Abstract
Background
Intragastric balloons have been used for weight loss with varying success. Widespread use of intragastric balloons has been limited because balloons must be placed in, and removed from, the stomach endoscopically. Development of a balloon that does not require endoscopy suggests that obesity treatment with intragastric balloons is feasible. The purpose of this study was to test the Ullorex® oral intragastric balloon (OIB) in a sample of human participants.
Methods
The Ullorex OIB is a large capsule that is injected with citric acid and swallowed. After 4 minutes, the balloon inflates to 300 cm3. Stomach acid degrades a plug on the balloon over 25–30 days, when the balloon deflates and passes in feces. The Ullorex OIB was tested in 12 humans (two participants received placebo capsules). Body weight was monitored before and after balloon placement, and test meals quantified food intake among 6 of the 12 participants, all of whom received one balloon.
Results
A single significant adverse event occurred. The one participant randomized to receive three balloons developed nausea and vomiting, requiring intravenous fluids, which was likely influenced by noncompliance (eating solid foods after balloon placement). Participants who received balloons had a significant mean weight loss over 2 weeks, amounting to 1.5 kg (p < 0.05). A marginally significant food intake reduction from baseline to week 1 was found (149 kcal, 24.4%) (p = 0.055).
Conclusions
The Ullorex OIB was successfully utilized in this study, with one serious adverse event that was likely influenced by noncompliance. Body weight and food intake data suggest that the Ullorex OIB be tested further as a possible treatment for obesity.
Keywords: balloon, device, food intake, gastric, obesity
Background
Gastric balloons were developed in the 1980s for the treatment of obesity based on the premise that they would act like a bezoar, take up room in the stomach, induce satiety, and cause weight loss.1 One of the first gastric balloons was the cylindrical Garren gastric balloon (GIB), which was approved by the U.S. Food and Drug Administration in 1985. This gastric balloon was inserted endoscopically, filled with 220 cm3 of air, and removed endoscopically after approximately 12 weeks.2 The GIB did not change gastrin or cholecystokin levels, was associated with gastric erosions and ulcers, and showed minimal weight loss.3 It was not until the GIB was in use that a double-blind, sham-controlled study was performed. The GIB and the sham groups both lost weight, but the weight loss was not different between the two groups.4 When the GIB was compared to gastric surgery, surgical patients lost significantly more weight in the first 12 weeks.5 Subsequently, food intake was not found to decrease with use of a balloon until the volume exceeded 440 ml, and obese individuals were found to have a greater gastric capacity than lean individuals.6 Because of its lack of efficacy and associated adverse events, the GIB was withdrawn from the market.
Several gastric balloons were developed when the GIB was withdrawn from the market. The Ballobes® gastric balloon decreased hunger over 2 months, was oval and smooth, and was filled with 400–500 ml of air.7,8 The Ballobes balloon had a reduced incidence of adverse events compared to the GIB, but increased gastroesophageal reflux.9 Only one of two double-blind, sham-controlled studies showed greater weight loss in the Ballobes-treated group compared to the sham group, and the difference in that group from the sham group was only 4 kg over 12 weeks.10,11
The Dow-Corning silicon gastric balloon was fashioned from a breast implant and was inflated with 300–400 ml of air, but, like the Ballobes balloon, gave minimal weight loss compared to control.12,13 Pear-shaped gastric balloons were also used, inflated with either air or fluid, and resulted in weight loss in uncontrolled trials.14,15 One of the most widely utilized gastric balloons, however, is the BioEnterics intragastric balloon (BIB).
The BIB is a smooth spherical balloon filled with 400–700 ml of fluid, placed endoscopically, and removed endoscopically after 3–6 months.16 A series of 2515 patients treated with the BIB had a mean excess weight loss of 34% and complications included gastric perforation (0.19%), death (0.08%), balloon rupture (0.36%), esophagitis (1.27%), and gastric ulcer (0.2%).17 The BIB and diet were compared to diet alone, and the BIB–diet group lost more weight in a shorter period of time, although weight was regained after balloon removal.18 Weight loss with the BIB was accompanied by an improvement of obesity-associated diseases, particularly diabetes.19 Improvement in sleep apnea was also reported as being related to BIB-induced weight loss.20 A double-blind, sham-controlled study over 3 months demonstrated that the BIB resulted in a 34% excess weight loss compared to 2.1% in the sham group.21 Another sham-controlled study accompanied by diet demonstrated significant weight losses in the BIB and sham groups. After 1 year of BIB treatment, participants lost 17% of their body weight (approximately 34% of excess body weight), but half that weight was regained in the year after balloon removal.22 These observations led to the use of the balloon as preoperative treatment prior to obesity surgery.
