Abstract
Background
Since the advent of subcutaneous glucose sensors, there has been intense focus on characterizing the delay in the interstitial fluid (ISF) glucose response and the effect of insulin to alter the plasma-to-ISF glucose gradient. The Medtronic MiniMed continuous glucose monitoring system (CGMS) has often been used for this purpose; however, many of the studies have used experimental conditions that fall outside its intended use, for example, studies that have assessed the delay during rapid glucose excursions brought about by intravenous infusion of glucose or insulin. Under these conditions, it is possible that the rate of glucose change may exceed that allowed by CGMS filtering routines. If so, the estimated delay may be because of the filter rather than the ISF. Also, sensor characteristics, such as nonspecific offset current or stability, may have been inadvertently attributed to changes in the plasma-to-ISF gradient. The potential for these issues to have confounded the understanding of ISF glucose delay and gradient is investigated.
Methods
An in vitro preparation in which no delay or gradient exists between sensor and measurement solution was used to recreate a rapidly changing glucose profile from a previously published in vivo study. The CGMS system (N = 6 sensors) was then used to estimate any artifactual delay and gradient introduced by the system per se.
Results
One-point calibration resulted in an apparent change in gradient as glucose was lowered from ∼100 to 50 mg/dl. After a two-point calibration, sensor glucose followed the glucose profile as it was decreased slowly from ∼100 to ∼60 mg/dl; however, when the glucose level was subsequently increased rapidly to ∼150 mg/dl, CGMS filtering routines limited the rate of change of sensor glucose and introduced a delay similar to that previously attributed to ISF glucose equilibration delay.
Conclusions
Studies that have previously used the Medtronic MiniMed CGMS system to assess changes in the plasma-to-ISF glucose gradient may need to be reassessed to ensure that the offset current was estimated accurately. Studies that have used the system to assess ISF glucose delay during rapid, unphysiologic changes in glucose and did not remove the CGMS smoothing filters may have attributed CGMS filter delay to ISF glucose equilibration.
Keywords: CGMS, delay, glucose gradient, glucose sensor, interstitial fluid glucose, one-point calibration, two-point calibration
Introduction
With the introduction of subcutaneous glucose monitoring, many attempts have been made to evaluate the relationship between blood and interstitial fluid (ISF) glucose. Primary focuses have been on assessing delays between blood and ISF readings and the impact of insulin on the plasma-to-ISF glucose gradient. Some studies1,2 have reported the delay between blood and ISF readings to be less than 10 minutes, irrespective of whether glucose is rising or falling, and the plasma-to-ISF glucose gradient to be stable. Other studies have shown long delays that differ depending on whether glucose is rising or falling3–6 and have argued that there is a marked effect of insulin on the plasma-to-ISF gradient.7
Most studies assessing ISF glucose delay have utilized measurement systems that are assumed not to contribute to the delay, for example, systems using amperometric glucose sensors. Generally, amperometric sensors do have in vitro response times that are fast enough to be ignored (time to reach 95% steady state, generally less than 1.5 minutes). However, systems using amperometric sensors may apply digital filters to remove any unwanted noise in the sensor signal and these filters can add substantial delay. For example, a simple three-point moving average on a signal obtained every 5 minutes will smooth the response but take 15 minutes to reach steady state. To avoid such a smoothing delay, rate-limiting filters that do not introduce any delay when the glucose is changing more slowly than a predefined threshold can be used.8 However, such filters introduce increasing longer delays as the signal begins to move faster than the maximum allowed rate. For example, if the glucose sensor is constrained not to increase faster than 3 mg/dl/min, an increase in glucose from 100 to 130 mg/dl in 10 minutes will not be delayed. The same increase in 1 minute will not be reported correctly until 10 minutes later, and if the increase is to 160 mg/dl, the filter will not reach steady state for 20 minutes. As a general rule, removing more noise will further smooth the signal but increase the delay.
