Abstract
Background
The incidence of type 2 diabetes is increasing disproportionately in individuals <65 years of age. It is not known whether disease characteristics in these younger patients are similar to “classic” late-onset type 2 diabetes.
Methods
In the epidemiological cohort study entitled “Retrolective Study: Self-Monitoring of Blood Glucose and Outcome in Patients with Type 2 Diabetes,” a total of 3268 patients from randomly contacted primary care practices were documented during a mean follow-up period of 6.5 years. All newly diagnosed patients of these practices were included.
Results
At diagnosis, 64.2% of the patients were aged ≤65 years. Thereof, 57.2% were male, whereas in the age group >65 years only 35.0% were male (p < 0.001). The younger group exhibited more severe metabolic deterioration at diagnosis and in the following years than the older group. Conversely, the older group presented at diagnosis with a higher prevalence of cardiovascular risk factors. Self-monitoring of blood glucose (SMBG) was more prominent in the younger group. In both age groups, the use of SMBG was associated with a significantly lower risk (p = 0.003) of a combined end point (severe diabetic complication or all-cause mortality).
Conclusions
There are considerable differences in disease characteristics between people diagnosed with type 2 diabetes during 45–65 years of age versus diagnosis at a later age. Type 2 diabetes diagnosed before the age of 65 years disproportionately affected men and exhibited a more severe disease course, but was characterized by significantly less cardiovascular risk factors in comparison to type 2 diabetes diagnosed at a later age. The use of SMBG was associated with a better clinical outcome in both age groups.
Keywords: diabetes therapy, diabetic complications, epidemiology, mortality, self-monitoring of blood glucose, type 2 diabetes
Introduction
Diabetes mellitus is a worldwide, pandemic disease. According to the last estimations of the International Diabetes Federation, 246 million people will have diabetes worldwide in 2007. The age group from 40 to 59 years has the largest number of persons suffering from diabetes with some 113 million; in the age group from 60 to 79 years, 97 million people have diabetes. By 2025, because of the aging of the world's population, there will be 166 million with diabetes aged from 40 to 59 years and almost as many aged from 60 to 79 years, approximately 164 million. This corresponds to an increase of 47% in the age group from 40 to 59 years and to an increase of 69% in the age group from 60 to 79 years.1
The incidence of type 2 diabetes is increasing preferentially in younger persons under 65 years.2 The earlier onset of type 2 diabetes appears to be associated with the increased prevalence of obesity at a young age, even in children and adolescents.3–5
Literature published so far focuses mainly on gender differences for several diabetes-related complications or diabetes management. There is a stronger effect of type 2 diabetes on the risk of coronary heart disease in women than in men,6 women suffering from diabetes have a higher morbidity rate than men,7 and elderly male patients with diabetes have a more favorable risk factor control than corresponding female patients.8,9 Albeit the age group from 45 to 65 years represents one of the largest fractions of the entire population afflicted by diabetes,1 it is not known whether disease characteristics in these younger patients are similar to the “classic” late-onset, senior age-related type 2 diabetes.
Materials and Methods
Study Design
This analysis included 3268 patients with type 2 diabetes from a retrospective, German multicenter cohort study entitled “Retrolective Study: Self-Monitoring of Blood Glucose and Outcome in Patients with Type 2 Diabetes” (ROSSO). Data were derived from patient medical records of randomly contacted primary care physicians. The mean follow-up period was 6.5 years. Further details of the underlying database are described elsewhere.10 The analysis considered two age groups (younger group: age at diagnosis between 45 and 65 years; older group: age at diagnosis over 65 years) in relation to the outcomes as documented in the patient medical records. Nonfatal end points (morbidity) were defined as myocardial infarction, stroke, foot amputation, blindness (one or both eyes), or end-stage renal failure requiring hemodialysis. The fatal end point was defined as all-cause mortality. These definitions are based on the occurrence of the event during the observation period. The course of clinical and biochemical parameters [body mass index (BMI), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), systolic and diastolic blood pressure, lipid levels, serum creatinine, uric acid], as well as diseases documented prior to diagnosis of type 2 diabetes (hypertension, coronary heart diseases, heart insufficiency, peripheral arterial occlusion, myocardial infarction, stroke, and antihypertensive drugs), was retrieved from patient medical records.