The BIB slows gastric emptying and reduces ghrelin, an orexigenic hormone originating from the stomach, but the BIB does not increase cholecystokinin, which is expected due to gastric distention caused by the balloon.23–25 The BIB has a place in the treatment of obesity when used for weight loss prior to obesity surgery.26,27 A case control study demonstrated that 6 months of preoperative weight loss using the BIB reduced the length of the hospital stay, operative time, intraoperative complications (0% vs 7%), and the conversion of laparoscopic to open procedures (0% vs 16.3%).28
Despite the success of the BIB for inducing preoperative weight loss in preparation for obesity surgery, several disadvantages remain. First, the placement requires insertion and removal endoscopically, which necessitates at least conscious sedation. Second, the stomach gradually adapts to the balloon, necessitating removal after 3–6 months, and, following removal, weight is regained. Development of a gastric balloon that does not require endoscopic placement and that has the potential to be used chronically might provide an effective long-term treatment of obesity. The Ullorex OIB is a large capsule that is injected with citric acid and swallowed within a 4-minute period.29 The citric acid reacts with bicarbonate in the capsule and forms carbon dioxide after a 4-minute delay, which slowly inflates a 300-cm3 round balloon. The balloon has a plug that is degraded by stomach acid over 25–30 days. This causes the balloon to deflate spontaneously and pass harmlessly from the body in the feces. Chronic use of the balloon and prolonged weight loss might be achievable if a single balloon is followed by the use of multiple balloons and if the stomach is allowed time to recover from the distention caused by the balloons.
The purpose of this study was to test the Ullorex OIB in a sample of human participants. It was hypothesized that body weight and food intake data would be reduced and that the Ullorex OIB would be demonstrated to be a safe and feasible option for the long-term treatment of obesity. This hypothesis was based on the premises that placement of the balloon does not require anesthesia or endoscopy, the risk of gastric ulceration is reduced due to the balloon being round, and tolerance will be improved due to reduced weight from inflation with air.
Methods
Preliminary Studies
Safety study in pigs
Three pigs had one, two, or three Ullorex intragastric balloons placed in the stomach and two pigs acted as untreated controls. The animals were monitored daily, and X-rays were performed twice during the 1-month trial. After the balloons passed spontaneously in the feces at the expected time, the animals were euthanized and a necropsy was performed with histological examination of the gastrointestinal tract. No adverse effects of the balloon placement were identified and the balloons functioned as expected.
Testing of the balloon material
The balloons are made from a plastic polymer and were tested in three different ways. First, new balloons were inflated, deflated, and sent for analysis to test for mechanical or chemical degradation. Second, balloons passed in the feces during the pig study were sent for analysis. Third, pairs of balloons were exercised in a bench-top apparatus containing 6 N hydrochloric acid (pH 1.0) for 1 month to simulate the stomach environment. The balloons were burst tested, tested for tensile strength, and tested for tear strength. The balloons were also analyzed by gas chromatography with mass spectroscopy and by infrared spectroscopy. The balloons did not experience any significant mechanical or chemical degradation in vitro or in the pigs.
Testing of the balloons in vitro
Inflated balloons were compared to never-inflated balloons using three procedures. First, the balloons were immersed completely in acid in a testing apparatus that exercised them mechanically with a force and rate comparable to the stomach for 1 month. Second, the balloons were partially immersed in acid and exercised in the same manner. Third, the balloons were rotated in a cylinder with force applied from various directions and speeds over 1 month. These tests confirmed that the plug dissolution was dependent on the pH and hydration. Burst pressure and gas loss did not differ significantly from balloons that were not exercised and were only inflated for evaluation of burst pressure and gas loss. The difference in burst pressure and gas loss between one balloon and two balloons together in the apparatus was not significant.
Biocompatibility studies
The polymer from which the Ullorex intragastric balloon was made was subjected to several forms of testing, including cytotoxicity testing; murine local lymph node testing using aqueous and nonaqueous methodology; intracutaneous testing according to the International Standards Organization (ISO) using aqueous and nonaqueous methodology; systemic toxicity testing according to the ISO and the United States Pharmacopeia; genotoxicity testing using bacterial reverse mutation by aqueous and nonaqueous methodology; mouse bone marrow micronucleus testing using aqueous and nonaqueous methodology; genotoxicity testing using in vitro chromosomal aberration in mammalian cells; and a 12-week subcutaneous implantation according to ISO. There were no signs of toxicity or adverse effects on living tissue. Because the 12-week subcutaneous implantation is three times longer than the balloons are expected to stay in the stomach, these results indicated that the balloon material is biocompatible.
