Abstract
Background
Glycohemoglobin A1c (HbA1c) is a universally accepted tool for glycemic control. Portable HbA1c devices for use in physicians' offices are desirable because they provide immediate results that physicians can share with their patients. This has been shown to enhance self-management in patients with diabetes. We undertook this study to evaluate the accuracy and precision of a recently introduced device, the A1cNow® InView™ capillary monitor.
Methods
Previously tested EDTA-preserved whole blood samples from our laboratory pool were preselected based on the results of HbA1c to cover a range from 4 to 13%. HbA1c was then measured using an A1cNow InView capillary monitor. Blinded aliquots of these samples were then sent to a National Glycohemoglobin Standardization Program (NGSP)-certified reference laboratory for comparison. One sample with a laboratory HbA1c result of 9.2% was measured with the InView device nine successive times to assess the device precision. The consistency between the measurement of HbA1c measured by the reference laboratory and the A1cNow InView device was analyzed via linear regression.
Results
Thirty-five samples were tested. The correlation between HbA1c measured by the InView device and the reference laboratory, as well as our own laboratory, was 0.96. The coefficient of variation was 2.71%.
Conclusions
Results of this study confirm the accuracy and precision of the InView capillary HbA1c monitor. However, the feasibility, reproducibility, and cost-effectiveness of this promising device in the real-life settings of physicians' offices must be verified by prospective clinical studies.
Keywords: A1c, A1cNow, diabetes, glycohemoglobin, point-of-care testing, portable A1c measurement
Introduction
About 21 million Americans (7.0 % of the population) are afflicted with diabetes mellitus at present,1 and this number has been escalating over recent years. The health care cost for diabetes is enormous: diabetes costs the nation nearly $132 billion a year.1,2 Poor glycemic control leads to micro- and macrovascular complications, including diabetic nephropathy, retinopathy, neuropathy, and cardiovascular disease. The demonstration that the development of microvascular complications in patients with diabetes can be slowed by treating hyperglycemia in patients with type 1 and type 2 diabetes3,4 has led to an increased use of intensive regimens to attain strict glycemic control in these patients.
The efficacy of the aforementioned regimens requires an accurate method to estimate the degree of control of diabetes, i.e., glucose monitoring. In patients with diabetes, glycohemoglobin A1c (HbA1c) is now well accepted as an index of control of hyperglycemia in the preceding 2 to 3 months. A strong correlation between mean blood glucose and HbA1c has been demonstrated previously.5,6
Several different methods are in use to measure HbA1c, each giving a different result. In the United States, the National Glycohemoglobin Standardization Program (NGSP) has led to most laboratories using NGSP-approved methods.7 This has alleviated previous concerns about a lack of standardization of HbA1c measurement.
Evidence8–11 suggests that regular measurement of HbA1c, along with the physician's immediate feedback to the patient, can lead to positive changes in diabetes treatment and improved metabolic control. Therefore, several diabetes clinical practices and diabetes centers have acquired such point-of-care instruments for on-site HbA1c measurement. Many of these methods require venipuncture, employ immunoassay or chromatography technology, and provide excellent specificity, but they either require a special instrument or require several days to obtain the final result.
It is notable that most of the diabetes care across the nation is currently provided in primary office care settings. In these settings, the rapid availability of HbA1c results, while the patient is in the office, results in appropriate therapeutic decisions and improved glycemic control.8–11 Recently introduced portable, user-friendly HbA1c devices for use in physicians' offices are desirable because they are easy for the clinical staff to use and provide immediate results that physicians can share with their patients.
The first generation of such devices was introduced in the 1990s.12,13 These innovations proved to be user-friendly, and for the first time finger stick, rather than venous sampling, was employed in HbA1c measurement, with results obtainable within 10 minutes. However, these methods still required an instrument, about the size of a 15-inch non-LCD computer monitor.
