Abstract
Background
Approved for treatment of treatment-resistant depression and for epilepsy, vagus nerve stimulation (VNS) therapy involves stimulation of the vagus nerve, affecting both mood and appetite regulating systems. VNS is associated with changes in food intake and weight loss in animals. Studies of its impact on food intake and weight with humans are limited. It is not known whether or how VNS influences emotional response to food, but vagus afferents project to regions in the insula involving satiety and taste.
Method
Thirty-three participants were recruited for three groups: depressed patients undergoing VNS therapy, depressed patients not undergoing VNS therapy, and healthy controls. All participants viewed images of foods twice in random order. When applicable, VNS devices were turned on for one viewing and off for the other. Participants were instructed to rate immediately after the viewings how each picture made them feel on a visual analog on three dimensions (unhappy to happy, calm to aroused, and small/submissive to big/domineering).
Results
Controlling for time since last meal, a significant main effect was found for arousal ratings in response to sweet food images. Post-hoc analyses indicated that the VNS group demonstrated significant changes in arousal ratings between paired food image viewings compared to controls. Sixty-four percent of VNS participants demonstrated increases and 36% demonstrated decreases in arousal. Higher body mass indexes and greater levels of self-reported sweet cravings were associated with increased arousal during VNS activation.
Conclusions
This study was the first to examine the effects of acute left cervical VNS on emotional ratings of food in adults with major depression. Results suggest that VNS device activation may be associated with acute alteration in arousal response to sweet foods among depressed patients. Future research is needed to replicate these findings and to assess how activation of the vagus nerve affects eating and weight.
Keywords: appraisal, brain stimulation, depression, emotions, food, obesity, vagus nerve, vagus nerve stimulation
Introduction
Obesity rates have increased dramatically over the last few decades. Approximately 66.3% of Americans are overweight or obese.1 A myriad of negative health consequences are associated with obesity, including type 2 diabetes mellitus.2 Obesity is associated with psychosocial consequences, including depression.3–6 Some types of depression (particularly the “atypical” variant) and obesity share similar pathophysiological and behavioral features,7 including increased consumption of calorie-dense foods and decreased physical activity. Research identifying factors that guide preferences for calorie-dense foods over lighter food options may help inform the development of more potent interventions.
The relationship between mood and food, particularly sweets, is complex and bidirectional in that mood states impact food selection and intake, and food selection and consumption impact mood.8–10 Negative mood is related to low energy,11 which can lead to increased consumption and preference for sweets and other energy-dense foods.12 Expectations about foods, which are based on past experience, may also play a role in which types of foods are selected and consumed under different emotional circumstances. As sweets are innately pleasant and have a high reward value,13 sweets may be more likely chosen in a negative mood state. For example, in one study women reported being more likely to consume sweets after a sad event.14
Food has also been found to impact emotional states in a variety of ways.15 Emotional reactions to foods are likely mediated by food properties and individuals' own cognitive and symbolic biases. Carbohydrate consumption—acting via insulin secretion and the “plasma tryptophan ratio”—increases serotonin release, which in turn is closely associated with mood and pain regulation.16 Similarly, chocolate is associated with elevated mood, increased activation, decreased tiredness, and joy.17 This contrasts with induced negative emotional states when food is presented to food-deprived females with bulimia nervosa18 or when the consumption of energy-dense foods is perceived as unhealthy among overweight individuals.9
A shared biological mechanism of mood and appetite regulation involves the vagus nerve.19 The vagus nerve is 1 of 12 cranial nerves and it carries information back and forth between the brain and major organs, including the heart, stomach, lungs, and esophagus. Electrical stimulation of the vagus afferents (nerves traveling to the brain from the body) results in activation or inhibition of brain stem structures such as the medulla and the nucleus of the tractus solitarius.20 These inputs are then conveyed to widespread bilateral areas of the cerebral cortex, diencephalons, and limbic lobe, particularly the insula.21–25 Appetite and hunger are, in part, regulated through the vagus pathways.26,27 Stimulation of the vagus nerve is associated with modulation of the orbitofrontal cortex, hippocampus, cerebellum, and striatum.28 These same regions have been implicated in drug cravings and addiction. Animal studies have provided evidence of the effect that vagus nerve stimulation (VNS) has on eating behavior and weight and have piqued interest in exploring VNS therapy as a potential treatment for obesity.29,30
Left cervical VNS was first established as a treatment for epilepsy,31,32 and research suggested that VNS affected mood over and above its effects on epilepsy.33 Preliminary studies suggest that long-term VNS therapy may be an effective treatment for depression and can aid in decreasing depressive symptoms in individuals with treatment-resistant depression (TRD).34–38 The study of the relationship between VNS and obesity and eating behaviors in humans is limited by the fact that the VNS leads in humans, when used for treatment of depression and epilepsy, are implanted in the cervical region. Therefore, activation of the vagus nerve may not have as specific an impact on eating as it would if the nerve were stimulated closer to the stomach. One study with epileptic patients did find an association between VNS and weight loss;39 however, other studies with VNS patients did not find such a relationship.36,37 In a study with patients receiving VNS for TRD, it was found that acute VNS had an effect on food cravings, specifically for sweets, in humans.40 Because VNS appears to affect areas of the brain associated with memory and pleasure,19 we hypothesized that VNS device activation among depressed patients would acutely affect emotional responses to foods compared to non-VNS-treated depressed individuals and to healthy controls.