Statistical Methods
Statistical analysis was performed using software SPSS 14.0 (SPSS, Chicago, IL). Comparison of groups of categorical variables was performed by χ2 statistics, comparison of binary variables with Fisher's exact test, and comparison of quantitative variables with the t test. All tests were performed two sided at a test level of <0.05. Continuous variables were presented as means ± standard deviation. Categorical variables were described as counts or relative frequency (%). Decremental life tables were drawn with regard to the time from diagnosis of diabetes to occurrence of a nonfatal or fatal end point as main target variable. Wilcoxon test analysis was used to test the difference in overall survival curves between the age groups. Cox proportional hazards modeling was performed to determine the impact of SMBG on events such as nonfatal and fatal end points, considering age as an influencing factor and adjusting for gender, FBG, hypertension, coronary heart diseases, heart insufficiency, peripheral arterial occlusion, myocardial infarction, stroke, antihypertensive drugs, and anamnesis.
Results
Age Distribution
At diagnosis of type 2 diabetes, 2099 patients (64.2%) of the total cohort were aged between 45 and 65 years (younger group). The mean age was 56.6 years compared to the mean age of 72.9 years for patients diagnosed after the age of 65 years (older group). The younger group was predominantly male (57.2% males) in contrast to the older group (35.0% males) (Table 1). The percentage of males in the German population in the year 2000 was 49.9% (age between 45 and 65 years) and 38.6% (age over 65 years).11 Differences between the study population and the general population are significant both for the younger group (p < 0.001) and for the older group (p = 0.012).
Table 1.
Demographic, Metabolic, and Cardiovascular Characteristics of Patients Aged 65 Years or Less/over 65 Years at Diagnosis of Type 2 DiabetesCharacteristic
| Baseline | ||||
|---|---|---|---|---|
| Characteristic | All patientsa | ≤65 yearsa | <65 yearsa | P value |
| 3268 (100%) | 2099 (64.2%) | 1169 (35.8%) | ||
| Malesa | 1609 (49.2%) | 1200 (57.2%) | 409 (35.0%) | <0.001 |
| (n = 3268) | (n = 2099) | (n = 1169) | ||
| Ageb (years) | 62.4 ± 9.6 | 56.6 ± 5.6 | 72.9 ± 5.5 | <0.001 |
| (n = 3268) | (n = 2099) | (n = 1169) | ||
| BMIb (kg/m2) | 29.8 ± 5.1 | 30.5 ± 5.1 | 28.5 ± 4.7 | <0.001 |
| (n = 2460) | (n = 1602) | (n = 858) | ||
| FBGb (mmol/liter) | 9.2 ± 3.7 | 9.3 ± 3.7 | 9.0 ± 3.7 | 0.016 |
| (n = 2868) | (n = 1838) | (n = 1030) | ||
| HbA1cb (adjusted)c (%) | 7.7 ± 2.1 | 7.8 ± 2.2 | 7.5 ± 1.9 | 0.003 |
| (n = 1486) | (n = 971) | (n = 515) | ||
| Systolic blood pressureb (mm Hg) | 149.2 ± 20.3 | 147.8 ± 20.3 | 151.6 ± 20.1 | <0.001 |
| (n = 2696) | (n = 1707) | (n = 989) | ||
| Diastolic blood pressureb (mm Hg) | 87.0 ± 10.9 | 88.0 ± 11.0 | 85.2 ± 10.4 | <0.001 |
| (n = 2697) | (n = 1708) | (n = 989) | ||
| Total cholesterolb (mmol/liter) | 6.1 ± 1.3 | 6.2 ± 1.3 | 6.1 ± 1.2 | 0.39 |
| (n = 2352) | (n = 1536) | (n = 816) | ||
| Low-density lipoprotein cholesterolb (mmol/liter) | 3.9 ± 1.1 | 3.9 ± 1.1 | 3.9 ± 1.1 | 0.40 |