Another innovation in HbA1c measurement has been recently introduced into the market. This new method employs finger stick sampling, but instead of a large instrument, a small device, similar to an average glucose meter, is employed. A1cNow® (Metrika, Inc., Sunnyvale, CA), one of the first such devices introduced into the market, was shown to be accurate in postmarketing testing.14 The latest version of A1cNow monitors, the A1cNow InView™ capillary monitor (Metrika, Inc.), was approved by the Food and Drug Administration (FDA) in the fall of 2005. However, no published studies have tested the accuracy of this newest version for use in clinical settings since FDA clearance.
Study Objective
We undertook this study to evaluate the accuracy and precision of the A1cNow InView capillary monitor (Metrika, Inc.). The objective of the study was to measure HbA1c by InView and compare results obtained with our local laboratory and to further validate the results with an NGSP-certified reference laboratory.
Methods
The study was performed at the clinical laboratory of Sparrow Hospital (Lansing, MI), a teaching hospital affiliated with Michigan State University in East Lansing, Michigan. Thirty-five venous whole blood samples anticoagulated with EDTA were supplied by the Sparrow Hospital laboratory. Samples were selected randomly from the patients' pool and had been tested previously using the local laboratory's analyzer, utilizing high-performance liquid chromatography (Tosoh A1c 2.2, Tosoh Biosciences, Inc., South San Francisco, CA). Samples were selected to cover the linear range of InView (4 to 13%). Samples were brought to room temperature and were mixed thoroughly.
Glycohemoglobin A1c was then measured on these samples using A1cNow InView by a representative from Metrika (the manufacturer) under supervision of the laboratory director, according to manufacturer's product insert directions. A blinded aliquot of each sample was sent to the NGSP secondary reference laboratory in Columbia, Missouri, and samples were tested using the Tosoh 2.2 analyzer (Tosoh Bioscience, Inc.). One sample was selected to be used for a secondary precision study and was tested with the A1cNow InView device nine successive times.
The study was approved by the Institutional Review Boards of Michigan State University, East Lansing, and Sparrow Hospital, Lansing, Michigan.
Results
Correlation
One sample (#30) was not sent to the NGSP laboratory and so was not included in all data analysis (Table 1). One sample (#15) was repeated at the request of the Sparrow laboratory director and was labeled as sample #36; the reason was the significant difference of the measurement from the laboratory prereported value (4.8% vs 6.7%, respectively). Sample #36 was thus used in the data analysis instead of sample #15.
Table 1.
Table 1. HbA1c Measurements by Different Methods of Testing
| Sample ID randomly assigned | HbA1c (%) by A1cNow Inview | HbA1c (%) by Sparrow laboratory | HbA1c (%) by NGSP laboratory |
|---|---|---|---|
| 1 | 7.7 | 7.0 | 6.8 |
| 2 | 11.0 | 12.1 | 11.5 |
| 3 | 6.5 | 6.5 | 6.4 |
| 4 | 8.2 | 7.8 | 7.5 |
| 5 | 6.2 | 6.0 | 5.9 |
| 6 | 6.5 | 6.7 | 6.5 |
| 7 | 6.0 | 6.1 | 5.8 |
| 8 | 6.5 | 6.9 | 6.8 |
| 9 | 7.8 | 8.8 | 8.4 |
| 10 | 9.4 | 9.2 | 8.8 |
| 11 | 6.7 | 6.8 | 6.6 |
| 12 | 5.4 | 5.5 | 5.3 |
| 13 | 6.2 | 6.3 | 6.1 |
| 14 | 6.9 | 7.6 | 7.2 |
| 15a | 4.8 | 6.7 | 6.4 |
| 16 | 7.6 | 8.6 | 8.1 |
| 17 | 7.2 | 7.4 | 7.1 |
| 18 | 8.1 | 8.7 | 8.3 |
| 19 | 8.3 | 9.1 | 8.6 |
| 20 | 10.3 | 10.2 | 9.6 |
| 21 | 5.6 | 5.6 | 5.4 |
| 22 | 5.9 | 6.3 | 6.1 |
| 23 | 7.4 | 8.1 | 8.1 |
| 24 | 6.3 | 7.0 | 7.1 |
| 25 | 9.7 | 9.7 | 9.6 |
| 26 | 4.2 | 4.1 | 4.3 |
| 27 | 5.1 | 5.2 | 5.0 |
| 28 | 6.1 | 6.1 | 5.9 |
| 29 | 6.9 | 7.0 | 7.1 |
| 30b | 6.3 | 6.9 | – |
| 31 | 4.9 | 5.2 | 5.2 |
| 32 | 8.5 | 9.5 | 9.0 |
| 33 | 8.2 | 7.9 | 7.9 |
| 34 | 7.4 | 6.9 | 6.9 |
| 35 | 5.6 | 6.1 | 6.1 |
| 36a | 5.5 | 6.7 | 6.3 |
| Mean | 7.03 | 7.30 | 7.10 |
Sample 15 was repeated as 36, and 36 was used in analysis instead of 15.