Method
Participants were recruited to participate in a study of the effects of VNS on food cravings and emotional responses to food images. Results from the study of the effects of VNS on food cravings are published elsewhere.40 This study used the same sample and methods but examined a different set of dependent measures (focusing on VNS effects on emotional responses to food images rather than food cravings).
Participants
Thirty-three participants were recruited to participate in the study from the Brain Stimulation Laboratory and Mood Disorders Clinic at the Medical University of South Carolina as described in more detail elsewhere.40 Eleven participants were recruited for each of three groups: participants receiving VNS therapy for depression, non-VNS patients with depression, and healthy controls (without VNS or depression). Depressed participants were recruited from the Mood Disorders Clinic where participants received treatment for treatment-resistant depression. The participants were contacted and, if interested in participating in the study, were scheduled for a single research session. Individuals must have had a past diagnosis of depression, specifically a treatment-resistant type (i.e., lack of response to trials of antidepressant medications). Groups were then created based on VNS treatment. Healthy controls were recruited from the medical university community. All VNS participants had the device implanted for treatment-resistant depression, and the background device settings employed in this study (i.e., output current, frequency, pulse width, on time, off time) were the clinical settings determined by the treating psychiatrist (and thus were different for each participant). The VNS settings for each individual along with the duration of VNS therapy for depression are listed in Table 1.
Table 1.
Individual Participants' Clinical VNS Settings and Number of Months Each Participant Had Been Undergoing VNS Therapy for Depression at Time of Study Enrollment Participant
| Participant | Output current(mA) | Frequency(Hz) | Pulse width(μs) | On time (seconds) | Off time (minutes) | Time with VNS (months) |
|---|---|---|---|---|---|---|
| 1 | 0.50 | 20 | 250 | 14 | 3 | 78.0 |
| 2 | 1.25 | 20 | 250 | 7 | 3 | 0.5 |
| 3 | 1.25 | 20 | 250 | 30 | 3 | 60.0 |
| 4 | 0.75 | 20 | 250 | 30 | 5 | 72.0 |
| 5 | 1.25 | 20 | 250 | 30 | 5 | 60.0 |
| 6 | 1.00 | 20 | 250 | 30 | 3 | 84.0 |
| 7 | 1.00 | 20 | 250 | 21 | 5 | 66.0 |
| 8 | 0.50 | 20 | 250 | 30 | 3 | 60.0 |
| 9 | 1.50 | 20 | 250 | 30 | 5 | 63.0 |
| 10 | 0.75 | 20 | 250 | 30 | 5 | 1.0 |
| 11 | 1.25 | 20 | 250 | 30 | 3 | 60.0 |
Of the 33 participants, 18 were women (54.5%) and 15 (45.5%) were men. The majority of participants were Caucasian (81.8%), and the mean age was 43.55 years (range = 23–64). Forty-two percent of participants had a body mass index (BMI) in the normal range and the majority did not smoke (87.9%). Self-reported antidepressant use was obtained from all participants. Eighty-two percent of participants (N = 9) from the VNS group, 55% (N = 6) of those in the depressed non-VNS group, and zero from the healthy control group reported taking antidepressants. See Tables 1 and 2 for participant characteristics of the overall sample and each group (depression VNS, depression non-VNS, and controls).
Table 2.
