Abstract
Background
A truly noninvasive glucose-sensing device could revolutionalize diabetes treatment by leading to improved compliance with recommended glucose levels, thus reducing the long-term complications and cost of diabetes. Herein, we present the technology and evaluate the efficacy of a truly noninvasive device for continuous blood glucose monitoring, the NBM (OrSense Ltd.).
Methods
In vitro analysis was used to validate the technology and algorithms. A clinical study was performed to quantify the in vivo performance of the NBM device. A total of 23 patients with type 1 (n = 12) and type 2 (n = 11) diabetes were enrolled in the clinical study and participated in 111 sessions. Accuracy was assessed by comparing NBM data with paired self-monitoring of blood glucose meter readings.
Results
In vitro experiments showed a strong correlation between calculated and actual glucose concentrations. The clinical trial produced a total of 1690 paired glucose values (NBM vs reference). In the paired data set, the reference glucose range was 40–496 mg/dl. No systematic bias was found at any of the glucose levels examined (70, 100, 150, and 200 mg/dl). The mean relative absolute difference was 17.2%, and a Clarke error grid analysis showed that 95.5% of the measurements fall within the clinically acceptable A&B regions (zone A, 69.7%; and zone B, 25.7%).
Conclusions
This study indicates the potential use of OrSense's NBM device as a noninvasive sensor for continuous blood glucose evaluation. The device was safe and well tolerated.
Keywords: noninvasive glucose monitoring, occlusion spectroscopy, self-monitoring of blood glucose
Introduction
Diabetes has evolved as one of the principal health care epidemics of the modern era. Worldwide the total number of people with diabetes is expected to rise from 171 million in 2000 to 366 million in 2030.1 Not only was diabetes the sixth leading cause of death listed on U.S. death certificates in 2002, but the estimated cost of diabetes in the United States in 2002 was $132 billion, consisting of both direct and indirect expenses (disability, work loss, premature mortality).2 Tight glycemic control is known to decrease the devastating and costly secondary micro- and macrovascular complications associated with diabetes, thus improving the quality of life for millions of diabetic patients and significantly reducing the health care expenditure.3–6 Tight glucose control has also been shown to provide clinical benefits in critically ill patients.7,8
Continuous glucose monitoring (CGM) provides additional temporal information, such as trends, magnitude, duration, and frequency of glucose level fluctuations. This information can aid in the identification and prevention of unwanted hypo- and hyperglycemic episodes (e.g., during sleep). Furthermore, it can activate alarm signals for extreme glucose levels, decrease the nursing workload in tight glycemic control, and facilitate in automatic feedback-controlled insulin delivery systems, such as an artificial pancreas. CGM can also help adjust therapy, quantify the response in diabetic therapy trials, and monitor conditions where tight control without hypoglycemia is sought (Intensive Care Units, gestational diabetes, pediatric diabetes).9
Self-monitoring of blood glucose (SMBG) levels is highly recommended by the American Diabetes Association as an integral part of the management strategy for diabetic patients using multiple insulin injections. For patients using less frequent insulin injections, oral agents, or medical nutrition therapy alone, SMBG is helpful in achieving glycemic goals as well.10 Nevertheless, because most patients use conventional glucometers, the number of daily measurements is limited by patient compliance and by the price of the disposable glucometer sticks.11 Consequently, only intermittent and incomplete profiles of blood glucose fluctuations are generated. A truly noninvasive glucose-sensing device could revolutionalize diabetes treatment by leading to improved compliance with recommended glucose levels, thus obtaining hemoglobin A1c at normal or near-normal levels without increasing the incidence of hypoglycemia due to intensive insulin therapy.12
Great advances have been made in glucose measurement techniques since the first chemical test were introduced in the early 19th century.13 Current cutting-edge developments in glucose monitoring include minimally invasive techniques using body fluids other than blood (e.g., sweat, saliva, interstitial fluid; compromising the skin barrier without puncturing blood vessels) and truly noninvasive glucose measurements, based on various optical methods. Noninvasive techniques include optical coherence tomography, near-infrared and Raman spectroscopy, polarimetry, light scattering, photoacoustic spectroscopy, thermo-optical studies on human skin, fluorescence measurements, and more.14–18
The goal of this article is to present a comprehensive survey of OrSense's noninvasive “occlusion spectroscopy” technology. The following sections describe the scientific background of the technology, including a report on in vitro experiments and a summary of the NBM performance in clinical trials.
