augmentation (AP) independent of any effects on blood flow or peripheral vascular resistance.20
Pulse wave analysis is a noninvasive and reproducible technique21–23 used to examine arterial elastic properties and has therefore been utilized in many clinical studies.24–26
The aim of this study was to investigate the arterial stiffness and endothelial function in patients with diabetes type 1 and to compare the effects of insulin during an euglycemic clamp in type 1 diabetic patients with healthy controls.
Patients and Methods
In an open phase II study the vascular effects of insulin in type 1 diabetes patients and healthy control subjects during an euglycemic clamp with two different insulin concentrations were investigated. Fourteen patients with type 1 diabetes (six male, eight female) with a mean age of 36.6 ± 11.8 years were included in the study. The mean HbA1c was 7.3 ± 0.7 % (normal value of the laboratory is 4.6–6.1%), the mean diabetes history was 23.5 ± 11.0 years, and 1 of the 14 patients showed clinical signs of a mild diabetic polyneuropathy. All diabetic patients were c-peptide deficient. Diabetic patients with known insulin resistance, strong variations in daily blood glucose profile and insulin dose, and severe microvascular complications of diabetes mellitus were excluded from the study. Eight of the diabetic patients received intensive insulin therapy with short acting insulin for the meals and one to three times long acting basal insulin and six patients were given insulin by continuous subcutaneous insulin pump therapy. Fourteen healthy subjects (seven male, seven female) with a mean age of 27.3 ± 5.5 years were included as the control group. All patients and controls were nonsmokers, normotensive, and did not receive any vasoactive substance or suffered from a clinical significant microvascular disease. The participants are characterized further in Table 1.
Table 1.
Main Characteristics of Patients and Healthy Controls
| Diabetes type 1 patients | Healthy controls | |
|---|---|---|
| Number | 14 | 14 |
| Male/female | 6/8 | 7/7 |
| mean age (years) | 36.6 ± 11.8 | 27.3 ± 5.5 |
| Mean body mass index (kg/m2) | 25.82 ± 3.55 | 23.82 ± 0.87 |
| Mean blood pressure (mm Hg) | 122/75 | 114/76 |
| Mean heart rate (bpm) | 65 | 69 |
| Mean c-peptide value (ng/ml) | 0.33 ± 0.06 | 2.05 ± 0.5 |
| Mean diabetes duration (years) | 23.5 ± 11.0 | – |
The diabetic patients and the healthy control subjects were informed of the nature, purpose, and possible risk involved in the study prior to giving their written consent to participate.
The study protocol was reviewed and approved by the local ethics committee of the University of Mainz.
Procedure
The study consisted of three visits, one screening visit and two investigational visits, which were performed 2–21 days following the previous visit.
During the screening visit the subjects were characterized by medical history, physical examination, electrocardiogram, and laboratory tests either as a patient with type 1 diabetes or as a healthy control fulfilling the study specific in- and exclusion criteria as just given.
All participants came to the study site after an overnight fast of at last 8 hours and after having skipped their breakfast and their regular antidiabetic therapy (if applicable). A Teflon catheter was placed in the cubital vein of each arm for the duration of the experiment. One catheter was used for the intravenous application of insulin and glucose and the other one served for blood sampling.
The insulin (Huminsulin, Eli Lilly) was infused with a controllable infusion device using a dosage of either 0.25 U/kg/min (low dose) or 1.0 U/kg/min (high dose) on the two different investigational days in a randomized manner. Glucose (concentrated 10%) was infused with a controllable infusion device (Infusomat). The glucose infusion was calculated individually by the investigator on the basis of actual blood glucose determinations to adjust the participants' blood glucose in a target range of 80–140 mg/dl. If this target could not be kept within 120 min the visit was discontinued and repeated another day.
Endothelial function was investigated by determination of serum plasma cGMP, nitrotyrosine, and asymmetric dimethylarginine (ADMA) at baseline and after 120 minutes.
Analysis
Cyclic guanosine monophosphate was measured in a radioimmunoassay (Immuno Biological Laboratories, Hamburg, Germany). Venous blood samples for the determination of cGMP were collected in EDTA tubes and immediately placed on ice. After separation, EDTA plasma was stored at −20°C in cryogenic tubes for subsequent analysis.