Participant Characteristics by Group (Mean and Standard Deviation)
| TRD VNS | TRD non-VNS | Controls | |
|---|---|---|---|
| Agea | 52.45(8.78) | 46.18 (11.57) | 32.00 (8.40) |
| Hours last meal | 3.59 (2.92) | 6.45 (5.98) | 4.91 (2.89) |
| Weight | 194.00 (29.27) | 189.86 (61.87) | 159.00 (30.36) |
| BMIa | 30.95 (3.58) | 27.09 (6.14) | 23.43 (3.43) |
| BDI scorea | 13.3 (8.88) | 26.10 (16.19) | 7.81 (6.83) |
| EES A/F | 13.72 (10.23) | 9.72 (11.03) | 4.63 (5.76) |
| EES depression | 8.90 (4.86) | 8.54 (5.22) | 5.09 (3.91) |
| EES anxiety | 10.54 (8.72) | 10.00 (7.98) | 3.90 (4.43) |
Significant difference between groups (p < 0.05)
Measures
Emotional responses to food
Participants viewed 22 images of foods from the International Affective Picture System (IAPS) and used a computerized visual analog scale (CVAS) to rate how each food made them feel using a visual analog on three dimensions (unhappy to happy, calm to aroused, and small/submissive to big/dominant). These visual ratings were converted to numbers ranging from 0 to 100 by a computer. There was a blank screen between pictures of the foods to prevent differential lengths of viewing of particular foods. Participants viewed and completed ratings for each picture immediately after it was presented. Research suggested that the variance in emotional assessments can be accounted for by three dimensions (valence, arousal, and dominance).41 The software for presenting the pictures, randomizing picture order, collecting CVAS ratings, and randomizing VNS on/off condition was custom developed using RealBasic5.5.5 (Austin, TX) on the Macintosh Platform and the program was run on a Macintosh G5 using the Mac OSX.4.9 operating system.
Beck Depression Inventory-II (BDI-II)
The BDI-II consists of 21 items assessing symptoms of depression experienced during the past 2 weeks.42 Each item contained four statements reflecting varying degrees of symptom severity, and respondents were instructed to circle the number (ranging from zero to three, indicating increasing severity) that corresponded with the statement that best described them. Ratings were summed to calculate a total BDI-II score, which ranged from 0 to 63. The BDI-II demonstrated high internal consistency, good test–retest reliability, and good construct and concurrent validity with other common measures of depression in clinical and nonclinical samples.42,43
Emotional Eating Scale (EES)
The EES is a 25-item scale that measures urges to eat when experiencing different emotions.12 The scale utilized a five-point Likert response format from “no desire to eat” to “a strong desire to eat.” Out of the 25 emotions listed on the measure, three factors emerged: anger/frustration, anxiety, and depression. The EES was found to have good internal consistency for the entire scale (coefficient α = 0.81) and for the three-factor (anger/frustration, anxiety, and depression) subscales with coefficient α values of 0.78, 0.78, and 0.72, respectively, and adequate test–retest reliability (r = 0.79).12
Procedure
All participants
Participants arrived at the Brain Stimulation Laboratory at the Medical University of South Carolina and written informed consent was obtained. The height of the participants was obtained via self-report and a scale was used to attain the participants' weights. BMI was calculated based on this information. The timing and content of their last meal were recorded.
After completing the initial assessment and questionnaires, the participants were taken to a laboratory room. Participants were seated at a computer where a series of standardized color food images from the IAPS were shown on the screen.41 Twenty-two images of foods (e.g., ice cream, cake, cheeseburgers, pizza, fruits, meats, vegetables) were presented for 4 seconds each. Each of the pictures was presented to each participant for two separate viewings within one session. That is, participants viewed the first 22 food images (presented in a random order) and then viewed the same pictures over again (a total of 44 food images) in the same order, in one block of time (close to 1 hour) without interruption. After completion of the questionnaires and laboratory part of the study, all participants received a $50 reimbursement.
VNS Participants
For VNS participants, information about the setting of the VNS device was obtained, and the participants' VNS device on time was set to 7 seconds for the duration of the study. The device parameters were otherwise unchanged from their standard outpatient settings. They are listed in Table 1. VNS participants' devices were turned on for one viewing of the food images and turned off for the other (randomly ordered). Participants were not told whether their devices were on or off for each trial.
Analytic Plan
Computerized visual analog scale difference scores for each emotional response to food type were computed by subtracting the score during the first viewing and the second viewing of the food images. As the VNS on/off conditions were randomized for participants in the VNS group, absolute values of the difference scores were used as the dependent variable to ensure a common metric.
