Abstract
Background
How smoothly insulin is injected is one of the major concerns when patients commence insulin injection therapy. Improving its usability may be important in initiation therapy and adherence, resulting in clinical benefits to the patient.
Methods
In a single-center, open-label and randomized two-period crossover trial, the effect of the tapered needle of NanoPass® (33 gauge, 5 mm) on usability in comparison with the standard needle of Micro Fine Plus® (31 gauge, 5 mm) was examined using a questionnaire. Patients with insulin-dependent diabetes (n = 40, self-injecting insulin four times daily for more than 3 months) were randomized to use NanoPass or Micro Fine Plus needles for 1 week and then use the alternative for 1 week. Patients completed the questionnaire before and after each test week. Each evaluation was scored from −100 (worst) to +100 (best) by a visual analogue scale. A higher score indicated a more favorable outcome compared with the other needle.
Results
The NanoPass needle was significantly less painful to insert and caused less bruising than the Micro Fine Plus needle. However, there was no significant difference in the overall patient satisfaction score between the two needles. Meanwhile, the NanoPass needle, which had less resistance in insertion with a new lubricant coating method, had a significantly superior (P < 0.001) overall patient's satisfaction score, including less frightening use, less bleeding, and less dribbling of injected insulin in comparison with the former evaluation.
Conclusions
For overall patient satisfaction in using an insulin needle, developing a thinner needle and improving other factors, such as lubricity coating the needle, are important.
Keywords: insulin, lubricant, microtapered needle, overall satisfaction, pain and bleeding
Introduction
Usability with insulin injections is one of the major concerns when patients are initiating insulin therapy. Improving the usability may be important in initiation therapy and adherence, resulting in clinical benefits to the patient.1,2
To achieve this purpose, a specific type of microtapered needle for pen-type insulin syringes in a variety of lengths and diameters (NanoPass®; 33 gauge and 5 mm in length, Terumo Corp., Tokyo, Japan) has been developed.3 The newly developed needle has the unique structure shown in Figures 1 and 2; 33 gauge from the tip to 2.75 mm is followed by a gradual taper to 3.5 mm, reaching 28 gauge at the cartridge. Thus, in the tip to 2.75 mm, the outer diameter of needle is thinner than that of a standard needle (Micro Fine Plus®; 31 gauge and 5 mm in length, Nippon Becton Dickinson Co., Ltd., Tokyo),4 while the inner diameter of the needle is same as a standard needle. In the cartridge, the outer and inner diameters of the needle are thicker than those of a standard needle. The NanoPass needle provided diabetic patients with less pain, and the usability of the NanoPass needle by patients for self-injection was better than the Micro Fine Plus needle.5
Figure 1.
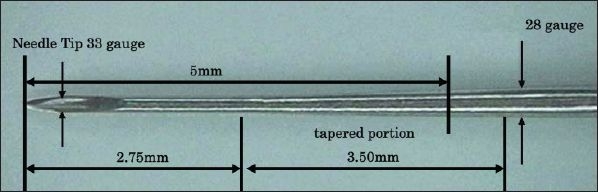
Microtapered structure of the NanoPass needle. Thirty-three gauge from the tip to 2.75 mm is followed by a gradual taper to 3.5 mm (tapered portion), which reaches 28 gauge at the cartridge. In the tip to 2.75 mm, the outer diameter of the needle is thinner than that of the 31 gauge, while the inner diameter of the needle is the same as the 31 gauge. In the cartridge, the outer and inner diameters of the needle are thicker than those of the 31 gauge. The portion inserted into the skin is 5 mm from the tip.
Figure 2.

Photographs of Micro Fine Plus (top) and NanoPass (bottom) needles by an optical microscope.
We reevaluated usability of the NanoPass needle in comparison with the Micro Fine Plus needle in insulin-dependent diabetic patients and examined the factors for the difference in usability between the two needles.