Nitrotyrosine
Nitrotyrosine is an index of peroxynitride, a cytotoxic compound formed from the superoxide anion and nitric oxide. For determination of nitrotyrosine, a competitive ELISA (nitrotyrosine assay kit, chemiluminescence detection, Upstate, USA) was used. Venous blood samples for the determination of nitrotyrosine were collected in EDTA tubes; after separation, EDTA plasma was stored at −20°C in cryogenic tubes for subsequent analysis.
Asymmetric Dimethylarginine
Asymmetric dimethylarginine, an endogenous inhibitor of endothelial NO synthase, was measured in an ELISA (Immundiagnostik, Bensheim, Germany). Venous blood samples for the determination of ADMA were collected in EDTA tubes; after separation, EDTA plasma was stored at −20°C in cryogenic tubes for subsequent analysis.
Pulse Waveform Analysis (PWA)
Arterial stiffness, which is predictive of vascular disease outcomes, can be measured by analysis of the arterial waveform to determine pulse wave form and augmentation index (Aix). In our study, arterial stiffness was assessed noninvasively with a commercially available SphygmoCor system (AtCorMedical, Australia) from the radial artery at the left wrist using applanation tonometry.
After 20 sequential waveforms were recorded, a validated generalized transfer function was used to generate the corresponding central aortic pressure waveform.27–29 On the generated central aortic pressure waveform, the merging curve of the incident and the reflected wave (the inflection point) was identified. Augmentation of the central aortic pressure is a manifestation of early wave reflection and is the boost of pressure from the first systolic shoulder to the systolic pressure peak.5 Augmentation was calculated as the difference between the second and the first systolic shoulders of the central pressure wave curve, and the Aix is expressed as the percentage of AP from total pulse pressure. Because it is known that Aix is influenced in an inverse and linear manner by heart rate, according to Wilkinson and colleagues,30 the Aix was normalized for a heart rate of 75 bpm (Aix@75). Higher values of Aix indicate increased wave reflection from periphery or earlier return of the reflected wave as a result of increased pulse wave velocity, which can be contributed to an increased arterial stiffness. Lower values for Aix indicate a good elasticity of the arterial wall.
All PWA recordings were performed on a recumbent subject by one of two trained investigators. Only high-quality recordings, controlled by internal quality definitions (in-device quality index >80 %) and an acceptable curve by visual inspection, were included in the analysis. Measurements of PWA were performed at baseline and at 120 min during the euglycemic clamp.
Statistics
All results are presented as mean ± 1 SD and as the number/proportion of patients with a characteristic for categorical variables. Differences in the mean values among the three groups were compared using the unpaired t test. The Shapiro–Wilk test was used for the characterization of data distribution. All analyses were performed in an exploratory and nonconfirmatory setting, and all p values <0.05 are interpreted as significant.
Results
Patients with diabetes type 1 showed increased values for AP with 3.5 ± 3.1 mm Hg and Aix with 12.5 ± 12.5% compared to healthy controls with −0.7 ± 2.6 mm Hg for AP and −4.2 ± 10.6% for Aix. This difference was statistically significant (p < 0.01). During the euglycemic clamp, insulin in low and high concentrations improved, but did not normalize the increased values for AP and Aix in patients with type 1 diabetes (low insulin: AP 2.1 ± 3.5 mm Hg in type 1 diabetes vs −0.1 ± 1.9 mm Hg in healthy controls; Aix 10.4 ± 16.1 vs −0.8 ± 8.1%; high insulin AP 3.3 ± 3.7 vs −1.2 ± 2.7 mm Hg; Aix 10.2 ± 12.2 vs −4-4 ± 11.6%). The improvement was statistically significant (p < 0.05). Results are shown in Figures 1 and 2.
Figure 1.