First use in humans
Two volunteers each swallowed one Ullorex oral intragastric balloon designed with a plug that would dissolve in 3 to 5 days. The participants lost 1.4 and 2.3 kg, respectively, and regained this weight within a week after passing the balloons in their feces. Both participants experienced mild indigestion the day after swallowing the Ullorex oral intragastric balloon and this indigestion was successfully relieved by antacids. No other adverse events were reported, but the participants did report eating less food due to early satiety. No changes in bowel habits were noted and the balloons passed spontaneously in the stool without symptoms. The findings from these two participants indicated sufficient safety to proceed to a safety study in 12 participants over 1 month.
Safety Study: Use of the Ullorex OIB in 12 Human Participants
Participants. Twelve participants participated in this safety trial. Participants were 21 through 64 years of age and generally healthy. Participants were required to have a body mass index (BMI; kg/m2) above 30 kg/m2 for more than 6 months and demonstrate their ability to swallow a placebo capsule of the same size as the Ullorex intragastric balloon within a 4-minute period. All participants provided written informed consent and the study was approved by the Pennington Biomedical Research Center Institutional Review Board. Participants were excluded who had sleep apnea, stroke, myocardial infarction, or cardiac revascularization within 6 months of randomization. Participants with a history of esophageal atresia, gastrointestinal stenosis, gastrointestinal obstruction, severe esophagitis, esophageal varices, dysphagia, achalasia, hiatus hernia, gastroparesis, gastric varices, adhesive peritonitis, or abnormalities of the esophagus, stomach, or pylorus were also excluded. The following medications were specifically excluded: chronic use of nonsteroidal anti-inflammatory drugs including aspirin, antiangina medications, antiarrhythmia medication, anticoagulants, or medications for congestive heart failure. Participants taking medications to control blood pressure or serum lipids were required to be on a stable dose for 3 months prior to the trial. Pregnant women, people who abuse substances, including alcohol, and people who regularly ate large quantities of sweet foods/drinks were also excluded.
Ullorex oral intragastric balloon
The Ullorex OIB device is an intragastric, thin-walled polyurethane balloon designed to be swallowed, self-inflate with carbon dioxide to approximately 300 cm3 with a diameter of 3 inches, and reside in the stomach for approximately 25–30 days, depending on gastric physiology and acid secretion.
The wall of the balloon contains a biodegradable plug with an injection port. Behind the plug and within the balloon is a pellet of compressed sodium bicarbonate to generate carbon dioxide gas. The pellet is encased in an inner pullulan capsule. Pullulan is a linear polysaccharide composed of linked maltotriose residues produced from yeast. The entire Ullorex OIB is folded and compressed to fit into a swallowable outer gelatin capsule, which is approximately 1.25 × 4.3 cm long.
The Ullorex OIB kit contains an injector that fits over the Ullorex capsule. A prefilled syringe containing citric acid and water is attached by luer lock to the injector. The Ullorex capsule is put into the injector and the syringe is emptied, causing the liquid to enter the capsule. The injected solution enters the interior of the balloon near the inner pullulan capsule, which encases the carbon dioxide generator. The pullulan begins to dissolve in approximately 4 to 5 minutes. After the solution is injected, which activates the device, the Ullorex OIB is immediately given to the patient to swallow. About 1–2 minutes after swallowing, the outer gelatin capsule begins to dissolve in the stomach. Several minutes thereafter, the inner pullulan capsule also dissolves, allowing reaction with the carbon dioxide generator and releasing carbon dioxide gas into the balloon. The reaction is gradual with the balloon inflating 90% approximately 9 minutes after the reaction begins.
The plug is composed of a nontoxic bioresorbable polymer. The polymer degrades in approximately 25–30 days at normal stomach acidity (pH 1–3). Degradation of the plug allows the carbon dioxide inside the balloon to escape and the balloon to deflate. Once deflated, the stomach muscles collapse the balloon, and the Ullorex OIB is passed into the gastrointestinal tract and excreted in the stool.
Procedure and randomization
Participants underwent a physical examination and laboratory tests, including chemistry panel with electrolytes, complete blood count (CBC) with platelets and white cell differential count, urine analysis, and occult blood analysis of the stool. Participants also received an electrocardiogram, a pregnancy test for women, an upper gastrointestinal radiology examination with oral barium contrast material, and an upper gastrointestinal endoscopy with particular attention to mucosal damage. The first six participants (cohort 1) were assigned randomly to receive a placebo capsule or one, two, or three balloons. These six participants and the study staff, with the exception of the study surgeon and study coordinator, were blind to treatment. The last six participants (cohort 2) all received one balloon and were not blind to treatment.