Subjects and Methods
Patients with insulin-dependent diabetes (n = 40) participated. There were 26 females and 14 males. Means ± SD of age, body mass index (BMI), diabetic duration with insulin injection, and glycated hemoglobin (HbA1c) were 45.8 ± 15.4 years old, 23.0 ± 3.1 kg/m2, 15.6 ± 9.8 years, and 7.3 ± 1.2%, respectively. All patients required four-daily insulin injections for more than 3 months and used rapid-acting and intermediate-acting (2 patients) or long-acting (38 patients) insulins via pens with replaceable cartridges. The mean ± SD of the dose was 46 ± 22 U/day. Before the study, patients had injected insulin into the subcutaneous tissue of the abdomen using the Micro Fine Plus needle and had never experienced injection of insulin into the subcutaneous tissue using a thinner needle than the Micro Fine Plus needle. There was no macrovascular disease, but 10 patients had simple, 5 preproliferative, and 1 proliferative retinopathies and 5 patients had albuminuria. However, all patients had no neuropathy with normal Achilles' tendon reflex and no factors that influenced the technique for self-injection of insulin, including loss of visual activity, less handling power, or tremor of finger. All patients gave written informed consent.
Study Design
Clinical usability of a microtapered needle of NanoPass in comparison with a standard needle of Micro Fine Plus in a single-center, open-label and randomized two-period crossover trial was evaluated using a questionnaire. Patients were randomized to use NanoPass or Micro Fine Plus needles for 1 week and then use the alternative for 1 week. The method was the same as reported previously.6 Patients completed the questionnaire before and after each test. The questionnaire consisted of six categories with 14 items: 4 items (Nos. 1–4) of category A (pain and frightening judged from appearance of the needle), 2 items (Nos. 5 and 6) of category B (installing and removing of needle), 3 items (Nos. 7–9) of category C (pain and sticking judged from inserting the needle into the skin), 2 items (Nos. 10 and 11) of category D (bleeding and bruising), 2 items (Nos. 12 and 13) of category E (dribbling of injected insulin and power of pushing inserting button), and 1 item (No. 14) of category F (overall patient satisfaction). Each evaluation was scored from −100 (worst) to +100 (best) by a visual analogue scale. A higher score indicated a more favorable outcome compared with the alternative needle.
There were two types (A and B) of NanoPass needles prepared for assessment. Type B, which is now available commercially in Japan, was a needle with less resistance of insertion by having a different coating method of lubricant than type A. Coating was performed with lubricant consisting of silicon and oil used widely anywhere1,2,4 and there was no difference in the ingredients of lubricant used between NanoPass A and B needles. However, a coated length of lubricant in the type B needle was longer than the type A needle by about 1 mm. The resistance difference of each needle was examined when they were pierced into a 5-mm-thick silicone sheet at a speed of 10 mm/second. The mechanical resistance value (unit of Newton; N) was measured with the AUTOGRAPH AGS-H machine (Shimadzu Corporation, Kyoto, Japan). The integrated area under the curves (IAC) described by the resistance level from 0 to 4 mm of displacement length in each needle was calculated based on the resistance value by the displacement length.
Data Analysis
All values are represented as mean ± SD. Mean values were compared using the Student's unpaired t test. Significant differences in scores obtained by a visual analogue scale in the randomized two-period crossover between the Micro Fine Plus needle and NanoPass needles A or B were analyzed using the nonlinear mixed effects model. P < 0.05 was considered statistically significant.
Results
Study 1
First, a trial using NanoPass needle A was performed. Patients were divided into two groups. In the first group, NanoPass needle A was initially used for 1 week and then the Micro Fine Plus needle for 1 week, while in the second group, the Micro Fine Plus needle was initially used for 1 week and then NanoPass needle A for 1 week.
The mean age (53.3 ± 15.8 years) in the former group was significantly (P < 0.01) higher than the age (38.3 ± 11.0 years) in the latter group. However, there were no significant differences in the means of BMI (23.3 ± 3.3 vs 22.7 ± 2.9 kg/m2), diabetic duration with insulin injection (20 ± 10 vs 11 ± 8 years), and HbA1c (7.2 ± 1.3 vs 7.4 ± 1.1%) between the two groups. In the former group, all patients used rapid-acting and long-acting insulin, whereas in the latter group, 2 of the 20 patients used rapid-acting and intermediate-acting insulin and the remaining patients used rapid-acting and long-acting insulin. There were no significant differences in the dose (47 ± 25 vs 44 ± 17 U/day) and in the incidence of diabetic complications between the two groups.