Scientific Background
OrSense's NBM device is based on occlusion spectroscopy, which is a proprietary technology19–21 aimed toward assuring accurate, convenient, and affordable glucose monitoring. The measurement is performed using an annular probe, which is positioned on the finger's root (Figure 1). The probe contains light sources and detectors operating in the red/near-infrared (RNIR) spectral region and pneumatic cuffs that produce oversystolic pressure to occlude blood flow. The occlusion of blood flow generates a dynamic optical signal (Figure 2), which facilitates accurate glucose monitoring. The probe has a special adaptive mechanism for easy positioning and a suitable grip for a wide range of finger sizes, thus assuring user convenience and compliance.
Figure 1.
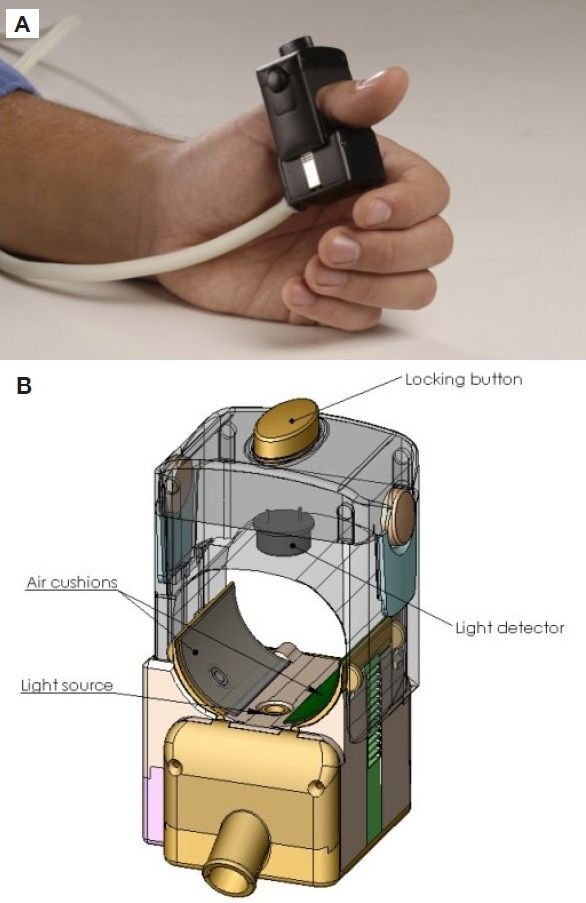
(A) Photograph and (B) schematic drawing of the noninvasive glucose monitoring probe from OrSense's NBM device.
Figure 2.
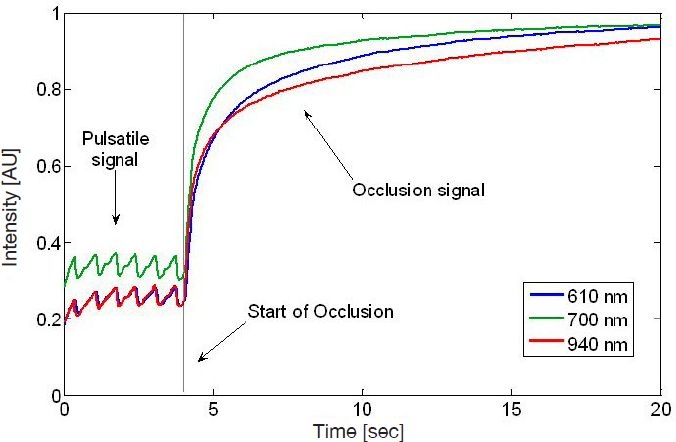
In vivo transmission signal recorded by the NBM device. From time t = 0 to 4 seconds, no pressure is applied, resulting in a pulsatile signal. After time t = 4, oversystolic pressure is applied to the finger. This pressure results in the occlusion signal from t = 4 to 20 seconds. The different curves relate to different wavelengths.
