Abstract
Background
The Diabetes Error Test Model (DETM) has been developed to characterize the clinical relevance of the large and varying margins of error of parameters affecting postprandial blood glucose (BG) levels, which increase the risk for hypo- or hyperglycemia.
Methods
The DETM is based on a treatment concept aimed at normoglycemia after meals. The model includes as parameters (a) preprandial BG measurement by patient self-monitoring (SMBG), (b) patient estimate of carbohydrate amounts (CARB-P) in food, (c) effect of CARB-P on maximum BG increase, (d) effect of insulin on maximum BG decrease, and (e) insulin dosage. Covering the relevant range of preprandial BG (30–330 mg/dl), the DETM simulates the maximum effect of these parameters and their margins of error on postprandial BG values.
Results
According to the DETM, a SMBG error of +20% results in normoglycemia (BG range: 60–160 mg/dl) as the postprandial outcome if preprandial BG values are in the range of 30–130 or 260–330 mg/dl, but can unexpectedly result in hypoglycemia if preprandial BG values are between 131 and 259 mg/dl. If the SMBG error of +20% is combined, e.g., with an error of CARB-P estimate in the food of +20%, hypoglycemia as the postprandial outcome is worsened. If one combines the effects of errors of more than two parameters, even with errors that are so small that they have no clinically relevant dysglycemic effect on postprandial BG per se (e.g., ±6%), this can result in postprandial hypo- or hyperglycemic values.
Conclusion
The DETM simulates the effects of errors of parameters affecting postprandial BG within the clinically relevant BG range. The DETM offers the opportunity to evaluate the clinical relevance of these errors and their contribution to the increased risk of meal-related excessive glucose excursions during intensified insulin therapy.
Keywords: glucose excursions, insulin therapy, prandial insulin therapy, SMBG
In recent years, considerable progress has been made in the development of diagnostic, therapeutic, and educational tools for diabetes self-management, particularly for intensified insulin therapy. However, even in people with diabetes (PwD) who have been well trained and regularly followed up in large intervention trials such as the Diabetes Control and Complications Trial1 or United Kingdom Prospective Diabetes Study,2 only a minority of patients was able to keep their blood glucose (BG) within the target range. PwD report repeatedly that they apply their BG meter and the recommended therapeutic tools for self-adjustment, e.g., at mealtimes, in good faith. However, they often fail to keep their metabolic control within their BG target limits without obvious errors or mismanagement. This is clearly a tremendous source of frustration for PwD and their treating physician.
The question is why is it so difficult to reach the goal, i.e., to achieve a postprandial glycemic control within the recommended BG target range? While the main focus is on the appropriate treatment adjustments to the variable requirements of daily life,3–14 it is less recognized that the diagnostic and therapeutic tools are characterized by rather large and variable margins of error. For example, in recent years it was studied more in detail how much impact the variability of insulin absorption and insulin action has on BG variability.15,16 Another source of error is related to self-monitoring of blood glucose (SMBG) as a consequence of technical and handling factors. However, a given error in the SMBG measurement result per se has no effect on the postprandial BG; this requires a therapeutic action. In the framework of intensified insulin therapy, the measured BG value is one of the factors that have to be taken into account by PwD to calculate the preprandial insulin dose. Ideally this will result in a postprandial glycemic excursion inside the target range.
Nevertheless, it is remarkable that the aforementioned algorithms used to calculate the preprandial insulin dose either ignore the margins of errors inherent to each of the factors or consider only errors of single factors within rather narrow ranges. The SMBG measurement error is the most recognized factor; however, up until now it was not studied systematically to what degree a single error or combined parameter errors result in postprandial glycemic excursions outside the target range.