| (n = 783) | (n = 523) | (n = 260) | ||
| High-density lipoprotein cholesterolb (mmol/liter) | 1.2 ± 0.6 | 1.2 ± 0.6 | 1.3 ± 0.7 | 0.001 |
| (n = 986) | (n = 663) | (n = 323) | ||
| Triglyceridesb (mmol/liter) | 2.6 ± 2.1 | 2.7 ± 2.4 | 2.3 ± 1.4 | <0.001 |
| (n = 1906) | (n = 1244) | (n = 662) | ||
| Serum creatinineb (mg/dl) | 84.2 ± 20.1 | 82.7 ± 19.9 | 86.8 ± 20.1 | <0.001 |
| (n = 2261) | (n = 1448) | (n = 813) | ||
| Uric acidb (mg/dl) | 5.8 ± 1.6 | 5.8 ± 1.6 | 6.0 ± 1.6 | 0.018 |
| (n = 2057) | (n = 1328) | (n = 729) | ||
| Hypertensiona | 2148 (65.7%) | 1302 (62.0%) | 846 (72.4%) | <0.001 |
| (n = 3268) | (n = 2099) | (n = 1169) | ||
| Coronary heart diseasesa | 733 (22.9%) | 320 (15.6%) | 413 (36.2%) | <0.001 |
| (n = 3194) | (n = 2052) | (n = 1142) | ||
| Heart insufficiencya | 501 (15.7%) | 174 (8.5%) | 327 (28.7%) | <0.001 |
| (n = 3188) | (n = 2049) | (n = 1139) | ||
| Peripheral arterial occlusiona | 189 (5.9%) | 96 (4.7%) | 93 (8.2%) | <0.001 |
| (n = 3192) | (n = 2053) | (n = 1139) | ||
| Myocardial infarctiona | 130 (4.1%) | 65 (3.2%) | 65 (5.7%) | <0.001 |
| (n = 3184) | (n = 2052) | (n = 1132) | ||
| Strokea | 102 (3.2%) | 44 (2.1%) | 58 (5.1%) | <0.001 |
| (n = 3192) | (n = 2054) | (n = 1138) | ||
| Antihypertensive drugsa | 1606 (49.1%) | 945 (45.0%) | 661 (56.5%) | <0.001 |
| (n = 3268) | (n = 2099) | (n = 1169) | ||
Data are valid n (%).
Data are means ± SD.
HbA1c was adjusted to 6.1% as the upper limit of normal range using the following formula: (HbA1c / upper limit of normal range of local laboratory) × 6.1.
Baseline Characteristics
Major age-dependent differences in disease characteristics at diagnosis were found. The younger group exhibited more severe metabolic abnormalities than the older group. They had higher mean values of BMI and mean concentrations of FBG and HbA1c (Table 1).
In contrast, the older group exhibited a higher prevalence of cardiovascular abnormalities at diagnosis than the younger group. This is evident from a higher frequency of hypertension, coronary heart diseases, heart insufficiency, peripheral arterial occlusion, myocardial infarction, stroke, and prescription of antihypertensive drugs (p < 0.001, each). More details about baseline characteristics are summarized in Table 1.
Patients Grouped by Sex
Because of the age-dependent difference in distribution between the sexes, all analyses were repeated for males and females separately.
Not all markers of diabetes quality were significantly worse in both younger male and female patients because of the lower number of patients per sex group, but the difference persisted as the trend. Differences remained significant for BMI and also for FBG in males. The age-dependent difference in the prevalence of cardiovascular risk factors remained significant in both sexes analyzed separately (data not shown).
Follow-up Characteristics
The difference in parameters of diabetes severity (BMI, FBG, HbA1c) persisted during the observation period (Figures 1A–1C). Also, the mean values of parameters of diabetes severity for the total observation period were significantly elevated in the younger group (Figure 1D).
Figure 1.