In practice, filter design is more involved than the moving average or rate-limiting examples just given, and the final filter can be expected to have different characteristics depending on how the device is intended to be used. Devices intended to highlight trends in the glucose profile retrospectively can be expected to have filters that produce very smooth signals but with longer delays. For these retrospective devices, delay is not an issue as the signal may not be analyzed for several days. Whereas devices used in “real time” will benefit from faster filter response times, the filter will still need to provide sufficient smoothing to prevent any undesirable patient intervention when the sensor is reacting to noise. In any case, without a priori knowledge of the filter used, it is not possible to separate filter delay from ISF glucose delay when analyzing sensor response curves. For the CGMS system per se, smoothing is performed with rate-limiting filters.8
Subcutaneous glucose sensors have also been widely used to assess the effect of insulin on altering the gradient between plasma and ISF. However, virtually all devices used to measure subcutaneous ISF glucose need to be calibrated in vivo using one or more blood glucose values. Unfortunately, amperometric glucose sensors often have a nonspecific background, or offset (OS), current, which means that the sensor current does not go to zero as glucose goes to zero even when assessed in vitro. In vivo, the OS current is difficult to ascertain without a large number of reference glucose values spanning a wide range of glucose values. Sensors that are calibrated based on a single point necessarily rely on an assumed OS current—with a typical assumption being zero. This can lead to systematic errors in glucose readings obtained above or below the calibration point, which can easily be misinterpreted as a change in the plasma-to-ISF glucose gradient. For example, if the ISF glucose gradient is 0.8 at all levels of glucose (stable), a sensor reading of 20 nA per 100 mg/dl with no OS would read 16 nA in vivo when plasma glucose is 100 mg/dl. This would result in a calibration factor (CF) of 6.25 mg/dl per nA (100 mg/dl/16 nA) and the calibrated sensor glucose (SG) would correctly report glucose at 50 and 150 mg/dl. A different sensor also reading 16 nA at 100 mg/dl but with 2 nA offset (sensor current = [(16 nA – 2 nA)/80 mg/dl × GISF + 2 nA], where GISF is interstitial glucose concentration) would read 9 nA when glucose falls to 50 and 23 nA when glucose is elevated to 150. If this latter sensor is calibrated at 100 mg/dl with an assumed OS of zero, it will also have a CF of 6.25, but read 56.25 when plasma glucose is 50 mg/dl and 143.73 when glucose is 150. This overestimation when glucose is below the calibration point and underestimation when glucose is above the calibration point can easily be misinterpreted as changes in glucose gradient.
The present study examined the impact of filter delay and sensor offset current on estimates of delay and gradient obtained with the Medtronic MiniMed continuous glucose monitoring system (CGMS). An in vitro system in which there is neither a gradient nor a delay was used to demonstrate the impact of the sensor offset current and device filters. While the CGMS system is used, the methodology developed can be applied to any device without need for proprietary filter information.
Methods
In vitro characterization of MiniMed CGMS system
In vitro, a glucose profile was created using pumps infusing 20% glucose and phosphate buffer into a well-stirred beaker (Figure 1). Infusion rates were calculated to obtain a profile reported previously in a study by Monsod and colleagues7 where the authors concluded that insulin effected a gradient change and that there was a variable delay in ISF glucose equilibration. The in vitro solutions were maintained at 37°C, and the glucose concentration in the beaker (GB) was confirmed by samples taken every 15 minutes from −20 to 190 minutes (same sample interval as reported previously7) and measured with a Yellow Spring Instrument (YSI) glucose analyzer (YSI Inc., Yellow Springs). Sensors were placed directly into the solution (no gradient; no delay). Unfiltered, 1-minute sensor current, filtered sensor current, and calibrated sensor glucose values were obtained from the CGMS system output.
Figure 1.
In vitro system used to evaluate CGMS delays. A CGMS sensor was placed in phosphate buffer, and infusion pumps with 20% glucose or buffer were used to manipulate the glucose level. A pipette was used to obtain samples that were measured with a YSI glucose analyzer (not shown), and a CGM system (shown on right) was used to obtain the sensor glucose profile.