Evaluations for between the two needles are shown in Table 1. Findings showed score values of item No. 1 of category A, item No. 7 of category C, and item No. 11 of category D in patients using NanoPass needle A were significantly increased than those in patients using the Micro Fine Plus needle.
Table 1.
Study 1: Each Score of Usabilties for NanoPass 33G Needle A Compared with Micro Fine Plus 31G Needle Evaluated by Visual Analogue Scale in 40 Patients with Insulin-Dependent Diabetes in a Single-Center, Open-Label and Randomized Two-Period Crossover Triala
| NanoPass (n = 20) | Micro Fine Plus (n = 20) | P value |
|---|---|---|
| Category A; pain and frightening judged from appearances of needle | ||
| 1. Did seeing the length of needle make you think it may be painful to insert? | ||
| 53.7 ± 41.0* | 43.7 ± 43.6 | 0.043 |
| 2. Did seeing the length of needle make you think it may be frightening to insert? | ||
| 53.1 ± 49.6 | 35.5 ± 53.1 | 0.071 |
| 3. Did seeing the thickness of needle make you think it may be painful to insert? | ||
| 55.1 ± 41.2 | 43.5 ± 44.1 | 0.129 |
| 4. Did seeing the thickness of needle make you think it may be frightening to insert? | ||
| 52.9 ± 46.4 | 41.2 ± 47.x | 0.103 |
| Category B; installing and removing of needle | ||
| 5. Is it easy installing the needle into a pen-type insulin syringe? | ||
| 73.2 ± 34.0 | 67.5 ± 39.1 | 0.202 |
| 6. Is it easy removing the needle from a pen-type insulin syringe? | ||
| 72.5 ± 34.8 | 65.8 ± 42.5 | 0.446 |
| Category C; pain and sticking judged from inserting the needle | ||
| 7. How often did you have pain when inserting the needle? | ||
| 41.6 ± 44.6* | 22.0 ± 47.3 | 0.030 |
| 8. How intense was the pain when inserting the needle? | ||
| 45.0 ± 43.2 | 27.5 ± 49.1 | 0.055 |
| 9. How do you feel sticking judged from inserting the needle? | ||
| 48.3 ± 42.7 | 36.3 ± 45.9 | 0.227 |
| Category D; bleeding and bruising | ||
| 10. How often did you bleed at the injection site? | ||
| 61.1 ± 33.5 | 41.8 ± 42.5 | 0.072 |
| 11. How often did you have bruising after injecting? | ||
| 68.8 ± 40.4 | 46.5 ± 49.6 | 0.025 |
| Category E; dribbling of injected insulin and power of pushing inserting button | ||
| 12. How many times do you have a dribbling of injected insulin from cutaneous tissue on average? | ||
| 61.1 ± 40.2 | 49.3 ± 46.3 | 0.471 |
| 13. How intense was the handling power of pushing inserting button? | ||
| 50.4 ± 55.0 | 55.9 ± 34.9 | 0.266 |
| Category F; satisfaction | ||
| 14. Overall satisfaction | ||
| 44.7 ± 46.4 | 40.9 ± 42.9 | 0.628 |
Numbers (n) in parenthedrodgises represent the number of participating patients. A higher score indicates a more favorable outcome compared with the alternative needle. *P < 0.05 was considered statistically significant for the favored needle (NanoPass 33G) vs the alternative needle (Micro Fine Plus 31G) compared using the nonlinear mixed effects model.
This indicates that NanoPass needle A was significantly less painful to insert judging from the appearance of the length of the needle, inserting the needle, and less bruising than the Micro Fine Plus needle. However, there was no significant difference in the overall patient satisfaction score including other items except the items just mentioned for use between the two needles.