The device was administered by the study coordinator, under the supervision of the study surgeon orally with water while an intravenous line was in place and the participant was in a sitting position. Thirty minutes after swallowing the Ullorex OIB, an abdominal X-ray (KUB) followed by a barium swallow was performed to assure proper placement of the device. All participants had the KUB, barium swallow, and laboratory testing repeated at baseline and weeks 2 and 4. If the balloon was still present in the stomach at week 4, the KUB and barium swallow were repeated weekly until week 6. If the balloon was still present at week 6, it was removed endoscopically or deflated to allow passage in the stool. Additionally, participants were instructed to contact the study coordinator if and when they passed deflated balloons in their stool to record when the balloons passed. Participants had a brief physical examination at week 2, and the physical examination done at baseline was repeated after passing the balloon. Adverse events were assessed at each visit.
Prior to randomization, a registered dietitian (RD) instructed participants to consume a liquid diet for the first day after swallowing the capsules to reduce the likelihood of stomach discomfort. On the second day, semisolid foods, e.g., soups, gelatins, and broths, could be consumed. The RD also reviewed a balanced diet to be followed during the study, provided the participant with printed material about the diet, instructed the participant in the use of antacids as needed for indigestion, and gave the participant a booklet in which to record food intake and any adverse events. The RD reviewed the dietary records and saw participants on the biweekly visits.
Food intake testing
The six participants in cohort 2 completed a food intake test at lunch before the single intragastric balloon was in place (week 0 or baseline), and the food intake test was repeated at weeks 1, 2, and 3. Food intake was measured at a lunch meal consisting of sandwiches, potato chips, and cookies served with water. Before each food intake test, participants were asked if they had a cold or any other condition, e.g., allergies, that might affect taste. Before and after each food intake test, participants completed computerized ratings of satiety on visual analogue scales (VAS), which consisted of a line anchored from 0 (“not at all hungry”) to 100 (“extremely hungry”).30
Results
Descriptive Characteristics of Participants
Twelve participants (eight women and four men) enrolled in this study, and all but two completed the trial. Eleven of the participants were African-American and one was Caucasian. Mean body weight was 146.7 ± 25.8 kg (mean ± standard deviation) and the mean BMI was 51 ± 3.5 kg/m2. Mean age was 36.8 ± 10.4 years. One participant that dropped out of the trial because of dissatisfaction with her weight loss received one balloon and gained 0.7 kg in the first 2 weeks of the trial. The only participant randomized to receive three balloons developed nausea, vomiting, and required hospitalization for intravenous fluids and deflation of the balloons by endoscopy. The balloons passed in the stool after deflation and the participant's symptoms resolved. Subsequent evaluation revealed that this adverse event likely resulted from participant noncompliance. The participant ate large amounts of solid fatty food almost immediately after the balloons were in place, despite being instructed to consume only liquids the day after balloon placement. He was removed from the trial for this adverse event, and his adverse experience prompted a change to the protocol. Specifically, the final six participants (cohort 2) received only one balloon. Additionally, the balloons for the first three participants in cohort 1 failed to fully inflate; therefore, the balloon was reengineered prior to being administered to the rest of the participants in cohort 1 and cohort 2.
Weight Loss
Two participants in cohort 1 were randomized to placebo, two to one balloon, one to two balloons, and one to three balloons. The number of balloons that each participant received and the time at which the balloon(s) deflated and passed are outlined in Table 1. Participants' weight loss data were analyzed who received balloons (the two placebo participants were excluded) and who did not experience a serious adverse event; therefore, the participant who experienced an adverse event and had the balloons deflated was not included in the analyses. Investigation of when the balloons deflated and/or passed in the stool indicated that of the nine participants' data eligible for analysis, only one participant passed the balloon prior to week 2 and one participant's balloon was partially inflated. Weight was recorded every 2 weeks; therefore, weight loss data were examined from baseline to week 2. This analysis indicated that participants lost a significant (p < 0.05) amount of weight during this 2-week period. Specifically, they lost a mean of 1.5 ± 1.7 kg. Because of the small number of participants in the placebo condition (n = 2), statistical analyses were not conducted on the placebo participants. The weight loss among cohort 2, who only received one balloon, was similar (−1.2 ± 1.5 kg). Weight loss for individual participants is depicted in Table 2.