The technology is based on the direct effect of glucose on the scattering properties of the organ. Glucose decreases the mismatch in refractive index between scatterers and their surrounding media, leading to a smaller scattering coefficient and, consequently, a shorter optical path (Figure 3). As a result, with the growing concentration of glucose, fewer photons are absorbed and the light intensity increases.22
Figure 3.
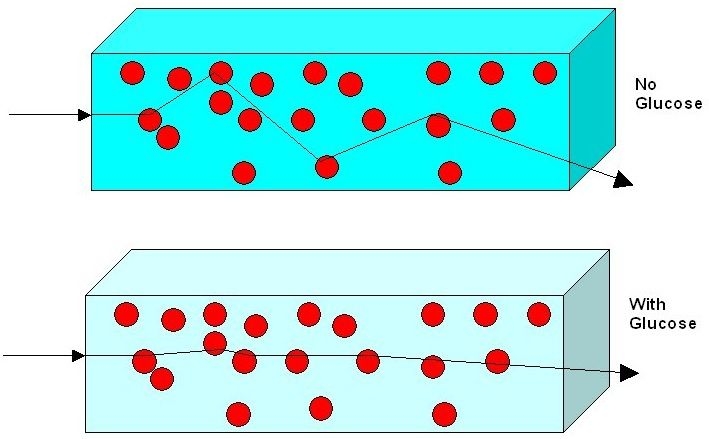
Schematic description of the influence of glucose on light propagation. More glucose results in less scattering, consequently shorter optical paths, and therefore less absorption.
To facilitate measurement accuracy, one needs to optimize for sensitivity and specificity. This is achieved by the following:
Transmission mode. In contrast to other configurations,. we use transmitted light in addition to reflected light for glucose measurement. In the transmission mode the light traverses the whole organ (finger), and the photons typically encounter many more glucose molecules along their paths than in the reflection mode. This enhances the sensitivity to glucose and clinically relates to an average over a whole organ rather than a highly localized measurement. In addition, the influence of local factors such as skin morphology and pigmentation is reduced considerably.
Dynamic signal. Occlusion spectroscopy is based on generation of an optical signal that changes with time. The signal is induced by oversystolic occlusion at the finger's root, which causes cessation of blood flow throughout the finger. This allows us to collect not only one data point per wavelength, but rather a whole function. The occlusion generates a signal whose magnitude of variation is typically 10 times stronger than that of the pulse signal (refer to Figure 2). This results in the better signal-to-noise ratio necessary for accurate measurement. Hence, the dynamic signal enhances the sensitivity to glucose and the robustness to interferences, resulting in a more accurate glucose measurement.
Multispectral data. We use multiple (typically 10). wavelengths of light sources. This is beneficial for specificity/selectivity, as the different behavior of the optical signal among wavelengths allows cleaning the influence of unwanted interferences, such as the absorption of hemoglobin and changes of oxygen saturation. The wavelengths are within the RNIR, allowing for transmission of light across the finger.
Sophisticated algorithms. The rich data gathered are processed with sophisticated algorithms, which use only a small number of parameters, hence avoiding overfitting and false correlations. The algorithms combine mathematical methods and physical insights, which contribute to the accurate prediction of glucose concentration.
In Vitro Experiments
In vitro phantoms are highly important in the development of accurate noninvasive glucose measurement devices. They provide a reasonably realistic physiological setting in which the basic physical principles, as well as the engineering and algorithmic concepts, can be verified.
Here we describe one of OrSense's finger phantoms (Figure 4). Other more advanced finger phantoms are described elsewhere.
Figure 4.