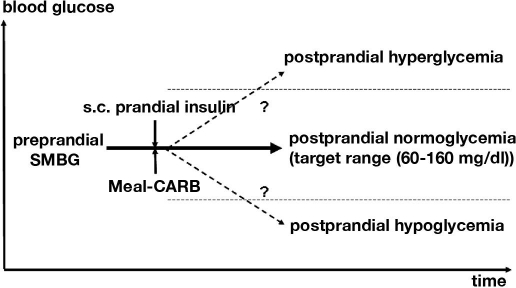
In order to analyze the impact of errors of different factors that affect postprandial glycemic excursions, the Diabetes Error Test Model (DETM) was developed. The DETM simulates the impact of these errors on postprandial BG as the outcome of the preprandial BG and the therapeutic action taken according to standard textbook guidelines and algorithms in order to achieve normoglycemia after meals by prandial insulin injections. The following factors are taken into account as model parameters: error of capillary BG measured preprandially by SMBG (calibrated for whole blood), variability of the BG effect of a carbohydrate portion (CARB-P) on maximum BG increase, degree of precision with which patients can estimate the carbohydrate content of a CARB-P within a given meal, variability of the BG effect of subcutaneously applied prandial insulin on maximum BG decrease, and the insulin dosing error (Figure 1). The impact of the errors of each of these parameters on postprandial glycemia was analyzed independently from and in combination with each other.
Figure 1.
Each meal and the related therapeutic actions represent a balance of BG-increasing and -decreasing factors: The prandial subcutaneous insulin parameter covers the variability of the BG-lowering effect (insulin absorption and insulin action). The BG-increasing effect of meals (CARB-P, patient's estimate of CARB-P, type of carbohydrate, and absorption in the gut) counteracts that of insulin. Depending on the preprandial SMBG value, the insulin dose and CARB-P will be adjusted by the patients with the goal of achieving a postprandial BG in the target range. All these factors have an inherent amount of error, which increases the risk of postprandial hypo- or hyperglycemia (BG below or above the target range). The DETM simulates the maximal combined effects of these parameters and their margins of error.
The aim of our simulation was to improve the understanding of the effects of errors of the parameters mentioned on postprandial BG and their contribution to the increased risk for meal-related hypo- or hyperglycemia during intensified insulin therapy.
Research Design and Methods
Diabetes Error Test Model
The treatment algorithms implemented in the DETM are those for adult PwD with type 1 diabetes with stable body weight, physical activity, and without diabetes-related complications or associated diseases/conditions; they are well trained on intensified insulin therapy. The prerequisite for these algorithms is that the basic treatment concept of an intensified insulin treatment with multiple daily injections of rapid-acting prandial insulin formulations aimed at normoglycemia (postprandial BG within the individualized target limits) has already been established successfully. The chosen basic treatment concept takes into account the dynamic nature of food absorption, insulin absorption, and insulin action by providing the individual insulin/CARB-P ratio for the individual PwD. It also considers the individual varying insulin sensitivity from diurnal counterregulatory hormone secretion, past hypoglycemia, and the critical timing of insulin injection to meal timing. The model algorithms are based on the extended clinical experience of the German Diabetes Research Institute/German Diabetes Center at the Heinrich-Heine-University of Düsseldorf for meal-related diabetes therapy17,18 and their regular successful application to the patients treated in this center over many years. Therefore, the prerequisite for the algorithms in the basic treatment concept using multiple daily injections had been already established.
The aim of the applied treatment algorithms is to achieve a postprandial BG in the normoglycemic range (60–160 mg/dl), irrespective of the preprandial BG value.
- X = number of carbohydrate portions (CARB-P: equivalent to 10 g carbohydrate content/portion) a person with diabetes should eat in addition or should omit from his meal in relation to his preprandial BG (mg/dl):
BG <40 40–59 60–120 121–160 161–200 201–240 241–300 301–330 CARB-P +2 +1 X −1 −2 −3 −4 −5 - Preprandial insulin dose self-adjustment starting from Y = 1 IU per CARB-P for BG 81–120 mg/dl. With a preprandial BG below or above this range the insulin dose has to be reduced or increased. If a meal contains more than 1 CARB-P, the insulin dose Y has to be adjusted accordingly.
BG <60 60–80 81–120 121–160 161–200 201–240 241–300 301–330 CARB-P 0 −1Y Y +1Y +2Y +3Y +4Y +5Y
If the preprandial BG is > 120 mg/dl, PwD can employ adjustment of carbohydrate intake, prandial insulin dose, or a combination of both.