Table 1.
Number of Balloons Administered to Each Participant, Inflation Status of the Balloons after Placement, and Week at Which the Balloons Passed out of the Body
| Subject identification | No. balloons administered | Balloon(s) fully inflated | Week balloon(s) deflated | Week balloon(s) passed |
|---|---|---|---|---|
| Cohort 1 | ||||
| 1 | 2 | No | Never inflated | 2.5 |
| 2 | 0 (3 placebo capsules) | – | – | – |
| 3 | 3 | Partial, 1 cm | 1 | 2 |
| 4 | 1 | Yes | 2 | 2 |
| 5 | 1 | Yes | 4 | 6 |
| 6 | 0 (3 placebo capsules) | – | – | – |
| Cohort 2 | ||||
| 7 | 1 | Yes | 2 | 6 (extracted) |
| 8 | 1 | Yes | 3 | 3 |
| 9 | 1 | Yes | 2 | 6 (extracted) |
| 10 | 1 | Yes | 2 | 4 |
| 11 | 1 | Yes | 4 | 4 |
| 12 | 1 | Yes | 2 | 6 (extracted) |
Table 2.
Body Weight and Energy Intake (Cohort 2 Only) over Time for Each Participanta
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
|---|---|---|---|---|---|---|---|
| Cohort 1 | |||||||
| Ss# 1 Wt. (kg) | 130.9 | – | 127.6 | – | 128.6 | – | 128.3 |
| Ss# 2 (placebo) Wt. (kg) | 116.4 | – | 112.8 | – | 112.7 | – | 111.4 |
| Ss# 3 Wt. (kg) | 168.9 | – | 167.1 | – | – | – | – |
| Ss# 4 Wt. (kg) | 147.0 | – | 147.7 | – | – | – | – |
| Ss# 5 Wt. (kg) | 131.8 | – | 128.3 | – | 129.4 | – | 129.2 |
| Ss# 6 (placebo) Wt. (kg) | 185.1 | – | 185.0 | – | 185.2 | – | 187.5 |
| Cohort 2 | |||||||
| Ss# 7 Wt. (kg) | 119.3 | – | 118.3 | 118.3 | 118.1 | – | 114.7 |
| EI (kcal) | 421 | 364 | 462 | 280 | – | – | – |
| Ss# 8 Wt. (kg) | 182.5 | – | 180.0 | – | 182.6 | – | 181.6 |
| EI (kcal) | 888 | 630 | 597 | – | – | – | – |
| Ss# 9 Wt. (kg) | 125.3 | – | 126.3 | 126.0 | 126.4 | – | 128.3 |
| EI (kcal) | 1063 | 736 | 615 | 610 | – | – | – |
| Ss# 10 Wt. (kg) | 181.3 | – | 178.3 | 179.0 | 179.3 | – | 181.9 |
| EI (kcal) | 589 | 422 | 592 | 620 | – | – | – |
| Ss# 11 Wt. (kg) | 131.5 | – | 131.4 | 132.9 | 132.7 | – | 130.2 |
| EI (kcal) | 401 | 232 | 210 | 352 | – | – | – |
| Ss# 12 Wt. (kg) | 140.1 | – | 138.5 | 136.9 | 136.4 | – | 136.6 |
| EI (kcal) | 304 | 388 | 883 | 439 | – | – | – |
Values in italics represent measurements collected when the balloons were not fully inflated or after the balloons passed from the body. Ss, subject, Wt. body weight in kilograms, EI, energy intake in kilocalories.
Food Intake
Food intake was tested among cohort 2 or the six participants who received one balloon (food intake data for individual participants is depicted in Table 2). Only one participant reported symptoms of a cold, although they were minor and did not affect her sense of taste or appetite. All six participants completed the baseline lunch test meal and all six retained the balloon through the first week, although by week 2 many of the balloons were deflated (refer to Table 1). As a consequence of the different times at which the balloons deflated and/or passed through the gatrointestinal tract, food intake was analyzed only from baseline to week 1. Energy (kcal) intake decreased by 149 ± 146 kcal (24.4%) from baseline to week 1 (p = 0.055) (Figure 1), with a significant decrease in kilocalories from fat and carbohydrate (p < 0.05), but not protein (p = 0.12).
Figure 1.