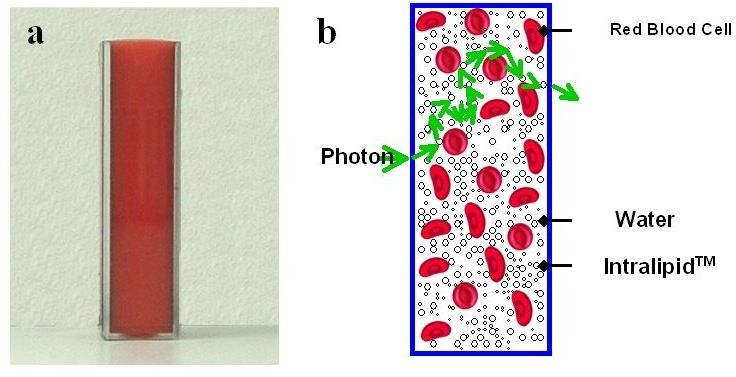
(A) A photograph of the finger phantom. (B) A schematic drawing of the phantom showing a potential photon path.
The finger phantom is designed to mimic the scattering and absorption properties of the finger in the red/near-infrared spectral region. Tissue scattering is modeled by use of an aqueous suspension of lipid droplets (Intralipid™), whereas absorption is accounted for by direct use of red blood cells (RBC). Both components are mixed to mimic the blood–tissue compound and are inserted into a rectangular cuvette. During the experiments, three physiological parameters are explored.
The concentration of glucose is varied and controlled by direct addition of glucose in the range of 0 to 1000 mg/dl.
The hematocrit level is determined by the addition of various RBC concentrations, representing hematocrit levels from 30 to 50%.
Deoxygenation is induced through nitrogen bubbling of the RBC suspension.
The finger phantom is illuminated with white light (Spectra Physics), and the transmitted light is measured using a high-performance spectrometer (Princeton Instruments). Measurements are performed for different concentrations of glucose with concurring variations in the hematocrit and oxygenation.
Analysis of transmission spectra (Figure 5) shows a decrease of the transmitted light intensity with increasing concentration of hematocrit. This is because of the absorbing nature of the hemoglobin molecules. More importantly, an increase of transmitted light is observed concurrently with the increase in glucose concentration for each of the hematocrit levels examined. This effect is in full accordance with the theoretical considerations outlined earlier. Namely, the reduction of the refractive index mismatch results from the reduction in scattering, induced by glucose dissolved in the aqueous solution.
Figure 5.
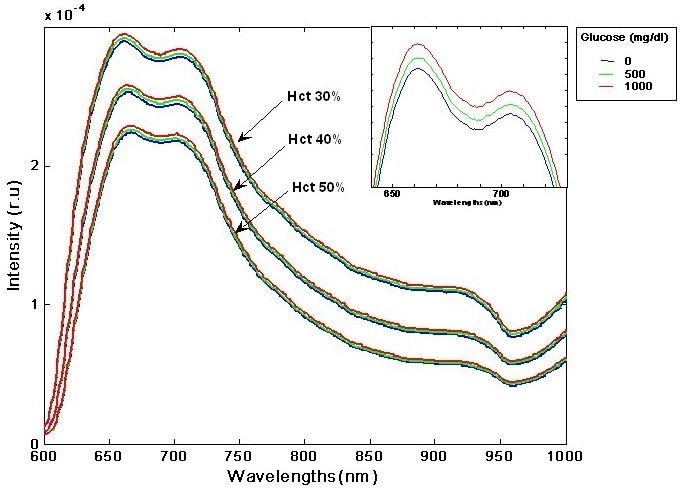
Transmitted light (in arbitrary units) as a function of wavelength for different hematocrit and glucose levels in the finger phantom. See inset graph for a close-up on glucose variation at a particular hematocrit level.
When the hematocrit and oxygen levels are not constant, the effect of glucose cannot be derived directly from transmission data. This mimics a real-life scenario, where physiological changes interfere with the glucose signal. To that end, OrSense's algorithm, devised for in vivo applications, was applied to the series of measurements. The correlation between the calculated glucose parameter (in arbitrary units) and the actual concentration of glucose was 0.95, demonstrating the ability to measure glucose concentration accurately even in the presence of strong physiological interferences. In addition, a parameter closely correlated with the reference hematocrit levels was extracted, and the correlation between the hematocrit and the calculated hematocrit parameter was 0.99.
