Abstract
Background
Overall obesity and, as it is increasingly appreciated, body fat distribution and ectopic fat deposition in liver and skeletal muscle, determine insulin resistance in humans. However, little is known about the independence of these relationships. Therefore, we determined the impact of different fat depots as well as fat accumulation in ectopic tissues such as liver and skeletal muscle in the prediction of insulin resistance in healthy humans.
Methods
Visceral and subcutaneous abdominal fat were determined by magnetic resonance (MR) tomography and liver fat and intramyocellular fat in the tibialis anterior muscle by 1H-MR spectroscopy in 220 subjects. Insulin sensitivity was estimated from the oral glucose tolerance test (OGTT) and measured by a euglycemic hyperinsulinemic clamp in a subgroup (n = 157).
Results
Insulin sensitivity estimated from the OGTT correlated negatively with total body fat (r = −0.27, p < 0.0001), subcutaneous abdominal fat (r = −0.35, p < 0.0001), and visceral fat (r = −0.43, p < 0.0001). Furthermore, insulin sensitivity correlated negatively with liver fat (r = −0.53, p < 0.0001) and intramyocellular fat (r = −0.26, p < 0.0001). In multivariate regression models, high liver and visceral fat emerged as the strongest predictors of low insulin sensitivity.
Conclusion
Among various fat compartments, high liver fat and high visceral fat are the strongest determinants of insulin sensitivity in humans.
Keywords: ectopic fat, insulin resistance, intramyocellular fat, liver fat, visceral fat
Introduction
Insulin resistance is a fundamental aspect of the etiology of type 2 diabetes.1,2 When insulin action starts to decline, increased insulin secretion of β cells compensates to decrease hyperglycemia. However, a failure to do so eventually results in manifestation of the disease.3,4 Obesity is an increasing worldwide problem that strongly determines insulin resistance. It is well known that the amount of adipose tissue, as well as its distribution, is of special importance in the pathogenesis of insulin resistance and type 2 diabetes. Visceral adipose tissue is metabolically highly active5 and its major role in the pathogenesis of insulin resistance is unchallenged. In addition, lipids in ectopic tissues such as liver and skeletal muscle are of increasing interest. It has been shown that liver fat is elevated in insulin-resistant subjects6–11 and, furthermore, is strongly correlated with the amount of visceral fat in a prediabetic population.11,12 In addition, high intramyocellular fat was found to be a marker of insulin resistance.13–17 Thus, determination of fat in the visceral depot and in the ectopic tissues liver and muscle may help to early identify subjects who are relatively lean, but are insulin resistant and have a high risk of type 2 diabetes. Magnetic resonance (MR) tomography and proton MR spectroscopy allow noninvasive quantification of these fat depots.
This study specifically addressed the following question: which of the fat depots and ectopic fat in liver and skeletal muscle are among the strongest determinants of insulin sensitivity in healthy humans who are at high risk to develop type 2 diabetes?
Materials and Methods
Subjects
We analyzed data from a total of 220 Caucasians from the southern part of Germany who participated in an ongoing study to investigate the pathophysiology of type 2 diabetes.18,19 Individuals were included in the study when they fulfilled at least one of the following criteria: a family history of type 2 diabetes, a body mass index (BMI) >27 kg/m2, previous diagnosis of impaired glucose tolerance, or gestational diabetes. The participants did not take any medication known to affect glucose tolerance or insulin sensitivity. None of the participants regularly consumed alcohol. They were considered healthy according to a physical examination and routine laboratory tests. Informed written consent was obtained from all participants and the local medical ethics committee approved the protocol.
Body Composition and Body Fat Distribution
Total body fat was measured by bioelectrical impedance (RJL, Detroit, MI). In brief, electrodes were attached to various parts of the body and a small electric signal was circulated. With this method the impedance or resistance to the signal was measured as it traveled through the water found in muscle and fat. The more fat a person has, the more resistance to the current exists.