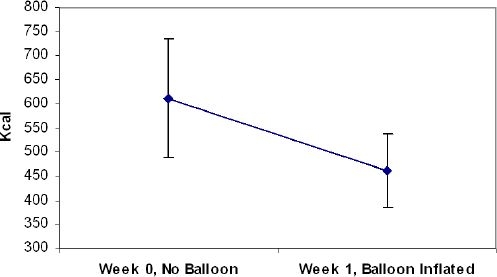
Mean food intake at baseline (week 0) and week 1, at which time the balloon was in place in the stomach.
The VAS, which measured subjective ratings of satiety before and after the meals at week 1, were compared to baseline values, e.g., the hunger rating taken before the meal at baseline was compared to the hunger rating taken before the meal at week 1. No significant differences were found (p > 0.20).
Safety
Physical examinations were unchanged during the study, and no new electrocardiographic changes occurred during the trial. The laboratory tests (chemistry panels, CBCs, and urinalyses) did not become abnormal during the study with the exceptions listed in Table 3, none of which were considered clinically significant. Pregnancy tests remained negative throughout the study, and the stools were negative for occult blood. A total of 67 adverse events (AEs) were reported during the study and 48 (72%) of these AEs were attributable to the device (see Table 4). One serious adverse event was reported during the trial. The participant who received three balloons developed nausea, vomiting, and dehydration, necessitating hospitalization, intravenous hydration, and deflation of the balloons with an endoscope. Patient noncompliance was the likely cause of the adverse experience, as it was discovered that the participant consumed nonapproved foods immediately after balloon placement.
Table 3.
Laboratory Values for Participants Who Experienced a Laboratory Value out of the Normal Range during the Triala
| Test | Week 0 | Week 2 | Week 4 | Normal range |
|---|---|---|---|---|
| Albumin | 3.1 | 3.0 | 3.1 | 3.1–5.4 g/dl |
| Chloride | 104 | 94 | – | 101–111 mmol/liter |
| Creatine phospho-kinase | 290 | 362 | 298 | 38–33 IU/liter |
| 221 | 392 | – | – | |
| High-density lipoprotein cholesterol | 30.5 | 29 | 30–70 mg/dl | |
| Lactate dehydro-genase | 173 | 232 | 175 | 82–195 IU/liter |
| Platelets | 394 | 424 | 452 | 150–450 × 103 |
| Urine protein | Negative | Negative | Trace | Negative |
The abnormal value is noted in bold text. Participant's values at other time points are also shown.
Table 4.
Number of Adverse Events (AEs) during the Study and Number and Percentage of AEs Attributable to the Device
| Type AE | No. AEs | No. AEs related to study device | % AEs related to study device |
|---|---|---|---|
| Gastrointestinal | 42 | 38 (2 unknown) | 90 |
| Head and neck | 9 | 4 | 44 |
| Vomiting | 5 | 5 | 100 |
| Skin | 3 | 1 | 33 |
| Other | 8 | 0 | 0 |
Discussion
This is the first study of the Ullorex oral intragastric balloon in a sample of human participants. The significant decrease in body weight from baseline to week 2 suggests that the balloons were having a positive effect on body weight. Additionally, use of a single balloon reduced food intake by 149 kcal (24.4%) at the lunch meal at 1 week compared to baseline and this decrease was marginally significant (p = 0.055), despite the small number of participants (n = 6). The significant decrease in body weight and food intake is surprising given the small sample size and is encouraging. Randomized controlled trials are needed to evaluate if use of the balloons results in significant weight loss and reduced food intake compared to placebo capsules.
Results of the study provide important information about the safety of intragastric balloons. First, although the literature suggested that balloons of 220 to 700 cm3 have been well tolerated, three balloons were not tolerated by the one participant who received three balloons in this study. Although one and two balloons were tolerated, the participant with three balloons developed nausea, vomiting, and dehydration. Even though patient noncompliance was the likely cause of this adverse event, we cannot rule out general nontolerance of the three balloons. This experience might suggest that the 900 cm3 displacement by three balloons is too great to be tolerated without a period of adaptation. This adverse event caused the protocol to be modified and the second six participants were all assigned to receive one balloon. Second, despite deflation of the balloons at 25–30 days in the pigs and the in vitro system, deflation occurred within the first 2 weeks in this safety study. This problem can be addressed by altering the plug to withstand stomach acid for a longer period of time.
Third, although the balloons deflated, some were slow to clear the stomach. It appeared on the radiographs that retained air in the balloon caused it to float on the top of gastric secretions, preventing it from passing the pylorus efficiently; therefore, the deflation mechanism will be redesigned in a manner to solve this problem.