A multivariate analysis of covariance (MANCOVA) was performed with the group (VNS, non-VNS depressed, and healthy) entered as an independent variable and change scores (absolute difference between viewing of food) in emotional responses (valence, arousal, and dominance) to different foods (proteins, vegetables/fruits, sweets) entered as dependent variables. Time since last meal was entered as a covariate for this model. A multivariate effect of group warranted examination of univariate analysis of variance (ANOVA) testing whether the mean change in the VNS group was different from healthy controls and whether the mean change in the VNS group was different from depression controls. Significant results were found for emotional responses by food groups, and analyses were conducted to assess variables associated with change in emotional response to food. Among VNS participants, an exploratory multiple regression analysis was performed to examine the association between the emotional response (dependent variable) and the following independent variables, including emotional responses to food, VNS device settings, depression, BMI, emotional eating, and craving.
Results
Group Characteristics
To assess groups differences at baseline, three separate ANOVA models were performed (see Table 2 for means and standard deviations for measures). Groups differed on age [F(2, 30) = 12.87, p =0 .000], BDI score [F(2, 28) = 7.21, p = 0.003], and BMI [F(2, 30) = 7.47, p = 0.002]. Least significant difference post-hoc analyses revealed that participants in the control group (M = 26.36) were significantly younger than participants in either depression VNS (M = 46.56) or depression non-VNS (M = 38.41) groups. No significant differences between depression non-VNS and depression VNS groups were found for age. For the BDI score, participants in the depression non-VNS group (M = 26.1) had significantly higher scores on the BDI (measure of depression) than those in the depression VNS (M = 13.3) and control (M = 7.81) groups. Significant differences in BDI scores were not found between depression VNS and control groups. Participants in the control group (M = 23.42) had significantly lower BMIs than those in depression VNS (M = 30.94) or depression non-VNS (M = 27.09) groups. No differences were found between depression VNS and depression non-VNS groups for BMI.
Changes in Emotional Responses between Food Picture Viewings (VNS on versus off)
Changes in ratings of emotional responses to food (valence, arousal and dominance) by food groups (proteins, vegetables/fruits, sweets) between paired pictures were assessed using MANCOVA controlling for time since last meal (in hours). Comparison across groups of these absolute values of differences permitted assessment as to whether the differences were random or related to turning the VNS device on or off (see Figures 1–3). The MANCOVA was significant for an effect of group across all food images, F(5, 27) = 3.42, p = 0.01. With respect to arousal ratings for “sweets,” there was a significant effect, F (2, 343) = 4.37, p = 0.022. Post-hoc analyses revealed significant differences on the arousal difference score between VNS (M = 20.26, SD 13.94, range 7.44–50) and nondepressed (M = 7.92, SD 4.45, range 2.22–14.89) groups. No significant differences were found between VNS and depressed groups (M = 14.32, SD 7.21, range 6.44–31.67) or between depressed and nondepressed groups for arousal to sweet foods. Results for arousal suggest that individuals in the VNS group had significantly larger difference scores between viewings of the pictures than those in the nondepressed group on the arousal rating (see Figure 1). Specifically, 36% (N = 4) of VNS patients experienced decreases in their arousal for sweet foods and 64% (N = 7) of VNS patients had increased arousal for sweet foods during acute VNS activation (device turned on). The range in change scores in arousal for sweets (VNS on versus VNS off) was −10.22 to 17.33. No significant differences were found for valence or dominance for sweet foods or any of the other emotional responses to either of the other food groups (vegetables/fruits and proteins).
Figure 1.
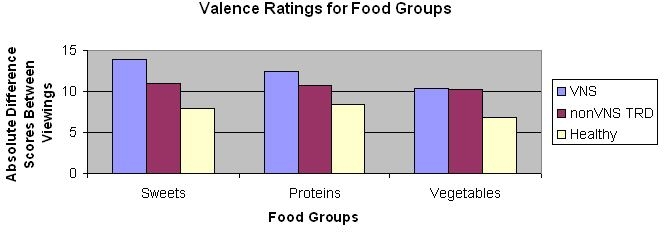
Absolute different scores in valence ratings for different food groups by participant group.
Figure 3.
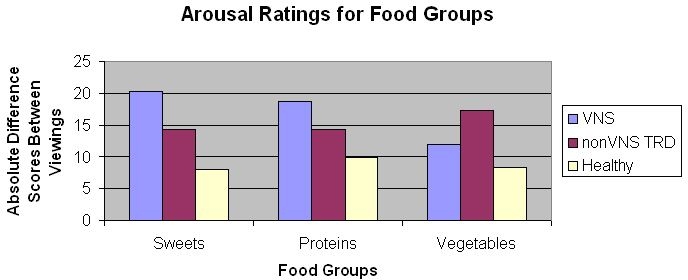
Absolute different scores in arousal ratings for different food groups by participant group.