Clinical Trial Design and Method
A total of 23 adult subjects with diabetes type 1 and
type 2 were enrolled in the clinical study. Twelve subjects had type 1 diabetes and 11 subjects had type 2 diabetes with a mean ± SD age of 42 ± 19 and a mean ± SD body mass index of 26.7 ± 6. Thirteen subjects were male and 10 subjects were female. Of the type 1 subjects, six used continuous subcutaneous insulin delivery (CSID) and six used multiple daily injections (MDI) of insulin. Six type 2 subjects used medications, apart from four subjects who used MDI and one subject who used CSID.
Noninvasive Device
The NBM device is used by placing the patient's thumb inside a circumferential probe placed on the proximal phalanx. For each session, calibration of the device requires four glucose reference values taken over the course of 3 hours, where the first point must be at least half an hour after application of the probe. In this study, each session began with a 3-hour calibration period consisting of four measurements taken every hour starting 30 minutes after the monitoring start time t0 (i.e., at t0+0:30, t0+1:30, t0+2:30, t0+3:30). All sessions began in the morning. Half an hour after the beginning of each session, the patients were instructed to eat and inject insulin as usual. Throughout the entire session, noninvasive data from the probe were collected every 10 minutes. Although the actual measurements lasted 2.5 minutes, a filtering algorithm was applied to reduce noise and remove outliers. Accordingly, the glucose value at time t: Gl(t) was available at time t+0:30, introducing an artificial delay.
Study Design
The study was conducted at an outpatient clinic. Subjects were blinded to NBM readings and used SMBG meters to guide treatment decisions. Reference glucose values were taken every 30 minutes using FreeStyle (Abbott Ltd.) SMBG fingersticks and monitor on capillary blood samples. The study was approved by the Kaplan Medical Center (Rehovot, Israel) ethics committee and all participating patients provided written informed consent before enrollment.
End Points
Establishing the clinical accuracy and efficiency of continuous glucose monitors is a complex task. In order to test our device, we compiled relevant data from the literature and existing standards to establish a baseline against which to test our device. As our primary efficacy end point we examined sensor bias as compared with SMBG measurements using FreeStyle (Abbott Ltd.) blood glucose fingersticks and monitor on capillary blood samples. This was done in the spirit of the research trial testing the Dexcom STS continuous subcutaneous glucose sensor.18 In order for the study to be deemed successful, bias had to be ≤15 mg/dl. This hypothesis was tested at glucose levels of 70, 100, 150, and 200 mg/dl. Secondary end points were clinical accuracy as assessed by Clarke error grid analysis (EGA), mean relative absolute difference (RAD), and Pearson correlation coefficient. Here, RAD is defined on paired data points as the absolute difference between NBM and SMBG meter glucose values divided by the SMBG meter value, yielding a percentage value.
Statistical Analysis
The significance level was 0.05. All hypothesis testing was done using two-sided tests unless stated otherwise. The comparison of continuous variables was conducted using independent sample t tests.
Bias estimations were based on a Deming regression analysis. Deming regression takes into account the error in the reference device (the FreeStyle SMBG meter). The variance ratio used in the Deming regression was 1 (NBM) to 1 (SMBG meter).
Results
On average, the subjects participated in 4.9 ± 3.0 (mean ± SD) sessions of 8–12 hours duration, yielding a total of 111 sessions, with a total of 1671 paired glucose values (NBM vs SMBG). It should be noted that the overall patient compliance was positive and that during the trial's measurements the patients ate and injected insulin according to their usual regimen.
In the paired data set, the reference glucose range was 40–496 mg/dl. In order to compare the NBM glucose monitor to a standard SMGB device, a Deming regression was performed on all paired glucose values and used to calculate bias of glucose over a range of values. The calculated bias in mg/dl at glucose levels 70, 100, 150, and 200 mg/dl was found to be (95% confidence intervals) −2.5 (−6.3 to 1.3), −2.5 (−5.7 to 0.6), −2.5 (−4.9 to −0.2), and −2.5 (−4.8 to −0.3), respectively. Based on these results, the null hypothesis (bias >15 mg/dl) was rejected at the α = 0.05 significance level.
The mean relative absolute difference was 17.2%. A Deming regression analysis yielded a slope of 1.00, reflecting a good overall correlation between NBM and SMBG devices. Furthermore, a Clarke EGA showed that 95.5% of the measurements fall within the clinically acceptable A&B regions (zone A, 69.7%; and zone B, 25.7%). These results are shown in Figure 6. Further accuracy measures are summarized in Table 1.