In summary, this safety study identified technical problems with the Ullorex OIB that require correction. Nevertheless, these problems appear to have solutions and are presently being addressed. It is anticipated that long-term treatment of obesity with the Ullorex OIB will be feasible following (1) reengineering the plug to prevent premature deflation of the balloon, (2) redesigning the deflation mechanism to facilitate efficient passage through the pylorus, and (3) beginning treatment with a single balloon and increasing the number of balloons as the stomach adapts to the presence of the balloons. Future trials are necessary to confirm this hypothesis, but the results of this first safety trial are encouraging for continued development of the concept.
Abbreviations
- AEs
adverse events
- BIB
BioEnterics intragastric balloon
- BMI
body mass index
- CBC
complete blood count
- EI
energy intake
- GIB
Garren gastric balloon
- ISO
International Standards Organization
- KUB
abdominal X-ray
- OIB
oral intragastric balloon
- RD
registered dietitian
- VAS
visual analogue scales
References
- 1.Brown TH, Davidson PF, Terrell S, Rayford P, Larson GM. The effect of an intragastric balloon on weight loss, gastric acid secretion, and serum peptide levels. Am Surg. 1988;54:109–112. [PubMed] [Google Scholar]
- 2.Kramer FM, Stunkard AJ, Spiegel TA, Deren JJ, Velchik MG, Wadden TA, Marshall KA. Limited weight losses with a gastric balloon. Arch Intern Med. 1989;149:411–413. [PubMed] [Google Scholar]
- 3.Lindor KD, Hughes RW, Ilstrup DM, Jensen MD. Intragastric balloons in comparison with standard therapy for obesity–a randomized, double-blind trial. Mayo Clin Proc. 1987;62:992–996. doi: 10.1016/s0025-6196(12)65069-1. [DOI] [PubMed] [Google Scholar]
- 4.Hogan RB, Johnston JH, Long BW, Sones JQ, Hinton LA, Bunge J, Corrigan SA. A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest Endosc. 1989;35:381–385. doi: 10.1016/s0016-5107(89)72839-x. [DOI] [PubMed] [Google Scholar]
- 5.Kirby DF, Wade JB, Mills PR, Sugerman HJ, Kellum JM, Zfass AM, Starkey JV, Birkenhauer R, Hamer RM. A prospective assessment of the Garren-Edwards Gastric Bubble and bariatric surgery in the treatment of morbid obesity. Am Surg. 1990;56:575–580. [PubMed] [Google Scholar]
- 6.Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- 7.Pasquali R, Besteghi L, Casimirri F, Melchionda N, Di Febo G, Zoccoli G, Barbara L, Tassoni U. Mechanisms of action of the intragastric balloon in obesity: effects on hunger and satiety. Appetite. 1990;15:3–11. doi: 10.1016/0195-6663(90)90095-p. [DOI] [PubMed] [Google Scholar]
- 8.Rigaud D, Trostler N, Rozen R, Vallot T, Apfelbaum M. Gastric distension, hunger and energy intake after balloon implantation in severe obesity. Int J Obes Relat Metab Disord. 1995;19:489–495. [PubMed] [Google Scholar]
- 9.Mathus-Vliegen EM, Tygat GN. Gastro-oesophageal reflux in obese subjects: influence of overweight, weight loss and chronic gastric balloon distension. Scand J Gastroenterol. 2002;37:1246–1252. doi: 10.1080/003655202761020498. [DOI] [PubMed] [Google Scholar]
- 10.Mathus-Vliegen EM, Tytgat GN, Veldhuyzen-Offermans EA. Intragastric balloon in the treatment of super-morbid obesity. Double-blind, sham-controlled, crossover evaluation of 500-milliliter balloon. Gastroenterology. 1990;99:362–369. doi: 10.1016/0016-5085(90)91017-z. [DOI] [PubMed] [Google Scholar]
- 11.Ramhamadany EM, Fowler J, Baird IM. Effect of the gastric balloon versus sham procedure on weight loss in obese subjects. Gut. 1989;30:1054–1057. doi: 10.1136/gut.30.8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geliebter A, Melton PM, McCray RS, Gage D, Heymsfield SB, Abiri M, Hashim SA. Clinical trial of silicone-rubber gastric balloon to treat obesity. Int J Obes. 1991;15:259–266. [PubMed] [Google Scholar]