Sample 30 was not sent to the NGSP laboratory and was not used in analysis involving NGSP laboratory results.
Least-squares linear regression including the slope, intercept, and correlation coefficient “r” were calculated for the various data sets. Mean bias and the 95% confidence interval (95% CI) were also calculated for the various data sets. These calculations are presented in Table 2. HbA1c results by A1cNow InView correlated well with HbA1c values reported by the reference NGSP laboratory (Figure 1) and the Sparrow Hospital laboratory (Figure 2); r = 0.96 for each. Figures 3 and 4 show bias plots for A1cNow InView against the NGSP and the Sparrow laboratories, respectively.
Table 2.
Table 2. Linear Regression and Bias Statistics of A1cNow InView
| Data set | n | 95% CI (%HbA1c) | Slope | Intercept | Correlation coefficient “r” | Mean bias (%HbA1c) |
|---|---|---|---|---|---|---|
| A1cNow vs NGSP | 34 | −0.90 to +0.81 | 0.97 | +0.16 | 0.96 | −0.04 |
| A1cNow vs Sparrow laboratory | 35 | −1.21 to +0.67 | 0.90 | +0.48 | 0.96 | −0.27 |
| Sparrow laboratory vs NGSP | 35 | −0.18 to +0.62 | 1.08 | −0.33 | 0.99 | +0.22 |
Figure 1.
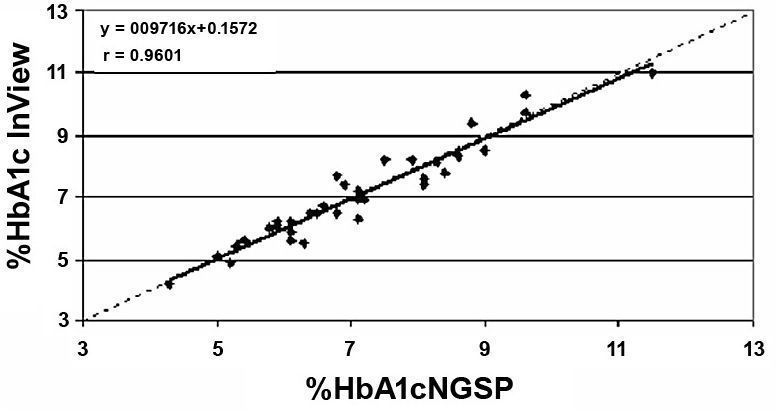
Figure 1. Correlation of HbA1c. A1cNow InView vs NGSP laboratory (n = 34).
Figure 2.