Determination of visceral fat and subcutaneous abdominal fat was performed by an axial T1-weighted fast spin echo technique as described previously using a 1.5-tesla whole body imager (Magnetom Sonata, Siemens Medical Solutions, Germany).20
1H Magnetic Resonance Spectroscopy
Liver fat and intramyocellular fat of the tibialis anterior muscle were determined by 1H magnetic resonance spectroscopy as described previously.19
Oral Glucose Tolerance Test
All individuals underwent a 75-gram oral glucose tolerance test (OGTT), and venous plasma samples were obtained at 0, 30, 60, 90, and 120 minutes for determination of plasma glucose and insulin. Glucose tolerance was determined according to 1997 World Health Organization diagnostic criteria.21 Insulin sensitivity was calculated from glucose and insulin values during the OGTT as proposed by Matsuda and DeFronzo (10,000 /□(mean insulin × mean glucose) × (fasting insulin × fasting glucose).22
Euglycemic Hyperinsulinemic Clamp
Insulin sensitivity was determined in 157 subjects as described previously.18,19 In brief, subjects received a primed insulin infusion at a rate of 40 mU·m−2·min−1 for 2 hours. Plasma was drawn every 5 minutes for determination of plasma glucose, and a glucose infusion was adjusted appropriately to maintain the fasting glucose level. An insulin sensitivity index (mol·kg−1·min−1·pM−1) for systemic glucose uptake was calculated as the mean infusion rate of glucose (in µmol·kg−1·min−1) necessary to maintain euglycemia during the last 40 minutes of a euglycemic hyperinsulinemic clamp divided by the steady-state plasma insulin concentration.
Analytical Procedures
Blood glucose was determined using a bedside glucose analyser (glucose–oxidase method; Yellow Springs Instruments, Yellow Springs, CO). For measurements of insulin, blood was placed on ice after drawing, transferred to the laboratory immediately, and subsequently analyzed. Plasma insulin was determined by a microparticle enzyme immunoassay (Abbott Laboratories, Tokyo, Japan).
Statistical Analyses
Data are given as mean ± SE. Data that were not distributed normally (Shapiro-Wilk W test) were transformed logarithmically. In multivariate regression models the dependent variables were adjusted for age, gender, and percentage of total body and for some analyses additionally for visceral fat. A p value <0.05 was considered statistically significant. The statistical software package JMP 4.0 (SAS Institute Inc., Cary, NC) was used.
Results
Demographics, Anthropometrics, and Metabolic Characteristics of Study Subjects
Anthropometrics and metabolic characteristics of the subjects (shown in Table 1) covered a wide range that was particularly large for body fat distribution and ectopic fat in liver and skeletal muscle. Total body fat measured by bioimpedance displayed the following correlations with the other measurement of adiposity: waist circumference: r = 0.66, p < 0.0001; subcutaneous abdominal fat: r = 0.81, p < 0.0001; visceral fat: r = 0.47, p < 0.0001; intramyocellular fat: r = 0.09, p = 0.18; liver fat: r = 0.33, p < 0.0001.
Table 1.
Demographics and Metabolic Characteristics of the Subjects
| Variablea | Mean ± SE | Range |
|---|---|---|
| Gender (89 male/131 female) | ||
| Age (years) | 46 ± 1 | 19–69 |
| Fasting plasma glucose (mg/dl) | 94.5 ± 0.7 | 76.0–142.9 |
| Fasting plasma insulin (pM) | 62 ± 3 | 19–246 |
| Waist circumference (cm) | 98 ± 1 | 64–138 |
| Total body fatbioimpedence (%) | 31.5 ± 0.6 | 9.3–50.0 |
| Visceral fatMRT (kg) | 3.5 ± 0.1 | 0.7–8.2 |
| Subcutaneous abdominal fatMRT (kg) | 12.5 ± 0.3 | 4.0–22.7 |
| Liver fat1MRS (%) | 5.8 ± 0.4 | 0.2–29.1 |
| IMCLtibialis anterior 1MRS(arb. units) | 4.0 ± 0.1 | 0.5–11.9 |
| Insulin sensitivityclamp (μM·kg−1·min−1·pM−1)b | 0.065 ± 0.003 | 0.013–0.347 |
| Insulin sensitivityOGTT (arb. units) | 12.72 ± 0.46 | 2.59–32.14 |
MRT(S), magnetic resonance tomography (spectroscopy).
Measured in 157 subjects.