- 13.Geliebter A, Melton PM, Gage D, McCray RS, Hashim SA. Gastric balloon to treat obesity: a double-blind study in nondieting subjects. Am J Clin Nutr. 1990;51:584–588. doi: 10.1093/ajcn/51.4.584. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JB, Schreiber H, Kolozsi W, Vasudeva R, Bacon BR, McCullough AJ, Holt S. A prospective, multi-center clinical trial of the Taylor intragastric balloon for the treatment of morbid obesity. Am J Gastroenterol. 1990;85:833–837. [PubMed] [Google Scholar]
- 15.Mathus-Vliegen EM, Tytgat GN. Intragastric balloons for morbid obesity: results, patient tolerance and balloon life span. Br J Surg. 1990;77:767–769. doi: 10.1002/bjs.1800770127. [DOI] [PubMed] [Google Scholar]
- 16.Doldi SB, Micheletto G, Di Prisco F, Zappa MA, Lattuada E, Reitano M. Intragastric balloon in obese patients. Obes Surg. 2000;10:578–581. doi: 10.1381/096089200321594200. [DOI] [PubMed] [Google Scholar]
- 17.Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L, Giardiello C, Angrisani L, Pecchioli L, Stornelli P, Puglisi F, Alkilani M, Nigri A, Di Lorenzo N, Furbetta F, Cascardo A, Cipriano M, Lorenzo M, Basso N. BioEnterics Intragastric Balloon: The Italian Experience with 2,515 Patients. Obes Surg. 2005;15:1161–1164. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- 18.Doldi SB, Micheletto G, Perrini MN, Librenti MC, Rella S. Treatment of morbid obesity with intragastric balloon in association with diet. Obes Surg. 2002;12:583–587. doi: 10.1381/096089202762252398. [DOI] [PubMed] [Google Scholar]
- 19.Doldi SB, Micheletto G, Perrini MN, Rapetti R. Intragastric balloon: another option for treatment of obesity and morbid obesity. Hepatogastroenterology. 2004;51:294–297. [PubMed] [Google Scholar]
- 20.Busetto L, Enzi G, Inelmen EM, Costa G, Negrin V, Sergi G, Vianello A. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128:618–623. doi: 10.1378/chest.128.2.618. [DOI] [PubMed] [Google Scholar]
- 21.Genco A, Cipriano M, Bacci V, Cuzzolaro M, Materia A, Raparelli L, Docimo C, Lorenzo M, Basso N. BioEnterics Intragastric Balloon (BIB): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes (Lond) 2006;30:129–133. doi: 10.1038/sj.ijo.0803094. [DOI] [PubMed] [Google Scholar]
- 22.Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19–27. doi: 10.1016/s0016-5107(04)02406-x. [DOI] [PubMed] [Google Scholar]
- 23.Bonazzi P, Petrelli MD, Lorenzini I, Peruzzi E, Nicolai A, Galeazzi R. Gastric emptying and intragastric balloon in obese patients. Eur Rev Med Pharmacol Sci. 2005;9:15–21. [PubMed] [Google Scholar]
- 24.Mion F, Napoleon B, Roman S, Malvoisin E, Trepo F, Pujol B, Lefort C, Bory RM. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes Surg. 2005;15:510–516. doi: 10.1381/0960892053723411. [DOI] [PubMed] [Google Scholar]
- 25.Boosalis MG, Gemayel N, Lee A, Bray GA, Laine L, Cohen H. Cholecystokinin and satiety: effect of hypothalamic obesity and gastric bubble insertion. Am J Physiol. 1992;262:R241–244. doi: 10.1152/ajpregu.1992.262.2.R241. [DOI] [PubMed] [Google Scholar]
- 26.Weiner R, Gutberlet H, Bockhorn H. Preparation of extremely obese patients for laparoscopic gastric banding by gastric-balloon therapy. Obes Surg. 1999;9:261–264. doi: 10.1381/096089299765553133. [DOI] [PubMed] [Google Scholar]
- 27.Alfalah H, Philippe B, Ghazal F, Jany T, Arnalsteen L, Romon M, Pattou F. Intragastric balloon for preoperative weight reduction in candidates for laparoscopic gastric bypass with massive obesity. Obes Surg. 2006;16:147–150. doi: 10.1381/096089206775565104. [DOI] [PubMed] [Google Scholar]
- 28.Busetto L, Segato G, De Luca M, Bortolozzi E, MacCari T, Magon A, Inelmen EM, Favretti F, Enzi G. Preoperative weight loss by intragastric balloon in super-obese patients treated with laparoscopic gastric banding: a case-control study. Obes Surg. 2004;14:671–676. doi: 10.1381/096089204323093471. [DOI] [PubMed] [Google Scholar]
- 29.Sampson D, Zanakis M. Office UPT. USA: 2003. Self-inflating intragastric volume-occupying device. [Google Scholar]
- 30.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]