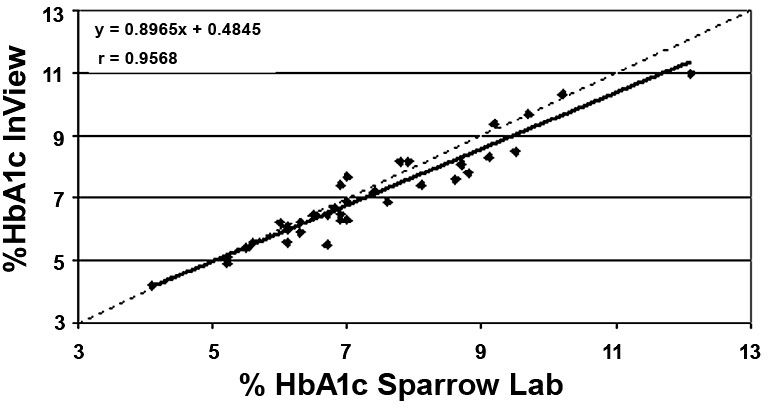
Figure 2. Correlation of HbA1c. A1cNow InView vs Sparrow Hospital laboratory.
Figure 3.
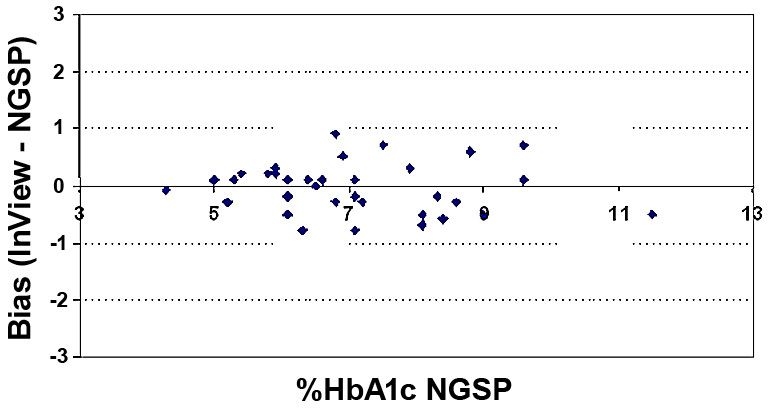
Figure 3. Bias of HbA1c. A1cNow InView vs NGSP laboratory (n = 34).
Figure 4.
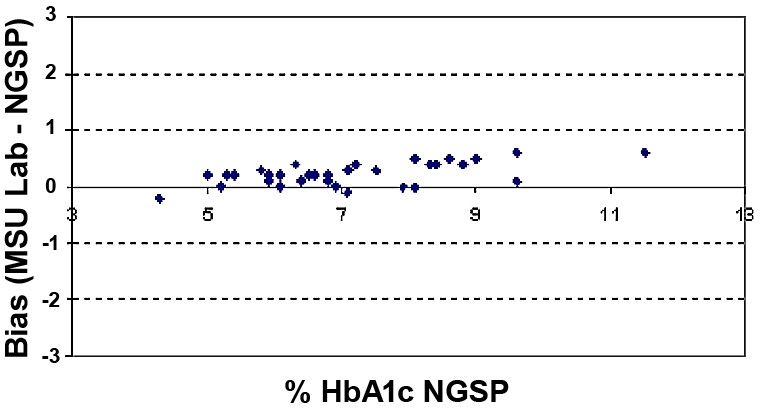
Figure 4. Bias of HbA1c. Sparrow Hospital vs NGSP laboratory (n = 34).
Precision
A secondary precision study was performed using a patient sample with a laboratory result of 9.2% HbA1c. The mean was 9.11% HbA1c, with a standard deviation (SD) of 0.25% HbA1c, resulting in a percent coefficient of variation (%CV) of 2.71% (Table 3).
Table 3.