Figure 2.
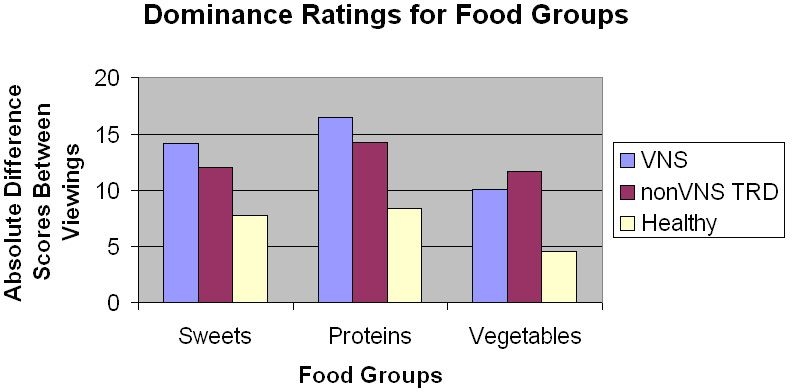
Absolute different scores in dominance ratings for different food groups by participant group.
Next, in order to understand factors related to the differential effect (increase versus decrease) of VNS on arousal for sweets among VNS participants, exploratory regression analyses were conducted. These analyses were performed to determine if variability in VNS-related changes in arousal ratings for sweets was associated with VNS device settings (output current and device on time), depressive symptoms, depressive emotional eating, sweet cravings, and BMI. The exploratory model was significant [F(3, 6) = 8.58, p = 0. 05]. A large proportion of individual variability in VNS-related changes in arousal for sweets was associated with cravings for sweets and BMI (adjusted R2 = 0.835). See Table 3 for parameters in the model. Specifically, we found that higher BMI (ß = 2.97, t = 3.51, p < 0.05) and higher levels of cravings for sweets (ß = 0.72, t = 3.8, p < 0.05) were associated with increases in arousal to sweets when the device was on (and conversely, lower BMI and lower sweets cravings were associated with decreases in arousal ratings for sweet food pictures during VNS device activation). The relationship between higher levels of current setting on the VNS device and arousal to sweets approached significance (ß = 23.11, t = 2.81, p = 0.06).
Table 3.
Parameters from Exploratory Regression Analyses Predicting VNS-Related Changes in Arousal for Sweet Foods among Participants Undergoing VNS Therapy for Depression at Time of Study (Adjusted R2 = 0.835). Variable
| Variable | β | Std. error | Std. β | t value | Sig. |
|---|---|---|---|---|---|
| Constant | −105.60 | 31.35 | −3.37 | 0.043 | |
| BMI | 2.97 | 0.85 | 0.77 | 3.51 | 0.04 |
| VNS device current | 23.11 | 8.22 | 0.54 | 2.81 | 0.06 |
| BDI | 0.05 | 0.28 | 0.03 | 0.17 | 0.88 |
| On-time VNS device | 0.264 | 0.30 | 0.15 | 0.87 | 0.45 |
| Depression emotional eating factor | −0.90 | 0.54 | −0.31 | −1.66 | 0.20 |
| Food cravings for sweets | 0.72 | 0.19 | 0.67 | 3.8 | 0.03 |
Discussion
This study was the first to examine the effects of acute left cervical vagus nerve stimulation on emotional ratings of food in adults with major depression. We found that the VNS-depressed group had greater differences in arousal ratings of sweets between viewings of images (when their device was on and then off) than healthy controls, but did not differ from non-VNS-depressed participants. A significant proportion of the variability in VNS-related changes in arousal for sweet foods was associated with cravings and BMI. No differences between the non-VNS depressed group and the other two groups were found. In addition, we did not find any differences in valence and dominance between groups and for other food categories (proteins and vegetables/fruits).