Results
Glucose concentration in the buffer (closed circles, Figure 2A) was approximately equal to that observed in the study by Monsod et al.7 (open circles, Figure 2A) at all measured values (r2 = 0.997; average glucose value not different P > 0.05). Using a one-point calibration at time = −20 minutes and an assumed OS current of 0 nA resulted in SG = 5.21 × ISIG, where 5.21 is the CF (mg/dl per nA) and ISIG is the sensor current signal. The calibrated SG (blue tracing left axis with corresponding current shown on the right in Figure 2A) overestimated glucose in the hypoglycemic region (64.72 ± 0.25 vs 58.03 ± 1.14; P < 0.01) and had a tendency to underestimate glucose at hyperglycemic levels (162.24 ± 2.4 vs 160.93 ± 3.2, P = 0.2728).
Figure 2.
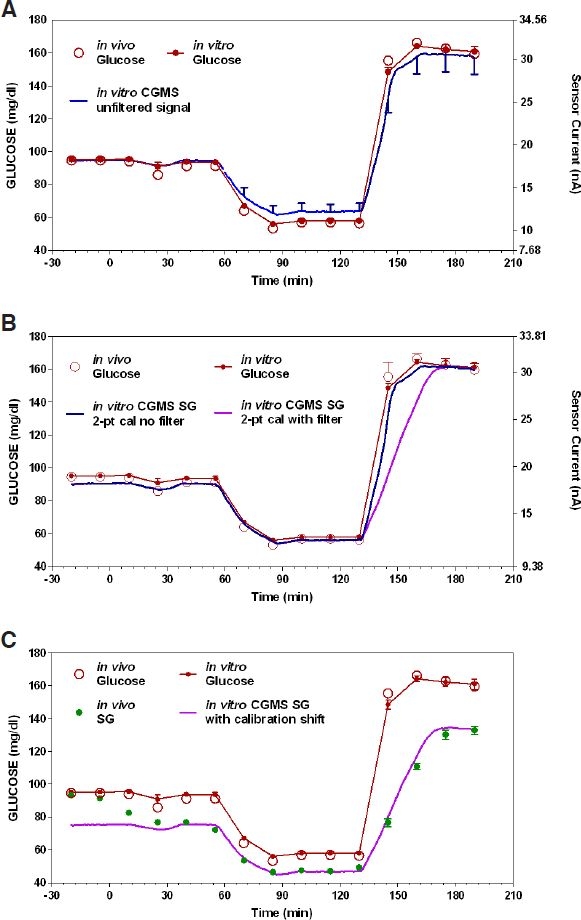
In vitro reproduction of the hypoglycemic glucose profile obtained in the study by Monsod et al.7 compared with (A) unfiltered sensor glucose (left axis) and sensor current (right axis) obtained with one-point calibration and zero offset, (B) same profile with two-point calibration, and (C) profile showing similarity of original in vivo profile together with In vitro profile if the sensor signal were to drift downward.
The tendency to overestimate glucose at hypoglycemia and underestimate glucose at hyperglycemia was corrected by two-point calibration at the 130 (hypo) and 190 (hyper) minute time points. With two-point calibration, sensor glucose (blue line left axis with corresponding current shown on right axis in Figure 2A) was calculated as SG = 5.73 × (ISIG – 2.4), where the CF is now 5.73 and the offset current 2.4 nA. Whereas two-point calibrations resulted in correct sensor glucose values at the two calibration points (by definition, two-point calibration will always yield correct readings at the calibration points), a small but statistically significant underestimation of glucose at t = −20 minutes was observed (89.91 ± 1.01 mg/dl for sensor glucose vs 95.175 ± 0.21 mg/dl for buffer glucose measured with the YSI analyzer; P = <0.01). This suggests that the sensor sensitivity increased slightly during the course of the experiment or that the sensor response is nonlinear.