Study 2
Second, because there was no significant difference in the overall patient satisfaction score between NanoPass needle A and the Micro Fine Plus needle, a trial using NanoPass needle B was performed. NanoPass needle B was coated by a different method of lubricant compared with NanoPass needle A. To examine the resistance level of needle distance when it was inserted into the skin, each needle from the tip into the cartridge was inserted into a 5-mm-thick silicone sheet at a velocity of 10 mm/second. The Micro Fine Plus needle could have been coated by the same ingredient of lubricant as the NanoPass needle. However, we do not have confirmed data. Despite this, the resistance value was also assessed by the same method using evaluation for the NanoPass needle. As shown in Figure 3, maximum peak resistance in all needles was recorded within 2.00 mm of displacement length, which appears to be the force to pierce the skin or be pressing against the skin before piercing (early phase). The resistance level in the Micro Fine Plus needle was greater than that in NanoPass needle A or B, whereas NanoPass needle A was identical with that in NanoPass needle B. The difference may be because of the different structure of needles as mentioned earlier, indicating that the NanoPass needle may have less insertion resistance compared with the Micro Fine Plus needle at the early phase when they were inserted into the skin. After that, the resistance level in all needles decreased gradually. From 2.00 to 4.00 mm of displacement length, which appears to be the time when the needles are moving through the skin, the resistance level in NanoPass needles A and B increased gradually (late phase). However, the increased level in NanoPass needle B was weaker than that in NanoPass needle A, although the resistance level in the Micro Fine Plus needle was almost identical with the level at 2.00 mm. This indicated that NanoPass needle B may have less insertion resistance compared with NanoPass needle A at late phase when they were inserted into the skin. The IAC in the Micro Fine Plus needle and NanoPass needles A and B were 14.54, 16.41, and 12.16 × 10−3 N · mm, respectively, indicating that NanoPass needle B is the lowest among the three needles. The finding also supports these views.
Figure 3.

Relationship between resistance value (unit of Newton; N) and displacement length (mm) of each needle insertion among three types of needles (Micro Fine Plus needle and NanoPass needles A and B). Evaluation was assessed on the resistance difference of each needle distance from the tip into the cartridge when the needle was pierced into a 5-mm-thick silicone sheet at the speed of 10 mm/second. Mechanical resistance levels were measured with the AUTOGRAPH AGS-H machine. The Micro Fine Plus needle and NanoPass needles A and B are shown as blue, yellow, and red lines, respectively. Maximum peak resistance was recorded within 2.00 mm of the displacement length, which appears to be the force necessary to pierce the skin or be pressing against the skin before piercing (early phase). The resistance level then decreased gradually, from 2.00 to 4.00 mm of the displacement length, which appears to be the time when needles are moving through the skin (late phase). Different resistance levels from maximum peak levels in all needles were observed. The IAC described by the resistance level from 0.00 to 4.00 mm of the displacement length in NanoPass needle B was lowest among the three needles.
Patients were divided into two groups the same as in study 1. The method was also the same as in study 1. Findings showed that the score values of items Nos. 3 and 4 of category A, items Nos. 7–9 of category C, items Nos. 10 and 11 of category D, item No. 12 of category E, and item No. 14 of category F in patients using NanoPass needle B were significantly increased than those in patients using the Micro Fine Plus needle. We did not compare scores in patients using the NanoPass needle as well as the Micro Fine Plus needle in study 1 and those in study 2 statistically because of the difficulty finding an appropriate statistical method for these comparisons.
This indicates that NanoPass needle B was significantly not only less painful to insert by judging from inserting the needle and less bruising, but also less painful to insert and less frightening to use based on the thick appearance of the needle and sticking of the needle, less bleeding, and less dribbling of injected insulin from the skin than the Micro Fine Plus needle. As a result, the overall patient satisfaction score using NanoPass needle B was significantly superior to that of the Micro Fine Plus needle, as shown in Table 2.
Table 2.