Results suggest that acute VNS and depression may impact individuals' self-reported arousal response to images of sweet foods. We are limited in the conclusions that can be drawn from our results because we did not find differences between depressed groups (VNS versus non-VNS). That is, the change in arousal ratings between viewings of sweet foods for VNS participants, where the VNS device was turned on for one viewing and off for the other, did not differ from a group of non-VNS-depressed patients who saw the same images twice without any manipulation. However, differences between the non-VNS depression group and the healthy control group on arousal to sweets were not found, which softly suggests that depression alone does not account for all of the variance in our finding. If depression alone were sufficient to account for differences in arousal to sweets, the non-VNS-depressed group would be expected to be significantly different from the healthy controls, especially as there was a significant difference between these groups with respect to depressive symptoms (M = 26.10 versus M = 7.81). There was also a significant difference in depression between the non-VNS-depressed group and the VNS group (M = 13.3), which also lends support to VNS impacting arousal to sweet food images. The small samples in this study allow for the possibility of a type II error, where there might be a difference but the small sample size precludes finding it. It is possible that there was an effect of VNS but because of the heterogeneity of our groups on several variables, including depression, weight, and age, along with the small sample size, we were unable to detect differences. Future research examining groups matched either on levels of depressive symptoms or nondepressed groups (i.e., epileptic patients with VNS) may help elucidate this relationship.
In our study, acute VNS did not have the same effect on arousal to sweets for all depressed patients. That is, 36% of the VNS participants experienced decreases in their arousal for sweets and 64% of the VNS participants had increased arousal for sweets during acute device activation. Differences in the effect of VNS on changes in arousal may be a function of the type of depression the person is experiencing, i.e., whether the symptomatology of their mood was typical (decreased appetite and sleep) or atypical (increased appetite and sleep).44 Activation of the vagus nerve may have a differential effect on arousal in individuals depending on their depressive state. As we did not assess depression type in the current study, future research should address the differential effects of VNS by subtype of depression and levels of depressive symptoms.
The effect that VNS has on arousal ratings of food may be especially pronounced in those individuals who are more obese and are “sweet cravers.” This is consistent with literature that suggests that emotional responses to eating high-energy dense foods (such as sweets and fats) are more intense among overweight individuals.9 As emotional responses to food are likely the result of complex bidirectional psychological (attitudes and perceptions related to foods)45 and physiological (tryptophan and serotonin) processes,46,47 it is difficult to ascertain from our study why arousal was more pronounced in these groups. It may be that obese individuals and/or sweet cravers have had past experiences with foods such as sweets that are different (i.e., eating as a response to mood) and more prominent than in lean individuals or nonsweet cravers. Depression level and emotional eating were not associated with a change in arousal ratings for sweets in patients receiving VNS as hypothesized. It may be that these variables are less important when looking at the effects of VNS on arousal to sweet foods.
In our initial study looking at the effects of acute VNS on food cravings we found that output setting was associated with cravings for sweets.40 Although the relationship between VNS device output setting and arousal to sweets among VNS participants was not statistically significant (p = 0.06), it does warrant mention. It is possible that with increased power as obtained through a larger sample size this association would have reached significance. It seems important to highlight the potential of VNS device parameters to produce specific brain effects that may be related to appetite and eating. As the only study to demonstrate a relationship between VNS and weight loss was among participants receiving VNS for epilepsy,39 one explanation for this could be that epilepsy patients tend to have a much higher output current programmed into their devices when compared to depressed patients. However, data examining the effects of VNS on weight change in patients with epilepsy (who had similar VNS parameters to the earlier study finding weight loss) found no change in weight.48 Future research is warranted examining the differential impact of VNS device parameters on food cravings, emotional responses to food, and eating behavior.
We did not find an effect for the valence and dominance of emotional responses to any of the food groups. It could be that the effects of depression and VNS are more potent on arousal then these other two emotional responses. Arousal is both an emotional response and a descriptor of a physiological state (i.e., one is aroused during a fight-or-flight response). It can be used to describe a state of high energy, thus the opposite of feeling tired or lethargic.
Limitations
There were several limitations to the current study. Because we utilized a small sample size of only 11 individuals in each group, power was limited. A larger N may have increased our ability to detect significant differences between groups. The groups differed significantly from each other with regard to depression, BMI, and age. Generalizability of these findings may be limited to participants with chronic, treatment-resistant depression receiving VNS treatment in the southeast United States. In addition, several variables that may have been used to explain individual differences in changes in emotional responses to food were not assessed, including type of depression, dietary restraint, and weight loss history. The categorization of patients into groups was based on past diagnoses of depression and not current depressive symptoms. The groups were categorized by past diagnoses using Structured Clinical Interviews based on DSM-IV conducted prior to participation in the current study (in VNS trials for depression). It is likely that the differences in depression scores for participants in the VNS and non-VNS-depressed groups were a result of their treatment response to VNS and/or antidepressants. Finally, we could not control for the impact that antidepressant treatment, the combination of antidepressants and VNS, and the chronic use of VNS has on eating behavior/food craving in patients. Therefore, it should be acknowledged that these variables could impact or change responses to foods.