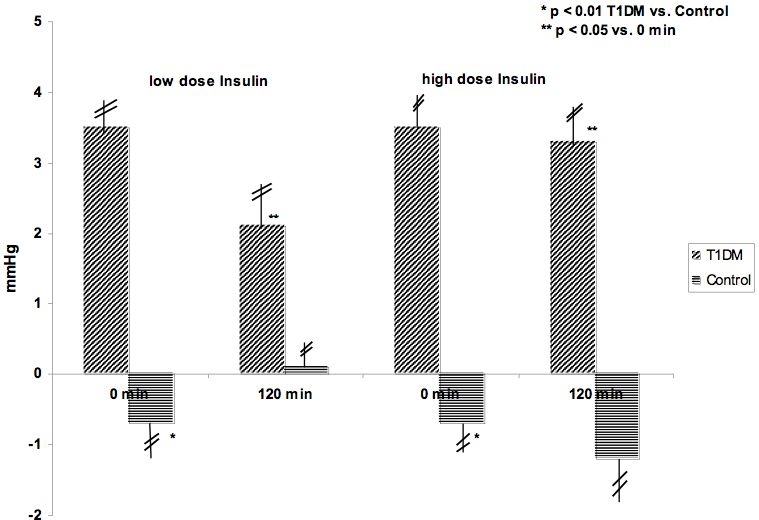
Augmentation (AP) in mm Hg during euglygemic clamp with low (0.25 mU/kg/min) and high (1.0 mU/kg/min) dose insulin infusions in patients with type 1 diabetes and healthy controls.
Figure 2.

Augmentation index (Aix@75) during euglygemic clamp with low (0.25 mU/kg/min) and high (1.0 mU/kg/min) dose insulin infusions in patients with type 1 diabetes and healthy controls.
Concerning parameters of endothelial function patients with type 1 diabetes showed statistically significant increased values for nitrotyrosine compared to healthy controls at baseline (low insulin: DM 1993.12 ± 1330.85 nmol/liter vs healthy controls 803.7 ± 726.91; high insulin: DM 2208.02 ± 1736.57 nmol/liter vs healthy controls 750.83 ± 426.03 nmol/liter)(p < 0.05).
During the euglycemic clamp, insulin in low and high concentrations improved the values for ADMA statistically significant in healthy controls, but not in patients with type 1 diabetes (Figure 3). Results of all determined endothelial parameters are given in Table 2.
Figure 3.
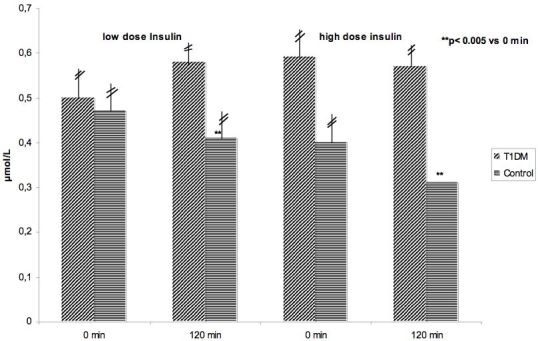
ADMA during euglygemic clamp with low (0.25 mU/kg/min) and high (1.0 mU/kg/min) dose insulin infusions in patients with type 1 diabetes and healthy controls.
Table 2.
Parameters of Endothelial Function, Given as Mean ± SD
| Diabetes type 1 | Controls | |||
|---|---|---|---|---|
| Insulin low | Insulin high | Insulin low | Insulin high | |
| cGMP 0 min (pmol/ml) | 4.15 ± 1.68 | 4.33 ± 1.59 | 4.91 ± 1.46 | 5.09 ± 2.07 |
| cGMP 120 min (pmol/ml) | 3.68 ± 1.68* | 3.37 ± 1.68 | 5.37 ± 1.48 | 5.50 ± 1.88 |
| ADMA 0 min (μmol/ml) | 0.50 ± 0.18 | 0.59 ± 0.36 | 0.47 ± 0.20 | 0.40 ± 0.21 |
| ADMA 120 min (μmol/ml) | 0.58 ± 0.32 | 0.57 ± 0.39* | 0.41 ± 0.21** | 0.31 ± 0.2** |
| Nitrotyrosine 0 min (nmol/liter) | 1993.12 ± 1330.85* | 2208.02 ± 1736.57* | 803.7 ± 726.91 | 750.83 ± 426.03 |
| Nitrotyrosine 120 min (nmol/liter) | 1718.65 ± 771.7* | 1718.44 ± 1250.69* | 806.41 ± 910.00* | 822.90 ± 780.18 |
p < 0.05 DM type 1 vs control.
p < 0.005 vs 0 min.