Depending on the time of the day, the prandial insulin dose has to be adjusted with respect to the diurnal changes in insulin sensitivity:
Morning 1.0–3.0 IU per CARB-P
Noon 0.5–1.5 IU per CARB-P
Evening 1.0–2.0 IU per CARB-P
However, for the subsequent analysis we always use an insulin/CARB-P ratio of 1.
Covering the clinically relevant BG range of preprandial values of 30–330 mg/dl the DETM calculates the maximum effect of all model parameters and their margins of error on postprandial BG in 1-mg/dl steps. The ranges of the margins of errors studied are known from clinical experience and published data,19–21 e.g., the clinical accuracy of SMBG measurement errors depends on the combined inaccuracies of the meter and strip system, as well as on user-handling errors in daily life.
| Margin of error | SMBG (%) | CARB-PBG increase (mg/dl) | CARB estimate (%) | Insulin BG decrease (mg/dl) | Insulin dosing (%) |
|---|---|---|---|---|---|
| Highest | +50 | 80 | 200 | 50 | +50 |
| No error | 0 | 40 | 100 | 40 | 0 |
| Lowest | −50 | 20 | 40 | 30 | −25 |
The effects of an error of preprandial SMBG measurement (as well as of the errors of the other parameters) were calculated under the condition that the errors of all other model parameters are kept at 0% if not stated otherwise. The focus of our simulation was on errors of SMBG measurement according to the approaches used by the ISO-NORM 15197:2003/E, the SMBG device-related CE procedure for the European Union, and Error Grid Analysis (EGA; zones A and B).22,23 The target limits of the postprandial lower/upper BG (derived from capillary whole blood and calibrated to whole BG in accordance to the usual calibration procedure for SMBG devices in Germany) have been set at 60/160 mg/dl. This BG range represented the 2 SD range of pre- and postprandial BG values from healthy subjects on two standardized diets during a 3-day observational period using continuous glucose monitoring.24 The upper postmeal target limit is also in accordance with the most recent recommendation of the American Diabetes Association for the peak postprandial capillary plasma glucose value (< 180 mg/dl).25 BG values above the upper limit and below the lower target limit have been defined as hyperglycemia and hypoglycemia, respectively. The use of plasma-referenced SMBG values would have no impact on the outcome of the model, but its BG target limits and the BG ranges of the therapeutic algorithms had to be adjusted accordingly.
The DETM does not calculate the postprandial glycemic excursion as a profile; it calculates the maximal postprandial effect but no time dimension is regarded. The program was written in Delphi 7. It allows simulation of different errors (and their combination) for the DETM parameters and displays the results numerically and graphically.
Results
Effects of SMBG Measurement Errors
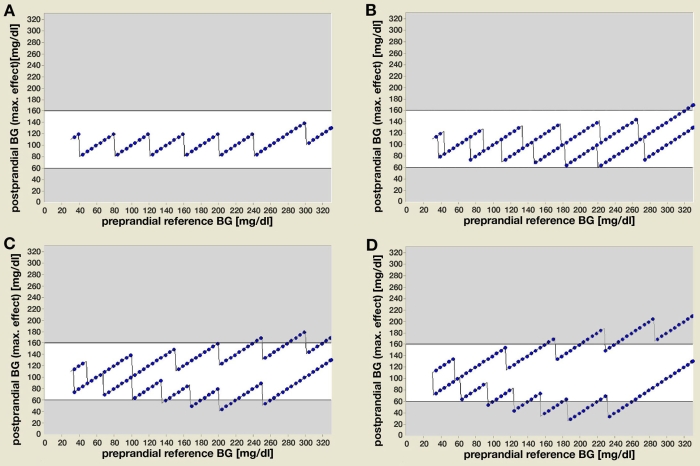
For all preprandial SMBG values within the defined hypo-, normo-, and hyperglycemic ranges, the DETM calculates the postprandial BG outcome. In the first step, all model parameter errors were kept at 0% (Figure 2a). Under these ideal conditions the postprandial outcome is normoglycemia, similar to an error of ±10% (Figure 2b), which represents an average BG result variability of a quality-controlled laboratory value. The stepwise changes within the treatment algorithms for CARB-P and insulin dose adjustment in relation to the preprandial BG values result in a sawtooth-like postprandial BG curve and not in a smooth line. When varying only the SMBG measurement error for a fixed preprandial hyperglycemic SMBG value (200 mg/dl), an error of +30% results in postprandial hypoglycemia, whereas a preprandial error of -30% results still in hyperglycemia instead of the predicted normoglycemic value (120 mg/dl) (Figure 2d).