While the sensor response was virtually superimposable with the in vitro glucose concentration during the fall (Figure 2B; 60 to 90 minutes), suggesting a minimal or nonexistent filter delay, a more substantial delay (time to 50% maximal response = 18.4 minutes; time to 90% response = 33 minutes) was observed in the smoothed CGMS output signal during the rapid rise in buffer glucose (Figure 2B; 130 to 190 minutes). With the signal processing delay included, the overall rise in sensor glucose was well correlated with the rise observed in the original Monsod et al.7 study (r2 = 0.86). The high correlation suggests that a simple shift in calibration can account for the transient hypo/hyperglycemic profile observed in the original Monsod study. Thus, reducing the CF used in Figure 2C from 5.73 to 4.77 [SG = 4.77 × (ISIG – 2.4)] resulted in a sensor glucose profile (Figure 2C) virtually superimposable with the profile in the original Monsod et al.7 in vivo study.
Discussion
In this study, we hypothesized that variability in the reported estimates of the ISF glucose delay and the effect of insulin on the plasma-to-ISF glucose gradient may have been artificially exaggerated by delays or other characteristics inherent in the devices used to measure ISF glucose. Using an in vitro system, in which there was no gradient or delay between measured glucose and sensor glucose (Figure 1), we generated data that could easily be misinterpreted as having changes in gradients (Figure 2A) and different delays depending on whether glucose is rising or falling (Figure 2B). The first of these misinterpretations is a consequence of an inappropriate assumption about the sensor offset current (assuming 0 when the true value was 2.4 nA); the second resulted from assuming no inherent delay in the CGMS noise reduction algorithm. Quantitatively, the observed system delays were virtually identical to those reported in the original Monsod et al.7 study (Figure 2C) with the difference being that here they are clearly attributed to the CGMS system per se, whereas in the Monsod et al.7 study they were attributed to a delay in the ISF glucose response.
The observation that CGMS introduced a different delay during the fall in glucose (Figure 2B; from time 45 to 90 minutes) versus the rise in glucose (from time 130 to 190 minutes) is a direct consequence of the type of filtering used by the CGMS system. The CGMS system utilizes thresholds to limit the maximum amount sensor glucose can change in any 5-minute period.8 The advantage of this type of filter, compared to filters that use weighted averages of past signal values (e.g., three-point moving average), is that for slow-moving smooth signals the filter does not introduce any delay. That the filter does not introduce any delay during a slow signal can be seen during a slow fall in buffer glucose (Figure 2B). Nonetheless, during a rapid change in glucose the delay can be quite substantial (e.g., the rise in glucose, Figure 2B). Generally, very rapid changes in glucose during normal day-to-day use indicate noise in the underlying sensor signal; however, infusion of intravenous (IV) glucose can introduce a rapid change that is real.