Conclusions and Future Directions
Our study provided initial evidence that VNS and depression may have an effect on self-reported arousal to images of sweet foods and that this relationship warrants further exploration. During acute vagus nerve activation, there were differences in whether participants had an increase or decrease in arousal to sweets, with higher BMIs and higher levels of sweet cravings being significantly related to increases in arousal levels. These findings add to the eating and mood literature by demonstrating that depression and stimulation of the vagus nerve may affect how people appraise sweet foods, which could potentially affect eating behaviors and possibly obesity. As this line of research is in its infancy, clearly more research is needed before we are able to draw any substantial conclusions. Replications of the current study in other samples of patients receiving VNS therapy for depression or epilepsy are needed. The use of physiological measures of arousal such as heart rate variability would be useful in measuring arousal. Future research would benefit from using larger samples, using real foods, and using groups of TRD patients who are more homogeneous in terms of their depressive symptoms. Further, as eating pathology (such as binge eating) may be more pronounced in obese patients, conducting a similar study with equal groups of normal weight and obese patients may also be beneficial.
Acknowledgment
This study was funded in part by an intramural grant through the University Research Committee at the Medical University of South Carolina.
Abbreviations
- ANOVA
univariate analysis of variance
- BDI-II
Beck Depression Inventory II
- BMI
body mass index
- CVAS
computerized visual analog scale
- EES
Emotional Eating Scale
- IAPS
International Affective Picture System
- MANCOVA
multivariate analysis of covariance
- TRD
treatment-resistant depression
- VNS
vagus nerve stimulation
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgibbon ML, Stolley MR, Kirschenbaum DS. Obese people who seek treatment have different characteristics than those who do not seek treatment. Health Psychol. 1993;12(5):342–345. doi: 10.1037//0278-6133.12.5.342. [DOI] [PubMed] [Google Scholar]
- 4.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65(5):634–651. doi: 10.4088/jcp.v65n0507. [DOI] [PubMed] [Google Scholar]
- 5.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagoto SL, Bodenlos JS, Kantor L, Gitkind M, Ma Y. Association of major depression and binge eating disorder on weight loss in a hospital-based weight loss program. Obesity Research. 2007 doi: 10.1038/oby.2007.307. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein SR, Schuppenies A, Wong ML, Licinio J. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene-environment interactions. Mol Psychiatry. 2006;11(10):892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 8.Kampov-Polevoy AB, Alterman A, Khalitov E, Garbutt JC. Sweet preference predicts mood altering effect of and impaired control over eating sweet foods. Eat Behav. 2006;7(3):181–187. doi: 10.1016/j.eatbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Macht M, Gerer J, Ellgring H. Emotions in overweight and normal-weight women immediately after eating foods differing in energy. Physiol Behav. 2003;80(2–3):367–374. doi: 10.1016/j.physbeh.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89(1):53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Thayer RE. Calm energy: how people regulate mood with food and exercise. New York: Oxford University Press; 2001. [Google Scholar]
- 12.Arnow B, Kenardy J, Agras WS. The Emotional Eating Scale: the development of a measure to assess coping with negative affect by eating. Int J Eat Disord. 1995;18(1):79–90. doi: 10.1002/1098-108x(199507)18:1<79::aid-eat2260180109>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite. 1992;18(3):207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- 14.Christensen L, Brooks A. Changing food preference as a function of mood. J Psychol. 2006;140(4):293–306. doi: 10.3200/JRLP.140.4.293-306. [DOI] [PubMed] [Google Scholar]
- 15.Benton D, Donohoe RT. The effects of nutrients on mood. Public Health Nutr. 1999;2(3A):403–409. doi: 10.1017/s1368980099000555. [DOI] [PubMed] [Google Scholar]
- 16.Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3(Suppl 4):477S–480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 17.Macht M, Dettmer D. Everyday mood and emotions after eating a chocolate bar or an apple. Appetite. 2006;46(3):332–336. doi: 10.1016/j.appet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Mauler BI, Hamm AO, Weike AI, Tuschen-Caffier B. Affect regulation and food intake in bulimia nervosa: emotional responding to food cues after deprivation and subsequent eating. J Abnorm Psychol. 2006;115(3):567–579. doi: 10.1037/0021-843X.115.3.567. [DOI] [PubMed] [Google Scholar]