Figure 2.
The DETM simulates for all preprandial BG values (x axis) within the clinically relevant range the postprandial BG outcome (y axis) as the combined maximum effect of all model parameters. (a) Assuming a zero percent error for all model parameters, the postprandial BG outcome is normoglycemia (target range 60–160 mg/dl) at all preprandial values; e.g., for a preprandial BG of 100 mg/dl, the postprandial BG is 100 mg/dl, for 130 mg/dl it is 90 mg/dl, and for 260 mg/dl it is also 100 mg/dl. (b) Assuming ±10% error (representing average result variability from CC laboratories), (c) ±20% error (representing margins of error for SMBG results according to the ISO-NORM 15197), or (d) ±30% error for preprandial SMBG measurements (representing clinical inaccuracy under daily life conditions) and keeping the remaining model parameters at 0% error, the postprandial BG outcome still results in normoglycemia for some preprandial BG ranges, whereas the other BG ranges result in postprandial hypo- or hyperglycemia.
Performing these calculations for a fixed SMBG error of +20% over the whole range of preprandial BG values results in the following postprandial outcome: normoglycemia with preprandial BGs in the range of 30–130 or 260–330 mg/dl, but occasional hypoglycemic values (50–60 mg/dl) if preprandial BG values are between 131 and 259 mg/dl (Figure 2c). A preprandial SMBG error of -20% results in postprandial normoglycemia for preprandial values < 240 mg/dl, but results in post-prandial hyperglycemia for preprandial values > 240 mg/dl. A SMBG error of +30% results postprandially in even lower hypoglycemic values for a wider preprandial BG range (90–265 mg/dl), whereas for preprandial BG values < 90 and > 265 mg/dl even such an error results in postprandial normoglycemia (Figure 2d).
Therefore, the same SMBG measurement error could be classified as neglectable (postprandial BG remains within the target range 60–160 mg/dl), as acceptable (BG within 50–200 mg/dl), and sometimes even as unacceptable (BG value < 50 or > 200 mg/dl), depending on the preprandial SMBG value.
Similar results with the same degrees of error are obtained for each of the other four model parameters under the condition that the errors are simulated only for one given parameter and the remaining parameters are kept at 0% error (data not shown).
Combined Effects of Parameter Errors
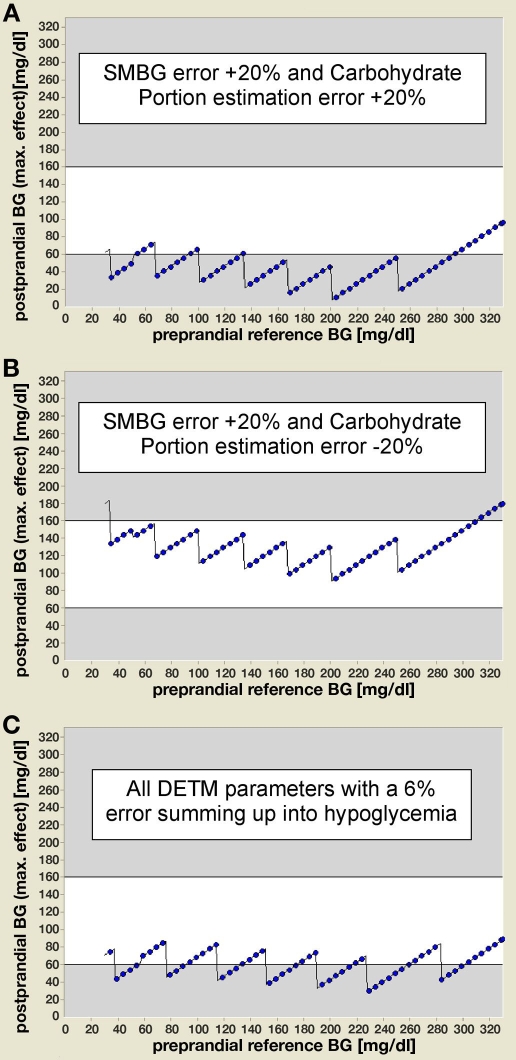
As in the daily life of PwD, not only SMBG measurement errors per se or single errors of the other model parameters are of interest but their combined effects, the DETM allows one to simulate all combinations of parameter errors. However, only some clinically relevant combinations are presented here. The combination of a SMBG error of +20% with an estimation error for CARB-P of +20% led to a further deterioration of the postprandial hypoglycemia compared to the single SMBG error (Figure 3a). However, the hypoglycemic result of the single SMBG error of +20% is ameliorated completely if combined with an estimation error for CARB-P of −20% (Figure 3b).