Relationships of Insulin Sensitivity with Demographics and Body Fat Depots
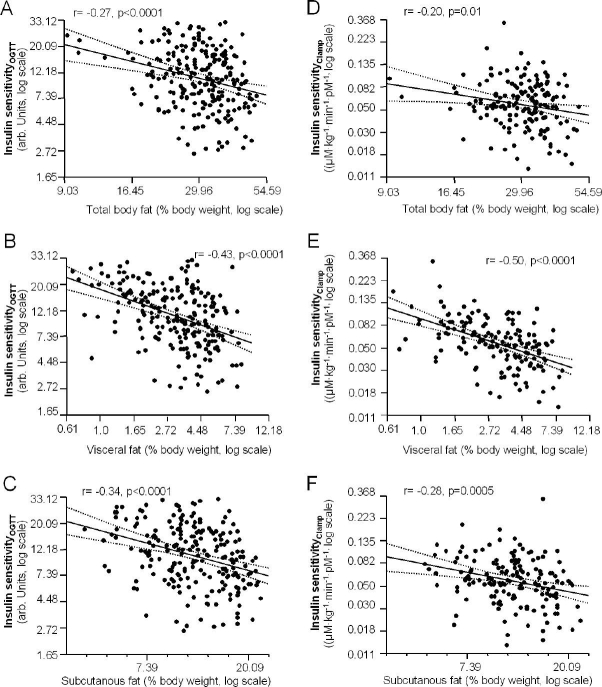
Insulin sensitivity estimated from the OGTT was not significantly associated with gender and age. In contrast, insulin sensitivity correlated negatively with waist circumference (r = −0.46, p < 0.0001), total body fat, visceral fat, and subcutaneous abdominal fat in univariate analyses (Figures 1A–1C). When insulin sensitivity was measured by a clamp in 157 subjects, similar relationships were found (waist; r = −0.48, p < 0.0001 and Figures 1D–1F).
Figure 1.
Relationship among total body fat (A and D), visceral fat (B and E), and subcutaneous abdominal fat (C and F) with insulin sensitivity estimated from the OGTT and measured by a clamp (n = 157, regression line and 95% confidence interval).
Relationships of Insulin Sensitivity with Ectopic Fat in Liver and Skeletal Muscle
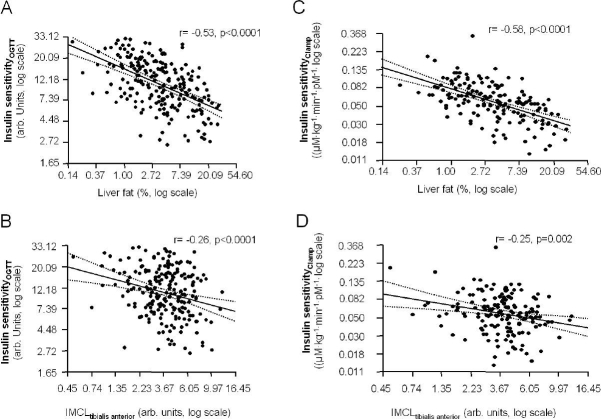
We further tested whether insulin sensitivity correlated with ectopic fat deposition in muscle and liver fat both measured by 1H magnetic resonance spectroscopy. Insulin sensitivity correlated negatively with liver fat and positively with intramyocellular fat of the tibialis anterior muscle (Figures 2A and 2B). Insulin sensitivity measured by a clamp in 157 subjects showed similar associations with these parameters (Figures 2C and 2D).
Figure 2.
Relationship between liver fat (A and C) and IMCL of the tibialis anterior muscle (B and D) with insulin sensitivity estimated from the OGTT and measured by a clamp (n = 157, regression line and 95% confidence interval).