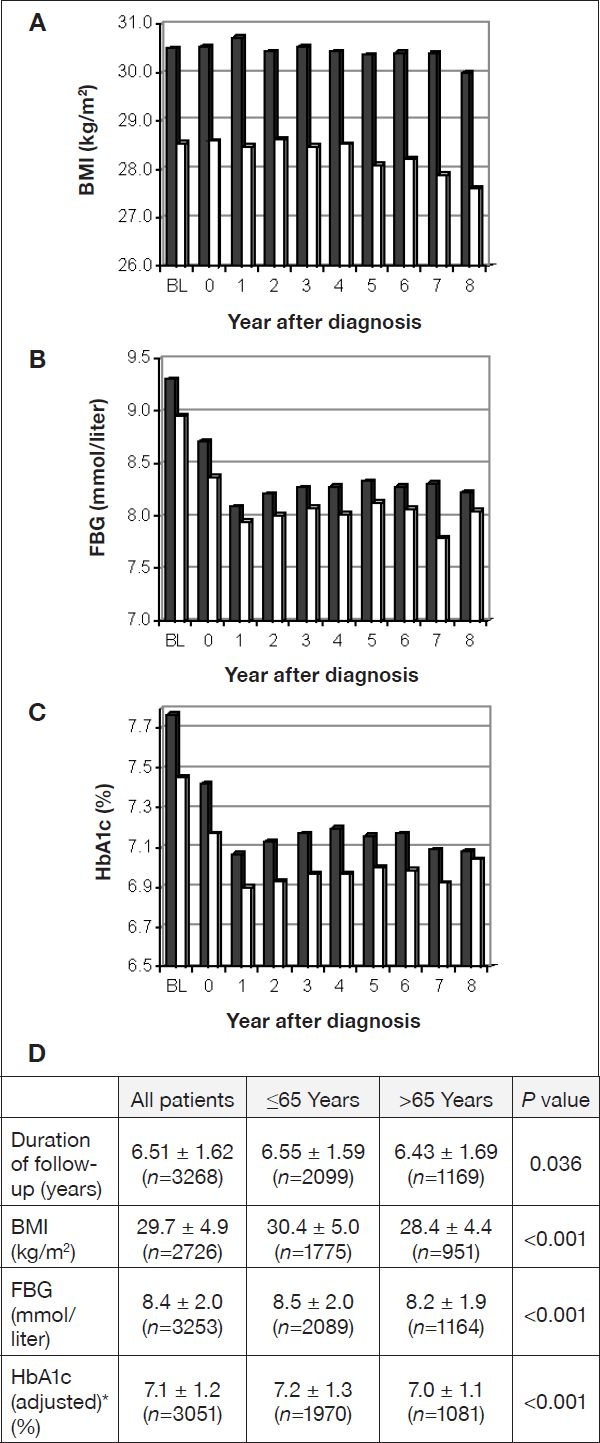
Follow-up values per year after diagnosis for BMI (A), FBG (B), and HbA1c (C). Black bars, age group ≤65 years; white bars, age group >65 years; BL, baseline. (D) Mean values ± SD for the duration of follow-up and for BMI, FBG, and HbA1c averaged over follow-up. *HbA1c was adjusted as in Table 1.
Pharmacotherapy
In both age groups, oral antidiabetic drugs (OADs) were the most commonly used treatment (about 60%), followed by the combination of insulin plus OADs and insulin alone (Table 3). The younger group received metformin (62.1% vs 44.8%, p < 0.001) and α-glucosidase inhibitors (19.5% vs 16.6%, p = 0.039) more often. In accordance with a milder form of diabetes-related metabolic dysfunction, the older cohort was treated more often with diet only, i.e., no medication. The mean duration from diagnosis until pharmacological therapy was similar in both age groups. However, thrombocyte aggregation inhibitors and antihypertensive drugs were prescribed more often in the older group, while the opposite was found for lipid-lowering drugs (Table 2).
Table 3.