That the CGMS system delay observed in the in vitro study used here and the delay observed by Monsod and colleagues7 in vivo were virtually identical during the recovery from low glucose (Figure 2C; from time 120 to 180 minutes) does not imply that there is no delay in the sensor itself or ISF glucose. Rather, smoothing algorithms based on limiting the maximal rate of change of signal tend to mask such delays whenever the delayed response is still faster than that allowed by the smoothing algorithm. Thus, with the smoothing algorithm in place, no definitive conclusions can be reached regarding sensor delay or ISF glucose delay during changes in plasma glucose that are faster than allowed by the smoothing routine. Based on data obtained here (Figure 2C), one could argue that the in vivo ISF glucose delay was actually zero; however, this is highly unlikely as similar clamps performed by our group in the absence of smoothing routines have shown an ISF glucose delay of 6–8 minutes.2
Determining how much the sensor is allowed to change in any 5-minute period is fundamental to the design of threshold filters. Severely limiting the maximum rate produces very smooth signals with virtually no noise, whereas allowing rapid changes yields very noisy signals with little or no delay. The thresholds used in CGMS are complicated,8 with the allowable changes limited sometimes by a fixed amount and sometimes by percentage changes—the choice being determined by both glucose levels and sensor sensitivity (smoothing shown in Figure 2 was based on fixed rather than percentage smoothing). The filter is arguably optimal for the device when used retrospectively to provide glucose trends under standard day-to-day living conditions, but is obviously less than ideal for research studies that utilize rapid changes in plasma glucose with IV insulin or glucose to assess ISF glucose delay. Unfortunately, rapid changes in plasma glucose are optimally suited to study such delays. The only study we have found in which the CGMS system was used during normal day-to-day living, i.e., when the smoothing algorithm is expected to introduce little or no delay, concluded that ISF glucose delay is minimal with no difference in rising or falling glucose.9
While results obtained with the CGMS system during changes in glucose brought about by IV insulin or glucose may not reflect one to one what is happening in the ISF space, the studies themselves are not invalid—they simply need to be reinterpreted as a product of “device behavior” and “ISF glucose behavior.” Thus, for the study by Monsod et al.,7 one can conclude that the CGMS response time when measuring ISF glucose during a rapid increase is delayed substantially. To assess the ISF glucose by itself, the filter needs to be removed. Absent the filter, delay and sensor offset current can be assessed independently using a model of the plasma-to-ISF glucose dynamics10 (delay and offset are identifiable independently, and any nonlinearity not in the model results in a poor model fit). With this methodology, we have shown previously that the ISF glucose response to hypoglycemia is rapid during both the fall and the recovery (6–8 minutes).2
Whereas the delay during the recovery from hypoglycemia observed by Monsod and colleagues7 can be explained by the delay introduced by signal smoothing, the decrease in sensor glucose observed during the initial hyperinsulinemic–euglycemic period (reproduced here in Figure 2C) cannot be attributed to either data smoothing or offset current as glucose was clamped. Further, in the original study,7 a similar drop in glucose was observed in microdialysis probes placed at the same time. Microdialysis data independently support the hypothesis that insulin can increase glucose uptake from subcutaneous tissues and that the need for increased glucose transport to the tissue bed necessitates an increase in the plasma-to-ISF glucose gradient. While we did not see the effect using SC glucose sensors in a similar hypoglycemic clamp study,2 our study used adult subjects whereas the study by Monsod et al.7 was conducted in children. It is possible that because the adult subjects had low insulin sensitivity, we were unable to show the effect. It is also possible that in both studies, changes in sensor sensitivity, offset current, or a prolonged run-in make such a determination difficult. Ideally, any future studies addressing this issue would use control subjects/sensors with no change in plasma glucose to rule out many out these confounding variables. Such a control experiment would also rule out any artifacts in the microdialysis system. Neither the Monsod et al. study7 nor our own study2 utilized such controls.
Finally, the need to accurately assess the nonspecific background current is clearly evident from in vitro data shown here. Assuming a zero OS current resulted in a clear overestimation of sensor glucose in the hypoglycemic range and a tendency to underestimate glucose in the hyperglycemic range (Figure 2A). This over/underestimation looks, on first pass, to be identical to that which would be evident if a variable gradient exists between the measured glucose concentration and the concentration as seen by the sensor. Estimation of the specific OS current associated with the sensors used in the present study eliminates any appearance of gradient (Figure 2B). That there is an OS current inherent in the CGMS is not a new finding—sensors of this type have long been know to exhibit nonspecific background current.11,12 Studies utilizing amperometric glucose sensors that have not used some method to assess the offset current independently and have subsequently concluded that there is an insulin effect to change the plasma-to-ISF glucose gradient will need to be reevaluated carefully. In practice, the CGMS obtains an estimation of the offset current during normal daily by requesting the patients take finger-stick glucose measurements across a range of low and high glucose values. Thereafter, retrospective optimization is applied to optimize the identification of both the offset current and calibration factors.13
In summary, results obtained in the present study highlight the need to separate “device properties” from “ISF glucose” properties. In vivo, the observed behavior of a sensing device is a product of both device characteristics and the ISF. Signal processing delays in the CGMS device can easily confound the estimation of ISF glucose delay, and errors in estimating sensor offset current can lead to erroneous conclusions regarding the plasma-to-ISF glucose gradient. Studies that have addressed ISF glucose kinetics using the CGMS device outside of its intended use description will need to be scrutinized carefully to determine if any confounding factors were introduced by the measurement device.