Discussion
The aim of our study was to investigate arterial stiffness measured by pulse wave analysis and parameters of endothelial function in patients with type 1 diabetes and healthy controls and to determine the effect of insulin during an euglycemic clamp with two different insulin concentrations on these parameters in both groups. Patients with type 1 diabetes showed an increased augmentation and augmentation index compared to healthy controls. Insulin application, even under euglycemic conditions, was associated with a statistically significant improvement of the increased augmentation in patients with diabetes type 1 and no further effect on the normal pulse wave analysis in healthy controls. The same method of pulse wave analysis has been used previously in type 231 and in type 1 diabetic patients.20 In contrast to our study, Westerbacka et al.20 found comparable augmentation and augmentation index either in patients with type 1 diabetes or in healthy controls at baseline, whereas we found increased values in the diabetic patients. After insulin infusion, our patients, not the healthy controls, showed a decrease in both parameters, whereas Westerbacka and colleagues20 detected a decrease in augmentation and augmentation index only in the healthy control subjects. They proposed that the type 1 diabetic patients were resistant not only to the glucose-uptake stimulating effect of insulin, but also to its ability to decrease augmentation; moreover, they found that the rate of insulin-stimulated glucose uptake correlated inversely with the change in augmentation index. However, in our present study, patients with type 1 diabetes showed an improvement, but not a normalization of arterial stiffness, which might be explained by the direct vasodilating effect of insulin32 and, if at all, only secondly by the improvement of preexisting slight hyperglycemia during the euglycemic insulin clamp.
Pulse wave analysis for determination of Aix has been used to assess endothelial function not only in combination with vasoactive substances, but to investigate relationships between Aix and endothelial function under baseline conditions. Weber et al.33 found that increased ADMA levels correlated positively to increased arterial wave reflections measured as augmentation and augmentation index in patients undergoing coronary angiography.
Therefore, we investigated several parameters of endothelial function in patients with diabetes mellitus type 1 compared to healthy controls and the effects of insulin application during the euglycemic insulin clamp.
Nitrotyrosine, which is an index of peroxynitride, a cytotoxic compound formed from the superoxide anion and nitric oxide,34 is a marker of oxidative stress.35 Evidence shows that oxidative stress is involved in the pathogenesis of cardiovascular diseases in diabetes mellitus.36 Nitrotyrosine was found to be elevated significantly in our type 1 diabetic patients compared to healthy controls. Increased nitrotyrosine could be demonstrated in patients with either type 137 or type 2 diabetes.38 Evidence in the literature shows that an acute increase in blood glucose causes an increase in nitrotyrosine.39 In insulin-treated type 2 diabetic patients, nitrotyrosine production increased during postprandial hyperglycemia, indicating the role of postprandial hyperglycemia in the pathogenesis of cardiovascular disease through the production of oxidative stress.38 During the present euglycemic clamp, under fasting conditions and only slightly elevated blood glucose levels at baseline in the diabetic patients, insulin application did not change significantly the increased nitrotyrosine levels in patients with type 1 diabetes or in healthy controls.
In the present study, cGMP levels, which are described to be reduced in patients with advanced stages of type 1 diabetes, suggesting that NO release or its action on guanylate cyclase is reduced,40 did not differ between type 1 diabetes and healthy controls.
Plasma levels of ADMA, an endogenous inhibitor of endothelial NO synthase, have been shown to be elevated in diseases related to endothelial dysfunction.41 Increased levels of ADMA are associated with endothelial vasodilator dysfunction and increased risk of cardiovascular diseases.41,42 Plasma levels are shown to be correlated positively with insulin resistance in several studies, including normo- and hypertensive, nondiabetic subjects.43,44 In vitro, hyperglycemia impairs dimethylarginine dimethylaminohydrolase activity in vascular smooth muscle cells and the endothelium, leading to elevated ADMA levels.45 Moreover, a significant relationship between increased ADMA concentrations and type 2 diabetes/insulin resistance has been described.46,47 In type 1 diabetes, ADMA is elevated in patients with diabetic nephropathy, whereas no relationship between ADMA and diabetic retinopathy was detected.48 According to this, in our present study, we could not find a statistically significant difference in ADMA in our relatively young diabetic patients without any microvascular complications compared to healthy controls. Furthermore, insulin application during the euglycemic clamp did not influence the ADMA plasma concentration in patients with type 1 diabetes; however, healthy control subjects showed a significant decrease in ADMA during the euglycemic clamp with both insulin concentrations.