- 19.Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006;103(42):15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lisanby S, Burt T, Goldman J, Ballenger JC. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47(4):287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- 21.Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, George MS. Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol. 2001;36(8):470–479. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, George MS. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003;37(6):443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 23.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6) Suppl 4:S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 24.Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36(4):219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 25.Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, Denslow S, Lomarev M, Moghadam P, Chae JH, George MS. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatry. 2004;55(8):816–825. doi: 10.1016/j.biopsych.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav. 2006;89(4):477–485. doi: 10.1016/j.physbeh.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81(2):249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, Walker J, Anderson B, Koola J, Kose S, Lomarev M, Bohning DE, George MS. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32(8):1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 29.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12(Suppl 1):12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 30.Roslin M, Kurian M. The use of electrical stimulation of the vagus nerve to treat morbid obesity. Epilepsy Behav. 2001;2:S11–S6. [Google Scholar]
- 31.Tecoma ES, Iragui VJ. Vagus nerve stimulation use and effect in epilepsy: what have we learned? Epilepsy Behav. 2006;8(1):127–136. doi: 10.1016/j.yebeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Uthman BM. Vagus nerve stimulation for seizures. Arch Med Res. 2000;31(3):300–303. doi: 10.1016/s0188-4409(00)00060-6. [DOI] [PubMed] [Google Scholar]
- 33.Harden CL, Lazar LM, Pick LH, Nikolov B, Goldstein MA, Carson D, Ravdin LD, Kocsis JH, Labar DR. A beneficial effect on mood in partial epilepsy patients treated with gabapentin. Epilepsia. 1999;40(8):1129–1134. doi: 10.1111/j.1528-1157.1999.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 34.George MS, Sackeim HA, Marangell LB, Husain MM, Nahas Z, Lisanby SH, Ballenger JC, Rush AJ. Vagus nerve stimulation. A potential therapy for resistant depression? Psychiatr Clin North Am. 2000;23(4):757–783. doi: 10.1016/s0193-953x(05)70196-9. [DOI] [PubMed] [Google Scholar]
- 35.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, Nahas Z, Haines S, Simpson RK, Goodman R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B, Carpenter L, Ninan P, Moreno F, Schwartz T, Conway C, Burke M, Barry JJ. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, Kling MA, Rittberg BR, Burke WJ, Rapaport MH, Zajecka J, Nierenberg AA, Husain MM, Ginsberg D, Cooke RG. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, Simpson RK, Goodman RR. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 39.Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59(3):463–464. doi: 10.1212/wnl.59.3.463. [DOI] [PubMed] [Google Scholar]
- 40.Bodenlos JS, Kose S, Borckardt JJ, Nahas Z, Shaw D, O'Neil PM, George MS. Vagus nerve stimulation acutely alters food craving in adults with depression. Appetite. 2007;48(2):145–153. doi: 10.1016/j.appet.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 41.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): instruction manual and affective ratings. University of Florida: Gainesville (FL); 2005. [Google Scholar]
- 42.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 43.Whisman MA, Perez JE, Ramel W. Factor structure of the Beck Depression Inventory-Second Edition (BDI-II) in a student sample. J Clin Psychol. 2000;56(4):545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 44.Paykel ES, Parker RR, Rowan PR, Rao BM, Taylor CN. Nosology of atypical depression. Psychol Med. 1983;13(1):131–139. doi: 10.1017/s0033291700050133. [DOI] [PubMed] [Google Scholar]
- 45.Booth DA. Psychology of nutrition. London: Taylor & Francis; 1994. [Google Scholar]
- 46.Wurtman JJ. Carbohydrate cravings: a disorder of food intake and mood. Clin Neuropharmacol. 1988;11(Suppl 1):S139–45. [PubMed] [Google Scholar]
- 47.Wurtman RJ, Wurtman JJ. Do carbohydrates affect food intake via neurotransmitter activity? Appetite. 1988;11(Suppl 1):42–47. [PubMed] [Google Scholar]
- 48.Koren MS, Holmes MD. Vagus nerve stimulation does not lead to significant changes in body weight in patients with epilepsy. Epilepsy Behav. 2006;8(1):246–249. doi: 10.1016/j.yebeh.2005.10.001. [DOI] [PubMed] [Google Scholar]