Major Angiopathic Risk Factors in Relationship to Use of SMBGCharacteristic
| Characteristic | Age | |||||
|---|---|---|---|---|---|---|
| ≤ 65 years (n = 2099) | >65 years (n = 1169) | |||||
| SMBG | No SMBG | P value | SMBG | No SMBG | P value | |
| 50.4% (n = 1058) | 49.6% (n = 1041) | 36.0% (n = 421) | 64.0% (n = 748) | |||
| Sociodemographic | ||||||
| Mean agea | 55.9 ± 5.7 (n = 1058) | 57.3 ± 5.4 (n = 1041) | <0.001 | 71.9 ± 5.0 (n = 421) | 73.4 ± 5.7 (n = 748) | <0.001 |
| Femaleb | 434 (41.0%) | 465 (44.7%) | 0.094 | 267 (63.4%) | 493 (65.9%) | 0.41 |
| Smokerb | 209 (19.8%) | 195 (18.7%) | 0.58 | 40 (9.5%) | 53 (7.1%) | 0.15 |
| BMIa (kg/m2) | 30.4 ± 5.0 (n = 817) | 30.7 ± 5.2 (n = 785) | 0.26 | 28.6 ± 4.5 (n = 307) | 28.5 ± 4.8 (n = 551) | 0.83 |
| History of cardiovascular risk factors | ||||||
| Hypertensionb | 619 (58.5%) (n = 1058) | 683 (65.6%) (n = 1041) | 0.001 | 285 (67.7%) (n = 421) | 561 (75.0%) (n = 748) | 0.008 |
| Coronary heart diseasesb | 156 (15.1%) (n = 1034) | 164 (16.1%) (n = 1018) | 0.54 | 149 (36.3%) (n = 410) | 264 (36.1%) (n = 732) | 0.95 |
| Heart insufficiencyb | 81 (7.8%) (n = 1036) | 93 (9.2%) (n = 1013) | 0.30 | 125 (30.7%) (n = 407) | 202 (27.6%) (n = 732) | 0.28 |
| Peripheral arterial occlusionb | 52 (5.0%) (n = 1035) | 44 (4.3%) (n = 1018) | 0.47 | 38 (9.3%) (n = 407) | 55 (7.5%) (n = 732) | 0.31 |
| Myocardial infarctionb | 33 (3.2%) (n = 1036) | 32 (3.1%) (n = 1016) | 1.00 | 27 (6.7%) (n = 406) | 38 (5.2%) (n = 726) | 0.35 |
| Strokeb | 27 (2.6%) (n = 1035) | 17 (1.7%) (n = 1019) | 0.17 | 17 (4.2%) (n = 407) | 41 (5.6%) (n = 731) | 0.33 |
| Antihypertensive drugsb | 409 (38.7%) (n = 1058) | 536 (51.5%) (n = 1041) | <0.001 | 209 (49.6%) (n = 421) | 452 (60.4%) (n = 748) | <0.001 |
Data are means ± SD.
Data are valid n (%).
Table 2.
Pharmacotherapy of Patients Grouped by Age at Diagnosis of Type 2 DiabetesCharacteristic
| Characteristic | Frequency valid n (%) | Interval to start of treatment following diagnosisa (years) | ||||
|---|---|---|---|---|---|---|
| ≤65 Years (n = 2099) | > 65 Years (n = 1169) | P value | ≤ 65 years | >65 years | P value | |
| Antidiabetic treatmentb | ||||||
| No medication | 316 (15.1%) | 247 (21.1%) | <0.001 | |||
| Insulin alone | 64 (3.0%) | 38 (3.3%) | ||||
| Insulin and OAD | 486 (23.2%) | 226 (19.3%) | ||||
| OAD alone | 1233 (58.7%) | 658 (56.3%) | ||||
| Thereof | ||||||
| α-Glucosidase inhibitors | 410 (19.5%) | 194 (16.6%) | 0.039 | 1.68 ± 1.99 (n = 410) | 1.52 ± 1.95 (n = 194) | 0.36 |
| Metformin | 1303 (62.1%) | 524 (44.8%) | <0.001 | 2.27 ± 2.20 (n = 1303) | 2.16 ± 2.15 (n = 524) | 0.34 |
| Sulfonylurea | 1200 (57.2%) | 672 (57.5%) | 0.88 | 1.68 ± 2.11 (n = 1200) | 1.63 ± 2.04 (n = 672) | 0.57 |
| Other OAD | 332 (15.8%) | 95 (8.1%) | <0.001 | 3.99 ± 2.04 (n = 332) | 4.05 ± 2.00 (n = 95) | 0.79 |
| Insulins | 550 (26.2%) | 264 (22.6%) | 0.023 | 3.29 ± 2.44 (n = 550) | 3.07 ± 2.43 (n = 264) | 0.22 |
| Additional therapy | ||||||
| Thrombocyte aggregation inhibitors | 220 (10.5%) | 200 (17.1%) | <0.001 | 2.62 ± 2.45 (n = 220) | 2.76 ± 2.48 (n = 200) | 0.57 |
| Lipid-lowering drugsc | 763 (36.4%) | 331 (28.3%) | <0.001 | 2.11 ± 2.27 (n = 763) | 2.06 ± 2.30 (n = 331) | 0.71 |
| Antihypertensive drugsd | 1616 (77.0%) | 1033 (88.4%) | <0.001 | 1.07 ± 1.89 (n = 1591) | 0.75 ± 1.56 (n = 1019) | <0.001 |
Data are means ± SD.