Figure 6.
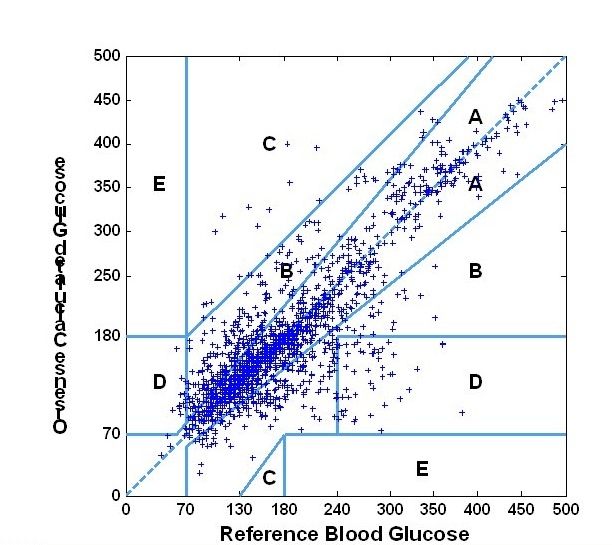
Clarke error grid analysis.
Table 1.
Summary of NBM Noninvasive vs FreeStyle Finger-Prick Accuracy Measures
| Statistic name | Value | Description |
|---|---|---|
| Mean RAD | 17.2% | Mean relative absolute difference |
| Slope | 1.0 | Slope of linear Deming regression |
| Intercept | -2.5 mg/dl | Intercept of linear Deming regression |
| Average error | -2.1 mg/dl | Average of the errors Y−X |
| N | 1671 | Number of samples |
In addition to the pooled data Clarke analysis, three daily sessions of a 75-year-old male with type 2 diabetes are displayed in Figure 7 where the continuous NBM glucose profile is compared to spot reference glucose data. Here, excellent continuous tracking of the subject's daytime behavior can be seen. Additionally, the device allows us to follow the subject's intradaily behavior, variability, and trends.
Figure 7.
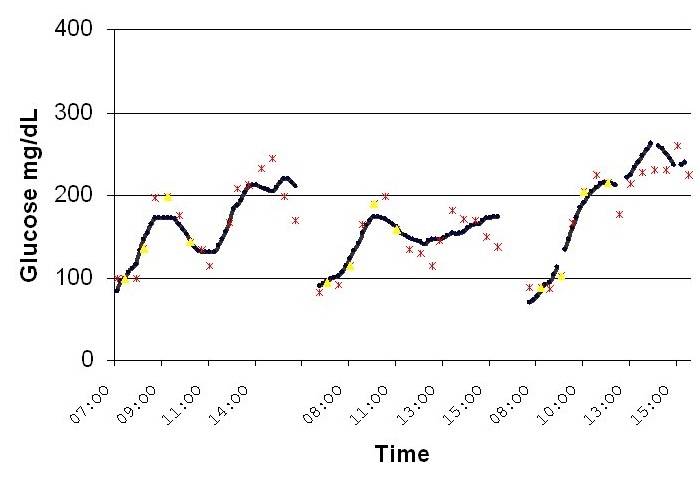
Three daily sessions of a 75-year-old male with type 2 diabetes.
Conclusions
We have presented the technology of the NBM (OrSense Ltd.) noninvasive device for continuous blood glucose monitoring, which is based on occlusion spectroscopy.
In vitro experiments applying in vivo-like algorithms showed a strong correlation between calculated glucose parameters and actual concentration in the presence of strong physiological interferences.
To evaluate the in vivo performance of the device, a clinical study was performed. The mean RAD was 17.2%. Clarke EGA on the paired data set consisting of 1671 NBM glucose values compared to reference SMBG measurements using the FreeStyle (Abbott Ltd.) showed that 95.5% of the measurements fall within the clinically acceptable A&B regions (zone A, 69.7%; and zone B, 25.7%).