Acknowledgement
This work was supported by National Institutes of Health Grant RO1 DK 0064567 to GMS.
Abbreviations
- CF
calibration factor
- CGMS
continuous glucose monitoring system
- ISF
interstitial fluid
- IV
intravenous
- OS
offset
- SG
sensor glucose
- YSI
Yellow Spring Instrument
References
- 1.Steil GM, Rebrin K, Mastrototaro J, Bernaba B, Saad MF. Determination of plasma glucose during rapid glucose excursions with a subcutaneous glucose sensor. Diabetes Technol Ther. 2003;5(1):27–31. doi: 10.1089/152091503763816436. [DOI] [PubMed] [Google Scholar]
- 2.Steil GM, Rebrin K, Hariri F, Jinagonda S, Tadros S, Darwin C, Saad MF. Interstitial fluid glucose dynamics during insulin-induced hypoglycaemia. Diabetologia. 2005 Sep;48(9):1833–1840. doi: 10.1007/s00125-005-1852-x. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen JK, Djurhuus CB, Gravholt CH, Carus AC, Granild-Jensen J, Orskov H, Christiansen JS. Continuous glucose monitoring in interstitial subcutaneous adipose tissue and skeletal muscle reflects excursions in cerebral cortex. Diabetes. 2005 Jun;54(6):1635–1639. doi: 10.2337/diabetes.54.6.1635. [DOI] [PubMed] [Google Scholar]
- 4.Fischer U. Continuous in vivo monitoring in diabetes: the subcutaneous glucose concentration. Acta Anaesthesiol Scand Suppl. 1995;104:21–29. doi: 10.1111/j.1399-6576.1995.tb04252.x. [DOI] [PubMed] [Google Scholar]
- 5.Aalders AL, Schmidt FJ, Schoonen AJ, Broek IR, Maessen AG, Doorenbos H. Development of a wearable glucose sensor; studies in healthy volunteers and in diabetic patients. Int J Artif Organs. 1991 Feb;14(2):102–108. [PubMed] [Google Scholar]
- 6.Sternberg F, Meyerhoff C, Mennel FJ, Mayer H, Bischof F, Pfeiffer EF. Does fall in tissue glucose precede fall in blood glucose? Diabetologia. 1996 May;39(5):609–612. doi: 10.1007/BF00403309. [DOI] [PubMed] [Google Scholar]
- 7.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care. 2002 May;25(5):889–893. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]
- 8.Mastrototaro JJ, Gross TM, Shin JJ. Glucose monitor calibration methods. US Patent No. 6424847;2002.
- 9.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003 Nov;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 10.Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000 Autumn;2(3):461–472. doi: 10.1089/15209150050194332. [DOI] [PubMed] [Google Scholar]
- 11.Ward WK, Wood MD, Troupe JE. Rise in background current over time in a subcutaneous glucose sensor in the rabbit: relevance to calibration and accuracy. Biosens Bioelectron. 2000 Mar;15(1–2):53–61. doi: 10.1016/s0956-5663(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 12.Velho G, Froguel P, Thevenot DR, Reach G. Strategies for calibrating a subcutaneous glucose sensor. Biomed Biochim Acta. 1989;48(11–12):957–964. [PubMed] [Google Scholar]
- 13.Shin JJ, Holtzclaw KR, Dangui ND, Kanderian S, Mastrototaro JJ, Hong P. Real time self-adjusting calibration algorithm. US Patent No. 6895263;5-17-2005.