Table 3. Precision Testing by A1cNow InView on Sample with HbA1c 9.2% (per Sparrow Hospital Laboratory)
| Replicate | Result |
|---|---|
| 1 | 9.4 |
| 2 | 9.2 |
| 3 | 9.2 |
| 4 | 8.8 |
| 5 | 8.7 |
| 6 | 8.9 |
| 7 | 9.2 |
| 8 | 9.3 |
| 9 | 9.3 |
| Mean | 9.11 |
| SD | 0.25 |
| %CV | 2.71 |
Discussion
On-site testing of HbA1c with immediate communications of results to patient is an efficient, faster, and much less expensive means of providing care to patients with diabetes mellitus. A1cNow InView is an instrument for HbA1c measurement that is simple to operate. The measurement involves a simple three-step procedure (remove from the wrapper, apply a diluted sample, and read results in 5 minutes) and requires only brief training (Figure 5). It provides quantitative results and does not require calibration, daily controls, or maintenance. It has recently received marketing clearance from the FDA. It is a small pager-sized device (Figure 6) and can be stored and used like a home blood glucose monitor. The blood sample can be obtained with a simple finger stick.
Figure 5.
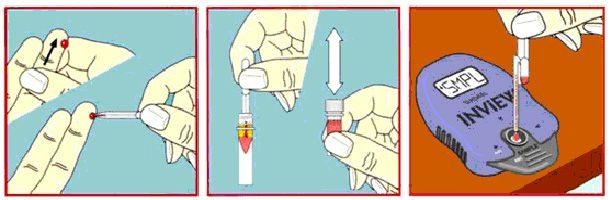
Figure 5. The simple three-step procedure of HbA1c testing by A1cNow InView monitoring device.
Figure 6.
Figure 6. A1cNow InView monitoring device developed by Metrika, Inc. (Sunnyvale, CA).
To be clinically useful, a reliable instrument to measure HbA1c should be accurate and precise. In general, accuracy is defined as how “true” a result is as compared to a gold standard test. Precision, or reproducibility, however, can be defined as how often a test can achieve the same result upon repetition.
In this study, which is the first postmarketing study of A1cNow InView, the overall performance of the device was comparable to those of the local and the reference laboratories. The slope and intercept values for A1cNow InView and the NGSP reference laboratory were 0.97 and +0.16, respectively, with a correlation coefficient (“r”) of 0.96. Average bias between the two data sets was -0.04% HbA1c. Similar results were noted when correlating with our local laboratory (Table 2).
Results indicate that HbA1c measured by A1cNow InView is accurate as shown by the correlation coefficient (“r”) of 0.96 with NGSP and our clinical laboratory, which also participates in NGSP. Mean biases among the three methods were also small, ranging from -0.27 to +0.22%. Results are reproducible, with the coefficient of variation (%CV) being quite low, 2.7%. A1cNow InView results were accurate and reproducible over the wide range of HbA1c (4.1 to 12.1%) that was observed in the study sample provided by our local laboratory.
One limitation of the study was that the assays were performed by a trained technician from the device manufacturer on previously collected whole blood samples. The procedure, though, was noted to be simple and it should be easy to train office staff to perform this test reliably. However, it remains to be established whether results obtained by office staff will be equally reliable. Similarly, it remains to be established whether capillary blood sampling, by finger stick, will compare reliably with whole blood testing. Theoretically, whole blood and capillary blood are expected to correlate reasonably well, as is the case with glucose testing.
It follows that studies are warranted to further test this device in actual clinical settings to validate its clinical usefulness. These studies should involve testing of capillary blood on actual patients at the point of care (i.e., in clinics), preferably by trained clinic personnel.
Conclusions
Results of this study confirmed the accuracy and reliability of the A1cNow InView monitor for HbA1c measurements. However, the feasibility, reproducibility, and effectiveness of the utilization of this promising device in real-life settings of physicians' offices have to be verified by prospective clinical studies.
Acknowledgments
This study was presented at the American Association of Clinical Endocrinologists (AACE) in the 2006 Poster Session of the Annual Meeting, Chicago, Illinois. We thank Jinie Shirey, Department of Medicine, Michigan State University College of Human Medicine, East Lansing, Michigan, for assistance with manuscript preparation.