On the contrary, elevated ADMA levels are associated with an increased risk for stroke and myocardial infarction in patients with diabetic nephropathy.48 As described earlier, ADMA levels are associated with increased arterial wave reflections, most likely to decreased NO activity in small arteries.33
Considering the literature and the results of our study, endothelial function measured by several plasma parameters such as nitrotyrosine, cGMP, and ADMA or noninvasively by pulse wave analysis is impaired in patients with uncomplicated type 1 diabetes. Application of insulin improves the arterial elastic properties, but was not able to normalize the vascular function in patients with type 1 diabetes.
Abbreviations
- ADMA
asymmetric dimethylarginine
- Aix
augmentation index
- AP
augmentation
- cGMP
cyclic guanosine monophosphate
- DM
diabetes mellitus
- NO
nitric oxide
- PWA
pulse waveform analysis
References
- 1.Letho S, Rönnemaa T, Pyoräkä K, Laakso M. Poor glygemic controls predicts heart disease events in patients with type 1 diabetes without nephropathy. Aterioscler Thromb Vasc Biol. 1999;19:1014–1019. doi: 10.1161/01.atv.19.4.1014. [DOI] [PubMed] [Google Scholar]
- 2.Asmar R, Benetos A, London G, Hugue C, Weiss Y, Topouchian J. Aortic distensibility in normotensive, untreated and treated hypertensive patients. Blood Press. 1995;4:48–54. doi: 10.3109/08037059509077567. [DOI] [PubMed] [Google Scholar]
- 3.O´Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2–9. doi: 10.1161/01.hyp.26.1.2. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WW, O´Rourke MF. Theoretical, experimental and clinical principles. 4th. London: Edward Arnold; 1998. McDonalds blood flow in arteries. [Google Scholar]
- 5.O´Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999;17:1–4. doi: 10.1097/00004872-199917010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Berry KL, Skyrme-Jones RA, Cameron JD, O´Brien RC, Meredith IT. Systemic arterial compliance is reduced in young patients with IDDM. Am J Physiol. 1999;276:H1839–1845. doi: 10.1152/ajpheart.1999.276.6.H1839. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Wallentsteen M, Genser G. Increased stiffness of the aorta in children and adolescents with insulin-dependent diabetes mellitus. Ultrasound Med Biol. 1996;22:537–543. doi: 10.1016/0301-5629(96)00040-3. [DOI] [PubMed] [Google Scholar]
- 8.Oxlund H, Rasmussen LM, Andreassen TT, Heikendorff L. Increased aortic stiffness in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:748–752. doi: 10.1007/BF00274536. [DOI] [PubMed] [Google Scholar]
- 9.Christensen T, Neubauer B. Increased arterial wall stiffness and thickness in medium-sized arteries in patients with insulin-dependent diabetes mellitus. Acta Radiol. 1988;29:299–302. [PubMed] [Google Scholar]
- 10.Ryden-Ahlgren A, Lanne T, Wollmer P, Sonesson B, Hansen F, Sundkvist G. Increased arterial stiffness in women, but not in men with IDDM. Diabetologica. 1995;38:1082–1109. doi: 10.1007/BF00402179. [DOI] [PubMed] [Google Scholar]
- 11.Giannattasio G, Failla M, Piperno A, Grappiolo A, Gamba P, Paleari F, Mancia G. Early impairment of large artery structure and function in type 1 diabetes mellitus. Diabetologica. 1999;42:987–994. doi: 10.1007/s001250051257. [DOI] [PubMed] [Google Scholar]
- 12.Westerbacka J, Uosukainen A, Mäkimattila S, Schlenzka A, Yki-Järvinen H. Insulin-induced decrease in large artery stiffness is impaired in uncomplicated type 1 diabetes mellitus. Hypertension. 2000;35:1043–1048. doi: 10.1161/01.hyp.35.5.1043. [DOI] [PubMed] [Google Scholar]
- 13.Jensen-Urstad KF, Reichard PG, Rosfors JS, Lindblad LE, Jensen-Urstad MT. Early artheriosclerosis is retarded by improved long-term blood glucose control in patients with IDDM. Diabetes. 1996;45:1253–1258. doi: 10.2337/diab.45.9.1253. [DOI] [PubMed] [Google Scholar]