Independent Relationships of Fat Compartments with Insulin Sensitivity
As insulin sensitivity correlated with several body fat compartments, we further investigated whether these relationships were independent of each other and tested the contribution of these parameters to the overall variability in insulin sensitivity. Insulin sensitivity was not measured by a clamp in subjects who refused to undergo this procedure. Because insulin sensitivity estimated from the OGTT is strongly correlated with insulin sensitivity measured by a clamp and because in our hands both measurements are good estimates of whole body insulin sensitivity, we performed these analyses in all subjects who had measurements of insulin sensitivity by the OGTT. In multivariate linear regression models (Table 2), age, gender, and total body fat explained merely 17% of the variability in insulin sensitivity (model 2). Additional inclusion of visceral fat and intramyocellular fat of the tibialis anterior muscle increased the r2 of the model to 0.30 (model 3) and 0.33 (model 4), respectively. Finally, inclusion of liver fat increased the r2 to 0.40 (model 5), explaining an additional 7% of the variability in insulin sensitivity. In the same model the contributions of total body fat, visceral fat, and intramyocellular fat were 2, 4, and 2%, respectively. Subcutaneous abdominal fat was not a significant determinant of insulin sensitivity in this model.
Table 2.
Determinants of Insulin SensitivityOGTT in Multivariate Linear Regression Models
| Covariates | Estimate ± SE | P |
|---|---|---|
| Model 1 (r2 = 0.008) | ||
| Female sex | 0.05 ± 0.04 | 0.19 |
| Age | 0.03 ± 0.14 | 0.81 |
| Model 2 (r2 = 0.17) | ||
| Female sex | 0.22 ± 0.04 | <0.0001 |
| Age | −0.002 ± 0.13 | 0.987 |
| Body fat | −0.996 ± 0.15 | <0.0001 |
| Model 3 (r2 = 0.30) | ||
| Female sex | −0.04 ± 0.06 | 0.46 |
| Age | 0.50 ± 0.14 | 0.0005 |
| Body fat | −0.40 ± 0.17 | 0.019 |
| Visceral fat | −0.62 ± 0.099 | <0.0001 |
| Model 4 (r2 = 0.33) | ||
| Female sex | −0.03 ± 0.06 | 0.64 |
| Age | 0.44 ± 0.14 | 0.002 |
| Body fat | −0.41 ± 0.17 | 0.02 |
| Visceral fat | −0.57 ± 0.097 | <0.0001 |
| IMCL | −0.21 ± 0.07 | 0.001 |
| Model 5 (r2 = 0.40) | ||
| Female sex | −0.02 ± 0.06 | 0.78 |
| Age | 0.38 ± 0.13 | 0.005 |
| Body fat | −0.37 ± 0.16 | 0.02 |
| Visceral fat | −0.35 ± 0.10 | 0.001 |
| IMCL | −0.16 ± 0.06 | 0.01 |
| Liver fat | −0.18 ± 0.04 | <0.0001 |
Discussion
Obesity plays an important role in the pathogenesis of insulin resistance and type 2 diabetes. It becomes evident that the amount of total body fat, as well as its distribution in different body compartments, is an important factor in the development of the disease. Particularly high visceral fat and liver fat were found to be strongly associated with insulin resistance.6,12 Furthermore, it is well accepted that visceral fat is a determinant of liver fat.23,24 It was hypothesized that an increased delivery of free fatty acids to the liver via the portal vein causes an increase in hepatic lipids. Whether visceral adipose tissue is an independent predictor of insulin sensitivity or exerts its effects on glucose metabolism only through increased liver fat is under investigation. We have shown that liver fat and visceral fat are associated with insulin sensitivity independently from each other.11 The present study addressed the following question: which fat depots and ectopic fat in liver and skeletal muscle are among the strongest determinants of insulin sensitivity? A cohort of people with an increased risk of type 2 diabetes was studied. In univariate analyses, insulin sensitivity estimated from the OGTT and measured by a clamp correlated negatively with body fat compartments such as total body fat, visceral fat, and subcutaneous abdominal fat, as well as with ectopic fat in liver and muscle. To investigate which of the fat compartments were among the strongest determinants of insulin sensitivity, we used multivariate linear regression models. Visceral fat contributed significantly to the variability in insulin sensitivity after additional adjustment for total body fat and intramyocellular fat that were determinants of insulin sensitivity. Additional inclusion of liver fat into the model that was significantly correlated with low insulin sensitivity largely enhanced the predictive value.