This study indicates the potential use of OrSense's NBM as a noninvasive sensor for continuous blood glucose monitoring. In addition to showing acceptable accuracy, the device was safe, painless, and well tolerated, as reflected by the high overall patient compliance. Development of the NBM device is currently focused on improving the performance and ease of use in the home-use setting.
Abbreviations
- CGM
continuous glucose monitoring
- CSID
continuous subcutaneous insulin delivery
- EGA
error grid analysis
- MDI
multiple daily injections
- NBM
noninvasive blood monitor
- RAD
relative absolute difference
- RBC
red blood cells
- RNIR
red/near infrared
- SMBG
self-monitoring of blood glucose
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.National diabetes information clearinghouse, NIH publication No. 2006 Sep; 06-3873. [Google Scholar]
- 3.Arnold MA, Small GW. Noninvasive glucose sensing. Anal Chem. 2005;77:5429–5439. doi: 10.1021/ac050429e. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes control and complications trial research group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes in America. 2nd ed. National Institute of Diabetes and Digestive and Kidney Diseases; 1995. National diabetes data group, National Institutes of Health. NIH Publication No. 95-1468. [Google Scholar]
- 6.American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005 May;28(5):1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Practice Recommendations Standards of Medical Care in Diabetes–2006 American Diabetes Association. Diabetes Care. 2006;29:4–42. [PubMed] [Google Scholar]
- 11.Weinzimer SA, Doyle EA, Tamborlane WV. Disease management in the young diabetic patient: glucose monitoring, coping skills, and treatment strategies. Clin Pediatr. 2005;44:393–403. doi: 10.1177/000992280504400503. [DOI] [PubMed] [Google Scholar]
- 12.Guerci B, Floriot M, Böhme P, Durain D, Benichou M, Jellimann S, Drouin P. Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analogs. Diabetes Care. 2003 Mar;26(3):582–589. doi: 10.2337/diacare.26.3.582. [DOI] [PubMed] [Google Scholar]
- 13.Steffes MW, Sacks DB. Measurement of circulating glucose concentrations: the time is now for consistency among methods and types of samples. Clin Chem. 2005;51(9):1569–1570. doi: 10.1373/clinchem.2004.044867. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg BH. An overview of minimally invasive technologies. Clin Chem. 1992 Sep;38(9):1596–1600. [PubMed] [Google Scholar]
- 15.Khalil SK. Non-invasive glucose measurement technologies: an update from 1999 to the dawn of the new millennium. Diabetes Technol Ther. 2004 Oct;6(5):660–697. doi: 10.1089/dia.2004.6.660. [DOI] [PubMed] [Google Scholar]
- 16.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive and minimally invasive methods for trans-dermal glucose monitoring. Diabetes Technol Ther. 2005 Feb;7(1):174–197. doi: 10.1089/dia.2005.7.174. [DOI] [PubMed] [Google Scholar]
- 17.Weiss R, Yegorchikov Y, Shusterman A, Raz I. Noninvasive continuous glucose monitoring using photoacoustic technology—results from the first 62 subjects. Diabetes Technol Ther. 2007 Feb;9(1):68–74. doi: 10.1089/dia.2006.0059. [DOI] [PubMed] [Google Scholar]
- 18.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006 Jan;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 19.Shvartsman L, Fine I. Optical Transmission of blood: effect of erythrocyte aggregation. IEEE Trans Biomed Eng. 2003 Aug;50(8):1026–1033. doi: 10.1109/TBME.2003.814532. [DOI] [PubMed] [Google Scholar]
- 20.Fine I, Shvartsman L. Non-Invasive method and system of optical measurements for determining the concentration of a substance in blood. US patent 6,400,972. 2002 Jun 4; [Google Scholar]
- 21.Fine I, Shvartsman L. Method for non-invasive optical measurements of blood parameters. US patent 6,587,704. 2003 Jul 1; [Google Scholar]
- 22.Kohl M, Essenpreis M, Cope M. The influence of glucose concentration upon the transport of light in tissue simulating phantoms. Phys Med Biol. 1995;40:1267–1287. doi: 10.1088/0031-9155/40/7/009. [DOI] [PubMed] [Google Scholar]