Figure 3.
The DETM allows simulating all combinations of parameter errors; examples are given for three clinically relevant combinations. (a) The combination of a SMBG measurement error of +20% with an estimation error for CARB-P of +20% results in a further deterioration of postprandial hypoglycemia compared to the single SMBG error. (b) The combination of a SMBG measurement error of +20% with an estimation error for CARB-P of -20% ameliorates the hypoglycemic result of the single SMBG error completely. (c) The combination of the effects of a 6% parameter error for carbohydrate content, estimate of carbohydrate content, prandial insulin effect, and an identical SMBG measurement error, which per se have no clinically relevant hypoglycemic effect on postprandial BG, results in severe postprandial hypoglycemia over a wide range of preprandial BG values. The 6% error was a positive or negative error for each given parameter with all errors inducing a blood glucose-lowering effect.
If one combines the effects of errors of more than two parameters, even with errors that are so small that they have no clinically relevant dysglycemic effect on postprandial BG per se, this can result in postprandial hypo- or hyperglycemic values. For example, combining a SMBG measurement error of 6% with the same degree of error for the other parameters results in severe postprandial hypoglycemia (Figure 3c).
Conclusions
The Diabetes Error Test Model allows characterizing simultaneously the clinical relevance of the margins of error of diagnostic as well as of relevant therapeutic parameters affecting postprandial blood glucose in PwD within well-defined clinical conditions. Like SMBG, the effect of errors for other parameters can be evaluated in an identical manner. The DETM also offers the opportunity for a detailed evaluation of errors of SMBG measurement and of other parameters affecting postprandial BG in combination.
The simulation of the postprandial effects of a wide range of SMBG errors (all other parameter errors kept at 0%) demonstrated that under the given conditions postprandial hypo- or hyperglycemia occurs only if the SMBG error is applied to preprandial BGs within certain ranges. Vice versa, even with a considerable SMBG measurement error of -50% the postprandial BG can still stay within the target range if the preprandial values are < 121 mg/dl. Obviously, the clinical evaluation of a SMBG measurement error with respect to the postprandial BG outcome has to take the actual preprandial BGs into account: The same error could be classified as neglectable, acceptable, and sometimes even unacceptable depending on the preprandial BG. While for SMBG values this is also the concept of the EGA, the DETM extends this concept to all model parameters.
The DETM is particularly well suited to evaluate the postprandial effects of even single SMBG measurement errors, as it always takes well-defined therapeutic measures into account. However, the DETM also provides evidence for the fallacies of an evaluation with the focus only on SMBG measurement errors: at the worst case an unacceptable postprandial BG outcome due to another unrecognized but common mistake, such as an estimation error of the CARB-P content by 20–30%, could be misinterpreted as an unacceptable SMBG measurement performance despite no SMBG error being present.
As errors of SMBG measurement and therapeutic interventions can occur during the same self-treatment action, their combined effects, according to the DETM, can worsen the postprandial dysglycemia or, to the contrary, the combination can diminish or even erase the individual BG effects. As the errors of the therapeutic DETM parameters can vary within considerable limits, DETM results support the hypothesis that their (potential) contribution to the postprandial BG outcome can outweigh the effect even of a considerable SMBG error. Assuming that certain error values occur more often than others for the same parameter in a given patient under daily life conditions, the DETM in principle can handle such information. Similarly, the model can analyze all potential combinations of errors, taking the frequency distribution of these errors into account. However, because of the fact that no reliable respective information is available, this has not been done so far.