- 14.De Jongh RT, Clark AD, Izerman RG, Serne EH, De Vries G, Stehouwer CD. Physiological hyperinsulinaemia increases intramuscular microvascular reactive hyperaemia and vasomotion in healthy volunteers. Int J Obes Relat Metab Disord. 2004;47:978–986. doi: 10.1007/s00125-004-1412-9. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg HO. Insulin effects on blood flow. Curr Opin Endocrinol Diabetes. 1999;6:135–140. [Google Scholar]
- 16.Vehkavaara S, Makimattila S, Schlenzka A, Vakkilainen J, Westerbacka J, Yki-Jarvinen H. Insulin therapy improves endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2000;20(2):545–550. doi: 10.1161/01.atv.20.2.545. [DOI] [PubMed] [Google Scholar]
- 17.Poldermann KH, Stehouwer CDA, vanKamp GJ, Gooren LJG. Effects of insulin infusion on endothelium-derived vasoactive substances. Diabetologia. 1996;39:1284–1292. doi: 10.1007/s001250050571. [DOI] [PubMed] [Google Scholar]
- 18.Flynn MD, Boolell M, Tooke JE, Watkins PJ. The effect of insulin infusion on capillary blood flow in the diabetic neuropathic foot. Diabet Med. 1992;9:630–634. doi: 10.1111/j.1464-5491.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 19.Tooke JE, Lins PE, Ostergren J, Adamson U, Fagrell B. The effects of intravenous insulin infusion on skin microcirculatory flow in type 1 diabetes. Int J Microcirc Clin Exp. 1985;4:69–83. [PubMed] [Google Scholar]
- 20.Westerbacka J, Wilkinson I, Cockcroft J, Utrianen T, Vehkavaara S, Yki-Järvinene H. Diminished wave reflection in the aorta: a novel physiological action of insulin on large blood vessels. Hypertension. 1999;33:1118–1123. doi: 10.1161/01.hyp.33.5.1118. [DOI] [PubMed] [Google Scholar]
- 21.O´Rourke MF, Gallgagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14:147–157. [PubMed] [Google Scholar]
- 22.Wilkinson IB, Crockfort JR, Webb DJ. Pulse wave analysis and arterial stiffness. J Cardiovasc Pharmacol. 1998;32:S33–37. [PubMed] [Google Scholar]
- 23.Filiposvsky J, Svobodova V, Pecen L. Reproducibility of radial pulse wave analysis in healthy subjects. J Hypertens. 2000;18:1033–1040. doi: 10.1097/00004872-200018080-00007. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson IB, MacCullum H, Rooijans DF, Murray GD, Crockfort JR, Scanlon MF. Increased augmentation index and systolic stress in type 1 diabetes. QJM. 2000;93:441–448. doi: 10.1093/qjmed/93.7.441. [DOI] [PubMed] [Google Scholar]
- 25.Smith JC, Page MD, John R, Wheeler MH, Crockfort JR, Scanlon MF. Augmentation of central arterial pressure in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:3515–3519. doi: 10.1210/jcem.85.10.6880. [DOI] [PubMed] [Google Scholar]
- 26.Kohara J, Jiang Y, Igase M, Hiwada K. Effect of reflection of arterial pressure on caroid circulation in essential hypertension. Am J Hypertens. 1999;12:1015–1020. doi: 10.1016/s0895-7061(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Nevo E, Fetics B. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: validation of a generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 28.Pauca AL, O´Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial arterial pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 29.O´Rourke MF, Kon ND. Generation of ascending aortic from radial artery pressure wavefrom. J Am Coll Cardiol. 2002;39(Suppl B):177B. [Google Scholar]
- 30.Wilkinson IB, MacCullum H, Flint L. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:63–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scharm MT, Henry RMA, van Djik RAJM, Kotense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Westerhof N, Stehouwer CDA. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes. Hypertension. 2004;43:176–181. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- 32.Joshua IC, Zhang Q, Falcone JC, Bratcher AP, Rodriguez WE, Tyagi SC. Mechanisms of endothelial dysfuction with development of type 1 diabetes mellitus: role of insulin and c-peptide. J Cell Biochem. 2005;96:1149–1156. doi: 10.1002/jcb.20620. [DOI] [PubMed] [Google Scholar]