Study 2: Each Score of Usabilties of NanoPass 33G Needle B Compared with Micro Fine Plus 31G Needle Evaluated by Visual Analogue Scale in 40 Patients with Insulin-Dependent Diabetes in a Single-Center, Open-Label and Randomized Two-Period Crossover Triala
| NanoPass (n = 20) | Micro Fine Plus (n = 20) | P value |
|---|---|---|
| Category A; pain and frightening judged from appearances of needle | ||
| 1. Did seeing the length of needle make you think it may be painful to insert? | ||
| 52.1 ± 40.1 | 43.5 ± 43.6 | 0.059 |
| 2. Did seeing the length of needle make you think it may be frightening to insert? | ||
| 49.7 ± 45.2 | 42.0 ± 47.6 | 0.114 |
| 3. Did seeing the thickness of needle make you think it may be painful to insert? | ||
| 58.0 ± 38.8* | 45.6 ± 42.0 | 0.010 |
| 4. Did seeing the thickness of needle make you think it may be frightening to insert? | ||
| 58.6 ± 39.8* | 43.7 ± 45.6 | 0.006 |
| Category B; installing and removing of needle | ||
| 5. Is it easy installing the needle into a pen-type insulin syringe? | ||
| 61.0 ± 40.0 | 59.1 ± 36.8 | 0.920 |
| 6. Is it easy removing the needle from a pen-type insulin syringe? | ||
| 65.5 ± 37.0 | 57.7 ± 37.9 | 0.418 |
| Category C; pain and sticking judged from inserting the needle | ||
| 7. How often did you have pain when inserting the needle? | ||
| 71.8 ± 25.4* | 32.4 ± 40.9 | 0.001 |
| 8. How intense was the pain when inserting the needle? | ||
| 67.4 ± 45.0* | 32.7 ± 47.3 | 0.001 |
| 9. How do you feel sticking judged from inserting the needle? | ||
| 70.1 ± 32.9* | 36.7 ± 45.3 | 0.001 |
| Category D; bleeding and bruising | ||
| 10. How often did you bleed at the injection site? | ||
| 78.1 ± 20.7* | 42.0 ± 40.6 | 0.001 |
| 11. How often did you have bruising after injecting? | ||
| 81.7 ± 19.3* | 47.9 ± 43.3 | 0.001 |
| Category E; dribbling of injected insulin and power of pushing inserting button | ||
| 12. How many times do you have a dribbling of injected insulin from cutaneous tissue on average? | ||
| 81.2 ± 21.8* | 54.6 ± 40.6 | 0.001 |
| 13. How intense was the handling power of pushing inserting button? | ||
| 61.9 ± 35.5 | 54.0 ± 36.8 | 0.053 |
| Category F; satisfaction | ||
| 14. Overall satisfaction | ||
| 73.1 ± 29.0* | 37.5 ± 44.9 | 0.001 |
Numbers (n) in parentheses represent the number of participated patients. NanoPass 33G needle B changed the method of lubricant coating. A higher score indicates a more favorable outcome compared with the alternative needle. *P < 0.05 was considered statistically significant for the favored needle (NanoPass 33G) vs the alternative needle (Micro Fine Plus 31G) compared using the nonlinear mixed effects model.
Discussion
We evaluated the usability of the NanoPass needle in comparison with the Micro Fine Plus needle in insulin-dependent diabetic patients in a single-center, open-label and randomized two-period crossover trial using a questionnaire. Although the mean age in the former group was significantly higher than that in the latter group, there were no significant differences in the means of BMI, diabetic duration with insulin injection, HbA1c, and dose of insulin between the two groups. Further, there were no differences in the method for injections of insulin and in the incidence of diabetic complications between the two groups. In this condition, the NanoPass needle was significantly less painful to insert, supporting the previous finding by Asakura et al.5 who used a shorter term than in this study. Furthermore, this study showed that the NanoPass needle causes significantly less bruising than the Micro Fine Plus needle. This finding may not be explained by the difference of age of patients but by the tapered structure characteristic of the NanoPass needle, which has a thinner outer diameter than that of the Micro Fine Plus needle, while the inner diameter is same. In fact, the difference of resistance in each needle at early phase when the needle was pierced into a 5-mm-thick silicone sheet was demonstrated in this study. The study by Arendt-Nielsen et al.7 supports this idea, although Hanas et al.8 reported that thinner needles do not influence injection pain, insulin leakage, or bleeding in children and adolescents with type 1 diabetes.