The interaction between combinations of errors also affects the clinical evaluation of SMBG measurement errors by other standard approaches for the clinical evaluation of errors such as the EGA or the new consensus error grids.8,9 The EGA evaluates the clinical implications of inaccurate SMBG values. This implies “… that they would lead to clinically correct decisions” for EGA zone A and that zone E “… is an erroneous treatment zone.” In this context the treatment algorithms applied by EGA and related parameters are not addressed in detail and their potential error contribution and combined error effects, as described with the DETM, are not considered at all. The EGA does not address postprandial outcomes specifically but certainly does not exclude them as they are an important part of preprandial SMBG measurements.
Therefore, these approaches do not address the real life of PwD sufficiently. However, there is a plethora of therapeutic model algorithms that have been developed for educational purposes, for use in automated pancreas systems, and to support decisions of PwD for their individual meal-related insulin dose adjustments in daily life. Interestingly, these algorithms do not address the effects of errors of the various parameters used in these therapeutic models. People with diabetes on intensified insulin therapy do report more or less regularly postprandial hypoglycemic events without obvious diagnostic or therapeutic mistakes and these events remain quite often unexplained. However, the DETM provides a reasonable explanation for such events by the observation that a combination of four relatively small errors such as 6% each, which per se have no clinically relevant effect on postprandial BG, can result in clinically relevant postprandial hypoglycemia if they add to each other.
To the best of our knowledge no systematic approach similar to the DETM has been developed to evaluate together the contribution of errors of major fundamental parameters affecting postprandial BG outcome. Most of the previous studies focused on the contribution of SMBG errors with the implicit assumption of no other contributing parameter error.22,23 Few studies considered the contribution of one or more additional errors of other parameters affecting BG.12–14,26,27 These authors also noted the potential of considerable deviations of the BG outcome from the intended one as a consequence of such errors. No systematic data are published on the frequency and magnitude of a given error per se, as well as the combined errors of the various combinations of the model parameters from the daily life of PwD.
The DETM can be used for educational purposes, as it allows simulating the effects of treatment changes according to the individual wishes/needs of the patient without the usual risk of real trial and error. At the same time, the DETM delivers its core message: it is not sufficient to focus on the performance of the SMBG system or on the availability of a “perfect” treatment algorithm alone, but it is also essential to take into account the potential effects of the various presented parameter errors on the intended BG outcome as they are equally effective at the same magnitude of error.
As a perspective, the concept of the DETM can also be applied to the development of a clinical evaluation tool for spot BG measurements, as well as for continuous glucose monitoring devices.
The DETM has several limitations, as its simulations are based on a number of assumptions and simplifications. At present the model is restricted to patients with type 1 diabetes mellitus with stable body weight and physical activity and without acute or chronic complications or stress on intensified insulin therapy. The main focus of the model is on meal-related effects on BG, as this represents a major challenge for this patient group. The DETM assumes that the dynamic time course of the BG-increasing effect of a carbohydrate-containing meal is perfectly matched by the BG-decreasing effect of the prandial insulin. This perfect match is assumed to be constant within the defined clinically relevant BG range.
The therapeutic algorithms implied in the model represent the accumulated experience of a German university hospital diabetes center over many years, but obviously more or less different algorithms are also used by other diabetologists; however, we assume that similar approaches are used worldwide, as they reflect the metabolic needs of the human body. A prerequisite for the DETM is that the basic treatment concept of multiple daily insulin injections aimed at normoglycemia (postprandial BG within the individualized target limits) has already been established successfully by the supervising physician that includes appropriate patient education and training. This procedure provides the basic clinical requirements, e.g., the actual insulin/CARB ratio among others, to apply the model algorithms. The algorithms implemented in the current version of the DETM increase or decrease the CARB-P or insulin dose in a sliding scale of 1 CARB-P or 1 IU per preprandial BG step (20–60 mg/dl per step). The consequence of this stepwise approach is that the postprandial BG outcome is not a flat line, but shows a sawtooth profile. The resulting profile would be flatter if one reduces the therapeutic step range, i.e., vary insulin doses in 0.5 IU/step. The DETM is flexible enough to substitute any reasonable therapeutic algorithm and BG target range for the implemented ones, and in this way it can also be applied to the specific requirements of an individual PwD. The DETM also supports (in addition to the already described possible clinical evaluations) the simulation of treatment alternatives, e.g., by providing a rational basis for changes of the algorithms (be they general or individualized) and of different insulin/CARB ratios with the goal of reducing the effects of errors as a prerequisite of a clinical study project for the improvement of therapeutic algorithms.