Patients were allocated to a given treatment group if the treatment was documented at least in 1 year.
Lipid-lowering drugs comprised fibrates, statins, and rarely other compounds.
Antihypertensive drugs comprised diuretics, β blocker, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor, and Ca antagonists.
Angiopathic Risk Factors and SMBG
The proportion of patients who performed SMBG during the observation period was higher in the younger group than in the older group (50.4% vs 36.0%, p < 0.001; Table 3). In the younger group, patients not performing SMBG were afflicted more often by myocardial infarction (3.7% vs 1.6%, p = 0.003), by other nonfatal events (8.5% vs 5.5%, p = 0.006), and with a nonfatal or fatal event combined (10.2% vs 7.2%, p = 0.016) than those who performed SMBG. Additionally, a higher proportion of fatal events in patients who did not perform SMBG (8.0% vs 5.0%, p = 0.055) than those who performed SMBG has been observed (Table 3).
Survival and SMBG
In both the younger group and the older group (p = 0.021), the proportion of patients without a nonfatal end point during the observation period was larger for patients performing SMBG than for patients not performing SMBG (Figures 2A and 2B). The proportion of patients surviving during the observation period was significantly larger for patients performing SMBG than for patients not performing SMBG in the older group, but not in the younger group (p = 0.001) (Figures 2C and 2D). The proportion of patients without a combined end point (i.e., fatal or nonfatal end point) during the observation period was larger for patients performing SMBG than for patients not performing SMBG in both the younger group and the older group (p = 0.003)(Figures 2Eand 2F).
Figure 2.
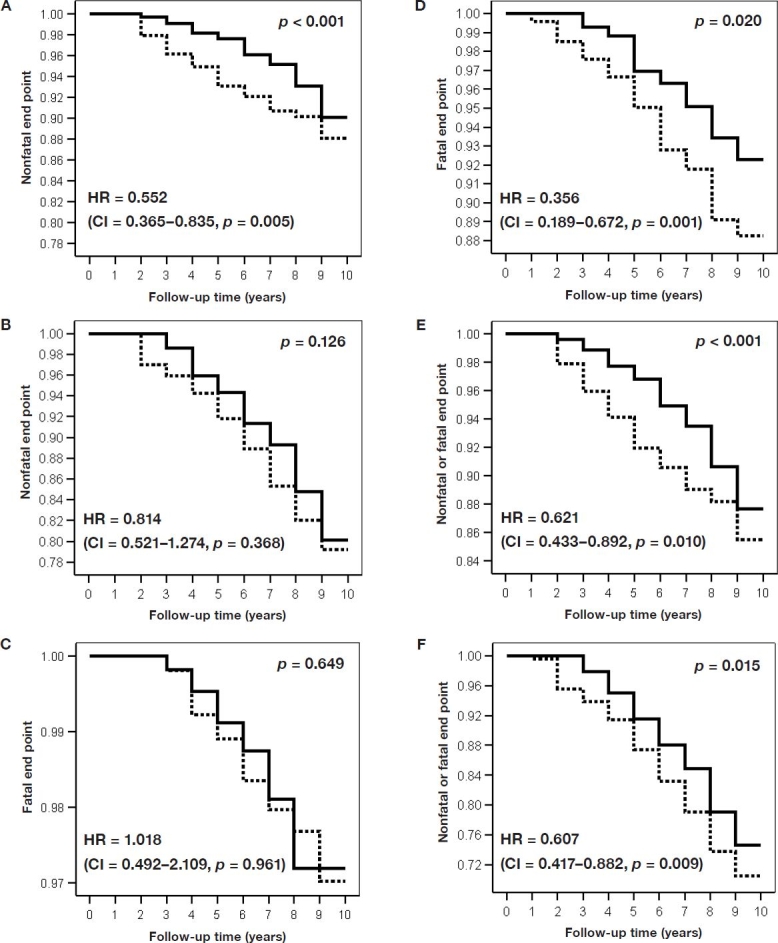
Decremental life tables for the age group ≤65 years (A, C, E) and for the age group >65 years (B, D, F). The y axis indicates the proportion of patients without a nonfatal end point (A, B), without a fatal end point (C, D), or without a fatal or a nonfatal end point (E, F). Subdivision of the follow-up time is 1 year after diagnosis. Solid line, patients performing SMBG; dotted line, patients not performing SMBG. The indicated Wilcoxon p values relate to the difference between patients performing SMBG and patients not performing SMBG. HR indicates the adjusted hazard ratios (as described in the text) calculated with Cox proportional hazards modeling (CI = 95% confidence interval of HR, p = test significance).