- 33.Weber T, Maas R, Auer J, Lamm G, Lassnig E, Rammer M, O´Rourke MF, Boger RH, Eber B. Arterial wave reflections and determinants of endothelial function as hypothesis based on peripheral mode of action. Am J. Hypertension. 2007;20:256–262. doi: 10.1016/j.amjhyper.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Eizirik DL, Flodstrom M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic β cells. Diabetolgica. 1996;39:875–890. doi: 10.1007/BF00403906. [DOI] [PubMed] [Google Scholar]
- 35.Ceriello A, Mercuri F, Qualiaro L, Motz E, Tonutti L, Toboga C. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologica. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 36.Giugliano D, Cervello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 37.Hoeldtke RD, Bryner KD, Mc Neil DR, Wareshime SS, Van Dyke K, Hobbs G. Oxidative stress and insulin requirements in patients with recent-onsent type 1 diabetes. JCEM. 2003;88:1624–1628. doi: 10.1210/jc.2002-021525. [DOI] [PubMed] [Google Scholar]
- 38.Ceriello A, Qualiaro L, Catone B, Pascon R, Piazzola DS, Bais B, Marra G, Tonutti L, Toboga C, Motz E. Role of hyperglycemia in nitrotyrosine generation. Diabetes Care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 39.Martella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001;108:635–636. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamata K, Miyata N, Abiru T, Kasuya J. Functional changes in the vascular smooth muscle and endothelium of arteries during diabetes mellitus. Life Sci. 1992;50:1379–1387. doi: 10.1016/0024-3205(92)90256-o. [DOI] [PubMed] [Google Scholar]
- 41.Schulze F, Lenzen H, Hanefeld C, Bartling A, Osterziel KJ, Godeva L, Schmidt-Lucke C, Kusus M, Maas R, Schedhelm E, Strodter D, Simon BC, Mugge A, Daniel WG, Tillmanns H, Maisch B, Streichert T, Boger RH. Asymmetric dimethylarginine is an independent risk factor coronary heart disease: results from the multicenter Coronary Artery Risk Determination investing the influence of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152:e1–8. doi: 10.1016/j.ahj.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Cooke JP. ADMA: its role in vascular disease. Vasc Med. 2005;10(Suppl 1):S11–17. doi: 10.1177/1358836X0501000103. [DOI] [PubMed] [Google Scholar]
- 43.Sydow K, Mondon CE, Cooke JP. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med. 2005;10(Suppl 1):S35–43. doi: 10.1177/1358836X0501000106. [DOI] [PubMed] [Google Scholar]
- 44.Takiuchi S, Fujii H, Kamide K, Horio T, Nakatani S, Hiuge A, Rakugi H, Ogihara T, Kawano Y. Plasma asymmetric dimethylarginine and coronary and peripheral endothelial function in hypertensive patients. Am J Hypertens. 2004;17:802–808. doi: 10.1016/j.amjhyper.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Lin KY, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–992. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 46.Abbassi F, Asagami T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, Stuhlinger MC, Tsao PS. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 47.Stulinger MC, Abbassi F, Chu JW, Lamendola C, McLaughlin TL, Cooke JP, Reaven GM, Tsao PS. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287:1420–1426. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 48.Tarnow L, Hovind P, Teerlink T, Stehouwer CAD, Pariving HH. Elevated plasma asymetric dimethylarginine as marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care. 2004;27:765–769. doi: 10.2337/diacare.27.3.765. [DOI] [PubMed] [Google Scholar]