It is of importance to note that the parameters chosen and their errors combine different factors, e.g., the diagnostic parameter SMBG measurement combines the preanalytical error of the capillary blood handling with the analytical error of the BG device and the postanalytical interpretation of the BG result by PwD. Another example is the combination of effects of compositional and physiological factors, e.g., the BG-increasing effect by the ingested carbohydrates is the result of the real amount of the carbohydrates in the meal, the type of carbohydrates that determines the rapidity of their absorption, and the physiological variability of the glucose absorption in the gut as a consequence of other factors such as total meal composition and so on. The obvious aim of the DETM is not a puristic scientific-detailed analysis of each aspect of the given model parameters, but a composite tool that takes into account their complex nature in the real life of PwD.
Treatment error is the cumulative result of several factors, not just SMBG error. The DETM provides a basic framework of four parameters to which additional parameters, such as exercise/changed physical activity with their respective BG changing effects and their potential error ranges, can be added. Their addition to the described DETM has been omitted to limit the complexity of this first model description as additional model parameters do not change the DETM results in principle.
In conclusion, the DETM represents a novel approach for the clinical evaluation of the effects of errors of diagnostic and therapeutic parameters on postprandial blood glucose in people with type 1 diabetes. Its features exceed the available standard systems with respect to the contributions and interactions of different errors of the described parameters and with respect to the documentation of the implied algorithms. Thereby, the DETM allows a broader understanding of the complex relationships of the diagnostic and therapeutic tools during meal-related adjustments of blood glucose. It provides a hypothesis generating potential for the improvement of meal treatment algorithms as a starting point for subsequent clinical studies. There is also a potential application for the adjustment of individual treatment regimens, but this requires additional validation studies. In addition, the DETM can be used for a diagnostic application, e.g., for the clinical evaluation of devices for SMBG or continuous glucose monitoring.28 It might also be of help for the development of an artificial pancreas.
Acknowledgments
The development of the DETM and the preparation of the manuscript were supported by a research grant by Roche Diagnostics GmbH, Mannheim, Germany. A compiled version of the program is available upon request. The intellectual property implemented in this program is patent protected. The other members of the Glucose Monitoring Study Group are listed on www.gm-study-group.com/members.de.
Abbreviations
- BG
blood glucose
- CARB-P
carbohydrate portion
- DETM
Diabetes Error Test Model
- EGA
Error Grid Analysis
- PwD
people with diabetes
- SMBG
self-monitoring of blood glucose
References
- 1.Diabetes Control and Complications Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Moberg EA, Lins PE, Adamson UK. Variability of blood glucose levels in patients with type 1 diabetes mellitus on intensified insulin regimens. Diabet Med. 1994;20:546–552. [PubMed] [Google Scholar]
- 4.Skyler JS, Skyler DL, Seigler DE, O'Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4:311–318. doi: 10.2337/diacare.4.2.311. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovic L, Peterson CM. Home blood glucose monitoring. Compr Ther. 1982;8:10–20. [PubMed] [Google Scholar]
- 6.Chanoch LH, Jovanovic L, Peterson CM. The evaluation of a pocket computer as an aid to insulin dose determination by patients. Diabetes Care. 1985;8:172–176. doi: 10.2337/diacare.8.2.172. [DOI] [PubMed] [Google Scholar]
- 7.Peterson CM, Jovanovic L, Chanoch LH. Randomized trial of computer-assisted insulin delivery in patients with type I diabetes beginning pump therapy. Am J Med. 1986;81:69–72. doi: 10.1016/0002-9343(86)90184-1. [DOI] [PubMed] [Google Scholar]