Discussion
Our study was the first one to analyze the following aspects of type 2 diabetes mellitus: (1) the dependence of disease characteristics on age and (2) the association with cardiovascular diseases in longitudinal cohorts of different age.
Our results suggest age-dependent differences in the pathogenesis of type 2 diabetes. Early onset of type 2 diabetes (i.e., diagnosis before the age of 65 years) is related to a more severe form of metabolic dysfunction. The mean values of BMI and mean concentrations of FBG and HbA1c are higher at baseline and during the mean follow-up time of 6.5 years. Men are afflicted more often by this early form of diabetes.
The more prominent use of metformin in the younger group can be attributed to the higher BMI in this age group. Conversely, the milder form of diabetes in the older group is reflected in the higher percentage treated with diet only during the observation period of 6.5 years from diagnosis.
Patients aged over 65 years at diagnosis of type 2 diabetes experience a milder form of diabetes. This may be partly because of the increased diagnostic vigilance of doctors when treating older patients. However, earlier diagnosis of the diabetic state should not account for the lower rate of male patients in this age group.
Interestingly, there is no obvious parallel in pathogenesis-related processes leading to type 2 diabetes and cardiovascular disease: The younger group with the more severe form of diabetes metabolic dysfunction does not exhibit more severe cardiovascular risk factors simultaneously. The prevalence of cardiovascular disease markers rather appears to increase with age. Therefore, cardiovascular diseases are advanced in the higher age group.
The benefits of SMBG in type 2 diabetes are still under discussion.12,13 However, a growing body of evidence—resulting from several meta-analyses—suggests that SMBG has a positive effect on metabolic control, independent of the type of antidiabetic treatment (oral antidiabetic drugs only or in combination with insulin).15–17 The positive effects of SMBG are supported by several observational studies,10,14,15 although some contradictory results have also been published.16,17 We find that performing SMBG showed a significant impact on the clinical outcomes in both age groups, although patients performing SMBG and patients not performing SMBG had comparable cardiovascular antecedents.
Self-monitoring of blood glucose was related to a significant reduction of nonfatal events in patients aged less than 65 years. However, SMBG had no significant impact on the already low overall mortality in these patients. In patients aged over 65 years the main effect of SMBG was a significant reduction of mortality, but no reduction of the number of nonfatal events. A possible interpretation is that patients with preexisting cardiovascular diseases had a better chance of surviving a cardiovascular event when they performed SMBG.
We should keep in mind that SMBG is a diagnostic procedure, which, by definition, is not an intervention such as a pharmaceutical treatment. Effects on disease course may only result because of actions taken as a consequence of SMBG results. For instance, diabetes patients must develop the ability to interpret correctly the output of the SMBG device and to translate this interpretation into an appropriate and sustained action(s). On that condition they can benefit from SMBG. Possible benefits for patients treated with oral antidiabetic drugs include a change in their oral therapy or the addition of short-acting insulin.18 Even more important than the enhanced pharmaceutical treatment may be the increased attentiveness of the treating physician. It is well known that depression is a major issue in older patients with type 2 diabetes and is related to higher mortality rates.19,20 Therefore, increased attentiveness of the treating physician could play a major role in this context.
Overall, our results demonstrate that disease characteristics of type 2 diabetes differ clearly between patients aged under or over 65 years. We think it is important to consider these age-related differences in the treatment of type 2 diabetes. Additionally, our results suggest that performing SMBG is associated with better clinical outcomes in both age groups.