However, there was no significant difference in overall patient satisfaction for use between the two needles. Osonoi9 reported that patients felt uncomfortable with insertions of a tapered needle because of the structure, such as like a wedge of needle. Furthermore, Nishimura et al.10 reported that the lubricity of needle has an effect on satisfaction of insulin injection. These views may explain the result obtained in this study. To resolve this problem, we reassessed usability of the NanoPass needle, which had less resistance of insertion, by changing the coating method of the lubricant, which was demonstrated by the resistance value in each needle at late phase when they were pierced into a 5-mm-thick silicone sheet. Surprisingly, the NanoPass needle was not only significantly less painful to insert by judging from inserting the needle and less bruising, but also less painful to insert and less frightening to use based on the appearance of needle thickness and sticking of needle, less bleeding, and less dribbling of injected insulin from the skin than the Micro Fine Plus needle. As a result, the overall patient satisfaction for using the NanoPass needle was significantly superior than that of the Micro Fine Plus needle. Concerning dribble from the tip of the needle after injection, Annersten and Frid11 reported that the difference in holding time of the needle influenced dribbling. However, in this study there was no difference in the holding time. Accordingly, less dribbling of injected insulin may be not explained by the holding time. Unfortunately, we did not compare evaluations for coated and uncoated NanoPass needles with the same criteria shown in Tables 1 and 2. The reason why we were not able to use an uncoated NanoPass needle in patients was because an uncoated needle cannot be inserted into the skin completely. All findings indicate that the difference may be explained by the resistance of needle insertion by lubricity due to the change in the coating method of lubricant as well as by the tapered structure of needles.
Conclusion
When patients commence and adhere to insulin therapy, devices should meet patient's requests, such as being painless, lubricated, or bleedless. Among these factors, being less painful is the most important in insulin therapy. However, in considering overall patient satisfaction when using insulin needles, developing a thinner needle and improving other factors such as lubricity are important.
Acknowledgement
This abstract was presented at the 6th Annual Meeting of the Japan Innovation Insulin Therapy, Niigata and October 28, 2006.
Abbreviations
- BMI
body mass index
- HbA1c
glycated hemoglobin
- IAC
integrated area under curves
References
- 1.Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999 Mar;3(1):41–49. doi: 10.1053/eujp.1998.0099. [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain perception of following controlled needle insertion, the impact of needle gauge. Diabetes. 2005;(54) suppl 1:A103–420. [Google Scholar]
- 3.Asakura T, Seino H. Basic study of micro-tapered needle (TN3305) using prefilled insulin. Jpn J Pham Health Care Sci. 2004;(30):368–376. [Google Scholar]
- 4.Schwartz S, Hassman D, Shelmet J, Sievers R, Weinstein R, Liang J, Lyness W. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004 Oct;26(10):1663–1678. doi: 10.1016/j.clinthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Asakura T, Seino H, Nunoi K, Hashimoto K, Mutou T, Yamazaki K, Kakutani M, Toraishi K, Kitaoka M, Daikoku H, Sugiyama K, Narasaki K, Tsuji K, Ohnishi S, Oto K, Tsujimoto T, Nakano R. Usability of a microtapered needle (TN3305) for insulin treatment in Japanese patients with diabetes mellitus: a comparative clinical study with a standard thin wall needle. Diabetes Technol Ther. 2006 Aug;8(4):489–494. doi: 10.1089/dia.2006.8.489. [DOI] [PubMed] [Google Scholar]
- 6.Iwanaga M, Kamoi K. Effect of Novo Fine® 32G 6mm and Micro Fine PIus® 31G 5mm needles on patient perceptions of pain and anxiety. Diabetes. 2006;55(Suppl 1):A458. doi: 10.1089/dia.2008.0027. [DOI] [PubMed] [Google Scholar]
- 7.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006 Mar–Jun;23(1–2):37–43. doi: 10.1080/08990220600700925. [DOI] [PubMed] [Google Scholar]
- 8.Hanas R, Lytzen L, Ludvigsson J. Thinner needles do not influence injection pain, insulin leakage or bleeding in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2000 Sep;1(3):142–149. doi: 10.1034/j.1399-5448.2000.010305.x. [DOI] [PubMed] [Google Scholar]
- 9.Osonoi T. Comparison of penetration pain between straight type and tapered type pen needle for insulin therapy. J Med Pharm Sci. 2006;55:381–386. HYPERLINK “ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi”. [Google Scholar]
- 10.Nishimura H, Eguchi A, Fukuda M, Jinnouchi H, Jinnouchi T. A comparison of 3 types of needle for insulin injection (31G 5mm, 32G 6mm, and 33G 5mm) with regard to frequency of pain due to needle insertion, insulin dripping and bleeding. J Nippon Hosp Pharm Assoc. 2006;(42):1601–1604. [Google Scholar]
- 11.Annersten M, Frid A. Insulin pens dribble from the tip of the needle after injection. Pract Diabetes Int. 2000;17(4):109–111. [Google Scholar]