- 8.Schifrin A, Mihic M, Leibel BS, Albisser AM. Computer-assisted insulin dosage adjustment. Diabetes Care. 1985;8:545–552. doi: 10.2337/diacare.8.6.545. [DOI] [PubMed] [Google Scholar]
- 9.Chiarelli F, Tumini S, Morgese G, Albisser AM. Controlled study in diabetic children comparing insulin-dosage adjustment by manual and computer algorithms. Diabetes Care. 1990;13:1080–1084. doi: 10.2337/diacare.13.10.1080. [DOI] [PubMed] [Google Scholar]
- 10.Beyer J, Schrezenmeir J, Schulz G, Strack T, Küstner E, Schulz G. The influence of different generations of computer algorithms on diabetes control. Comput Methods Programs Biomed. 1990;32:225–232. doi: 10.1016/0169-2607(90)90104-h. [DOI] [PubMed] [Google Scholar]
- 11.Schrezenmeir J, Dirting K, Papazov P. Controlled multicenter study on the effect of computer assistance in intensive insulin therapy of type 1 diabetics. Comput Methods Programs Biomed. 2002;69:97–114. doi: 10.1016/s0169-2607(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 12.Owens CL, Zisser H, Jovanovic L, Srinivasan B, Bonvin D, Doyle FJ., 3rd Run-to-run control of blood glucose concentrations for people with type 1 diabetes mellitus. IEEE Trans Biomed Eng. 2006;53:996–1005. doi: 10.1109/TBME.2006.872818. [DOI] [PubMed] [Google Scholar]
- 13.Zisser H, Jovanovic L, Doyle F, Ospina P, Owens C. Run-to-run control of meal-related insulin dosing. Diabetes Technol Ther. 2005;7:48–57. doi: 10.1089/dia.2005.7.48. [DOI] [PubMed] [Google Scholar]
- 14.Palerm CC, Zisser H, Jovanovic L, Doyle FJ., 3rd A run-to-run framework for prandial insulin dosing: handling real-life uncertainty. Int J Robust Nonlinear Control. 2007;17:1194–1213. [Google Scholar]
- 15.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25:905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 16.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type-1 diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2005;52:3–12. doi: 10.1109/TBME.2004.839639. [DOI] [PubMed] [Google Scholar]
- 17.Gries FA, Koschinsky T. In: Diabetes mellitus (internistisch). Therapie-Handbuch, 3. Aufl , Krück Hrsg. F, Kaufmann W, Bünte H, Gladtke E, Tölle R, editors. München: Verlag Urban & Schwarzenberg; 1989. pp. 1041–1071. [Google Scholar]
- 18.Berger W, Gries FA, Koschinsky T, Toeller M. In: Diabetes mellitus: Lehrbuch der inneren Medizin, 3. Aufl , Siegenthaler Hrsg. W, Kaufmann W, Hornbostel H, Waller HD, editors. Stuttgart: Georg Thieme Verlag; 1992. pp. 1277–1304. [Google Scholar]
- 19.Toeller M, Koschinsky T, Gries FA. Diabetes mellitus neue Entwicklungen. Basistherapie–Schulung und Erfolgskontrolle. Therapiewoche. 1983;33:2633–2646. [Google Scholar]
- 20.Mehnert Hrsg. H, Standl E, Usadel K-H, Häring H-U., editors. Diabetologie in Klinik und Praxis, 5. Aufl. Stuttgart: Georg Thieme Verlag; 2003. [Google Scholar]
- 21.Keith K, Nicholson D, Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr. 2004;43:69–74. doi: 10.1177/000992280404300109. [DOI] [PubMed] [Google Scholar]
- 22.Clarke WL, Cox D, Gonder-Frederik LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 23.Parkes JL, Statin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 24.Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, Haug C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1(5):695–703. doi: 10.1177/193229680700100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2007;30(Suppl 1):S4–41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 26.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001;47:209–214. [PubMed] [Google Scholar]
- 27.Kildegaard J, Randlov J, Poulsen JU, Hejlesen O. The impact of non-model-related variability on blood glucose prediction. Diabetes Technol Ther. 2007 Aug;9(4):363–371. doi: 10.1089/dia.2006.0039. [DOI] [PubMed] [Google Scholar]
- 28.Koschinsky T, Heckermann S, Heinemann L. A standardized evaluation of continuous glucose monitoring (CGM) techniques: a new application of the Diabetes Error Test Model (DETM) Presented at the 2007 DTM.