Acknowledgements
This study was supported by the Ministry of Science and Research of the State North Rhine-Westphalia, Düsseldorf, the Federal Ministry of Health, Bonn, Germany, and an unrestricted grant from Roche Diagnostics, Mannheim, Germany.
Abbreviations
- BMI
body mass inde
- FBG
fasting blood glucose
- HbA1c
hemoglobin A1c
- OAD
oral antidiabetic drugs
- ROSSO
Retrolective Study: Self-Monitoring of Blood Glucose and Outcome in Patients with Type 2 Diabetes
- SMBG
self-monitoring of blood glucose
References
- 1.International Diabetes Foundation. Prevalence and projections. In: Delice G, editor. Diabetes atlas. 3rd ed. 2006. pp. 16–103. Brussels. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Bloomgarden ZT. Type 2 diabetes in the young: the evolving epidemic. Diabetes Care. 2004;27:998–1010. doi: 10.2337/diacare.27.4.998. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 6.Sarafidis PA, McFarlane SI, Bakris GL. Gender disparity in outcomes of care and management for diabetes and the metabolic syndrome. Curr Diab Rep. 2006;6:219–224. doi: 10.1007/s11892-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 7.Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27:2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson PM, Theobald H, Journath G, Fritz T. Gender differences in risk factor control and treatment profile in diabetes: a study in 229 Swedish primary health care centres. Scand J Prim Health Care. 2004;22:27–31. doi: 10.1080/02813430310003264. [DOI] [PubMed] [Google Scholar]
- 9.Shalev V, Chodick G, Heymann AD, Kokia E. Gender differences in healthcare utilization and medical indicators among patients with diabetes. Public Health. 2005;119:45–49. doi: 10.1016/j.puhe.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum W. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49:271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 11.Federal Statistical Office Wiesbaden. Statistical yearbook 2002 for the Federal Republic of Germany. Stuttgart, Metzler-Poeschel. 2002:59. [Google Scholar]
- 12.Davidson MB. Counterpoint: self-monitoring of blood glucose in type 2 diabetic patients not receiving insula waste of money. Diabetes Care. 2005;28:1531–1533. doi: 10.2337/diacare.28.6.1531. [DOI] [PubMed] [Google Scholar]
- 13.Ipp E, Aquino RL, Christenson P. Point: self-monitoring of blood glucose in type 2 diabetic patients not receiving insulthe sanguine approach. Diabetes Care. 2005;28:1528–1530. doi: 10.2337/diacare.28.6.1528. [DOI] [PubMed] [Google Scholar]
- 14.Karter AJ, Ackerson LM, Darbinian JA, D'Agostino RB, Jr, Ferrara A, Liu J, Selby JV. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 15.Karter AJ, Parker MM, Moffet HH, Spence MM, Chan J, Ettner SL, Selby JV. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care. 2006;29:1757–1763. doi: 10.2337/dc06-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis WA, Bruce DG, Davis TM. Is self-monitoring of blood glucose appropriate for all type 2 diabetic patients The Fremantle Diabetes Study. Diabetes Care. 2006;29:1764–1770. doi: 10.2337/dc06-0268. [DOI] [PubMed] [Google Scholar]
- 17.Meier JL, Swislocki AL, Lopez JR, Noth RH, Bartlebaugh P, Siegel D. Reduction in self-monitoring of blood glucose in persons with type 2 diabetes results in cost savings and no change in glycemic control. Am J Manag Care. 2002;8:557–565. [PubMed] [Google Scholar]
- 18.Kolb H, Schneider S, Heinemann L, Lodwig V, Scherbaum WA, Martin S. Altered Disease course after initiation of self-monitoring of blood glucose in noninsulin-treated type 2 diabetes (ROSSO 3) J Diabetes Sci Technol. 2007;1:487–495. doi: 10.1177/193229680700100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pouwer F, Beekman AT, Nijpels G, Dekker JM, Snoek FJ, Kostense PJ, Heine RJ, Deeg DJ. Rates and risks for co-morbid depression in patients with Type 2 diabetes mellitus: results from a community-based study. Diabetologia. 2003;46:892–898. doi: 10.1007/s00125-003-1124-6. [DOI] [PubMed] [Google Scholar]
- 20.Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, Kinder L, Young B, Von KM. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]