Our findings first confirm the majority of data in the literature that particularly excess of visceral fat in contrast to excess of subcutaneous fat may decrease insulin sensitivity most probably via increased lipolysis and inflammation.25 In addition, we provide new data that ectopic fat accumulation in the liver over and above the other fat compartments has the strongest effect on the induction of insulin resistance. This suggests that fat accumulation in the liver, independent of the other fat compartments, is directly involved in the induction of insulin resistance. This hypothesis is supported by data showing that fat in the liver induces the production of fetuin-A, which impairs insulin signaling in insulin-sensitive tissues.18
In conclusion, while visceral fat, intramyocellular fat in skeletal muscle, and liver fat are all related strongly and independently to insulin sensitivity, liver fat appears to play a predominant role in the determination of insulin sensitivity.
Acknowledgements
We thank all the research volunteers for their participation. Studies were supported by a grant from the Deutsche Forschungsgemeinschaft (KFO 114) and the European Community's FP6 EUGENE6 (LSHM-CT-2004-512013).
Abbreviations
- BMI
body mass index
- IMCL
intramyocellular lipids
- MR
magnetic resonance
- OGTT
oral glucose tolerance test
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000 Aug;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Defronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Stumvoll M, Tataranni PA, Stefan N, Vozarova B, Bogardus C. Glucose allostasis. Diabetes. 2003 Apr;52(4):903–909. doi: 10.2337/diabetes.52.4.903. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999 Sep;104(6):787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002 Mar;25(3):431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 6.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002 Jul;87(7):3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E906–16. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 8.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006 Dec;91(12):4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 9.Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes–pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006 Jun;2(6):335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 10.Kantartzis K, Rittig K, Balletshofer B, Machann J, Schick F, Porubska K, Fritsche A, Haring HU, Stefan N. The relationships of plasma adiponectin with a favorable lipid profile, decreased inflammation, and less ectopic fat accumulation depend on adiposity. Clin Chem. 2006 Oct;52(10):1934–1942. doi: 10.1373/clinchem.2006.067397. [DOI] [PubMed] [Google Scholar]
- 11.Thamer C, Machann J, Stefan N, Haap M, Schafer S, Brenner S, Kantartzis K, Claussen C, Schick F, Haring H, Fritsche A. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 2007 Feb;15(2):531–538. doi: 10.1038/oby.2007.568. [DOI] [PubMed] [Google Scholar]
- 12.Thamer C, Machann J, Haap M, Stefan N, Heller E, Schnödt B, Stumvoll M, Claussen C, Fritsche A, Schick F, Haring H. Intrahepatic lipids are predicted by visceral adipose tissue mass in healthy subjects. Diabetes Care. 2004 Nov;27(11):2726–2729. doi: 10.2337/diacare.27.11.2726. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999 May;48(5):1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 14.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999 Jan;42(1):113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–346. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- 16.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999 Aug;48(8):1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 17.Virkamäki A, Korsheninnikova E, Seppälä-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Häkkinen AM, Yki-Järvinen H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001 Oct;50(10):2337–2343. doi: 10.2337/diabetes.50.10.2337. [DOI] [PubMed] [Google Scholar]
- 18.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006 Apr;29(4):853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 19.Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O, Spieth C, Weigert C, Fritsche A, Stumvoll M, Häring HU. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia. 2005 Nov;48(11):2282–2291. doi: 10.1007/s00125-005-1948-3. [DOI] [PubMed] [Google Scholar]
- 20.Machann J, Thamer C, Schnoedt B, Haap M, Haring HU, Claussen CD, Stumvoll M, Fritsche A, Schick F. Standardized assessment of whole body adipose tissue topography by MRI. J Magn Reson Imaging. 2005 Apr;21(4):455–462. doi: 10.1002/jmri.20292. [DOI] [PubMed] [Google Scholar]
- 21.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997 Jul;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda A, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 23.Garg A, Misra A. Hepatic steatosis, insulin resistance, and adipose tissue disorders. J Clin Endocrinol Metab. 2002 Jul;87(7):3019–3022. doi: 10.1210/jcem.87.7.8736. [DOI] [PubMed] [Google Scholar]
- 24.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005 Feb;288(2):E454–61. doi: 10.1152/ajpendo.00203.2004. [DOI] [PubMed] [Google Scholar]
- 25.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec 14;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]