The study materials were provided by the device manufacturer, Metrika, Inc. (Sunnyvale, CA), and samples were tested by a Metrika representative (Ms. Jill Lilton) in our local laboratory under direct supervision of the laboratory director (Dr. Anthony Koller, Technical Director of Clinical Chemistry, Sparrow Hospital Laboratory), who is a coinvestigator in the study and a coauthor of the manuscript.
The blinded aliquots were shipped to the reference laboratory by the Metrika representative. Data were collected and analyzed by Metrika personnel (Ms. Annette Clark) and provided to the investigators. The statistical calculations were then reviewed thoroughly and approved by one of the coinvestigators/coauthors (Dr. David Solomon).
Abbreviations
- CV
coefficient of variation
- HbA1c
glycohemoglobin A1c
- NGSP
National Glycohemoglobin Standardization Program
References
- 1.American Diabetes Association. Diabetes statistics. [accessed 2007 May 31]. Available from: http://www.diabetes.org/diabetes-statistics.jsp. [Google Scholar]
- 2.Hogan P, Dall T, Nikolov P, American Diabetes Association Economic costs of diabetes in the US in 2002. Diabetes Care. 2003 Mar;26(3):917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 3.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993 Jul 29;329(5):304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care. 1987 Jan–Feb;10(1):1–19. doi: 10.2337/diacare.10.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002 Feb;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 7.Little RR. Glycated hemoglobin standardization – National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003 Sep;41(9):1191–1198. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- 8.Thaler LM, Dunbar VG, Ziemer DC, Phillips LS, Gallina DL, El-Kebbi IM, Cook CB. Diabetes in urban African-Americans. XVII. Availability of rapid HbA1c measurements enhances clinical decision making. Diabetes Care. 1999 Sep;22(9):1415–1421. doi: 10.2337/diacare.22.9.1415. [DOI] [PubMed] [Google Scholar]
- 9.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin treated type 2 diabetic patients. Diabetes Care. 1999 Nov;22(11):1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 10.Ferenczi A, Reddy K, Lorber DL. Effect of immediate hemoglobin A1c results on treatment decisions in office practice. Endocr Pract. 2001 Mar–Apr;7(2):85–88. doi: 10.4158/EP.7.2.85. [DOI] [PubMed] [Google Scholar]
- 11.Miller CD, Barnes CS, Phillips LS, Ziemer DC, Gallina DL, Cook CB, Maryman SD, El-Kebbi IM. Rapid A1c availability improves decision making in an urban primary care clinic. Diabetes Care. 2003 Apr;26(4):1158–1163. doi: 10.2337/diacare.26.4.1158. [DOI] [PubMed] [Google Scholar]
- 12.Marrero D, Vandagriff J, Gibson R, Fineberg S, Fineberg N, Hiar C, Crowley L. Immediate HbA1c results. Performance of new HbA1c system in pediatric outpatient population. Diabetes Care. 1992 Aug;15(8):1045–1049. doi: 10.2337/diacare.15.8.1045. [DOI] [PubMed] [Google Scholar]
- 13.Guerci B, Durain D, Leblanc H, Rouland JC, Passa P, Godeau T, Charbonnel B, Mathieu Daude JC, Boniface H, Monnier L, Dauchy F, Slama G, Drouin P. Multicenter evaluation of the DCA 2000 system for measuring glycated hemoglobin. DCA Study Group. Diabetes Metab. 1997 Jun;23(3):195–201. [PubMed] [Google Scholar]
- 14.Sicard DA, Taylor JR. Comparison of point of care HbA1c test versus standardized laboratory testing. Ann Pharmacother. 2005 Jun;39(6):1024–1028. doi: 10.1345/aph.1E504. [DOI] [PubMed] [Google Scholar]


