Abstract
Continuous subcutaneous insulin infusion therapy (CSII) is an increasingly popular form of intensive insulin administration in pediatric patients. The use of CSII commenced at our large tertiary referral diabetes clinic as recently as 2002. In the intervening years, demand and enthusiasm from both patients and physicians alike have resulted in a steady ongoing increase in CSII use at our clinic. We currently have >200 active patients using insulin pump therapy. This article reviews our experience with CSII and outlines our current multidisciplinary approach to optimizing glycemic control and outcomes in this patient group.
Keywords: continuous subcutaneous insulin infusion, insulin pump therapy, pediatrics, type 1 diabetes
Introduction
Continuous subcutaneous insulin infusion (CSII) was first introduced as a management strategy for both adult1 and pediatric2 patients with type 1 diabetes mellitus (T1DM) in the late 1970s. However, it was not until the Diabetes Control and Complications Trial3,4 and Epidemiology of Diabetes Interventions and Complications5 studies confirmed and reaffirmed the preeminent role of glycemic control in the pathogenesis of microvascular complications that use of insulin pump therapy as “intensive therapy” in young people with diabetes has become increasingly widespread.
The potential benefits of CSII have been well canvassed. CSII is the most physiological method of insulin delivery currently available and offers more precision in insulin delivery than twice-daily or multiple daily injections (MDI) of insulin. Observational studies in pediatric age groups have reported lower hemoglobin A1c (HbA1c) and decreased hypoglycemia rates following commencement of CSII.6–9 While the potential for improvement in metabolic control offered by CSII seems intuitive, well-designed prospective randomized controlled trials (RCTs) of long-term glycemic outcomes have yet to test this hypothesis in pediatric patients. This may reflect the relative infancy of CSII in many centers around the world. Short-term RCTs comparing CSII with MDI in children and adolescents show either comparable efficacy10–12 or at best a modest improvement13 in the CSII group. Despite this, the perceived potential for improved metabolic control, coupled with improved flexibility in daily living and the associated potential for improved quality of life (QOL), has proved to be enticing for patients with T1DM.
History of CSII Experience at the Royal Children's Hospital, Melbourne
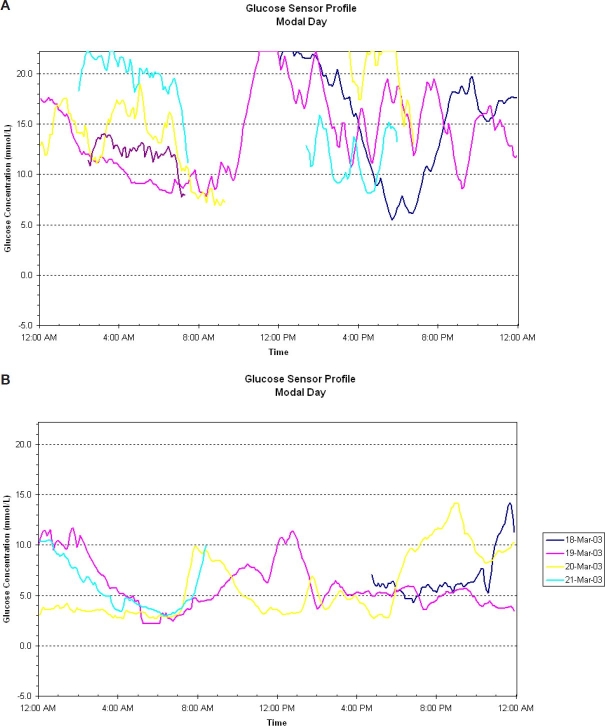
The diabetes clinic at the Royal Children's Hospital, Melbourne, Australia has approximately 1400 active patients (age 0–18 years) with T1DM. Use of CSII in our pediatric and adolescent population began relatively recently, in 2002. Early indications for CSII included enthusiastic motivated patients and patients for whom problematic recurrent hypoglycemia was precluding an increase in total daily insulin dose, despite suboptimal HbA1c (see Figure 1). In the intervening years, the number of patients commencing CSII has increased from 3 in 2002 to a cumulative total of >230 by October 2007. This rise has been facilitated by the introduction of a national reimbursement scheme for costs associated with CSII consumables, which has meant that monthly running costs are comparable to those associated with the use of MDI or needles and syringes. The initial purchase price of the insulin pump does not receive any government funding however, so patients without private health insurance are rarely in a position to avail of this technology at our center.
Figure 1.
(A) Continuous glucose monitoring system on subcutaneous insulin: HbA1c 9.2%; dose increases resulted in recurrent hypoglycemia. (B) Same patient on a continuous glucose monitoring system 3 months after initiation of CSII: HbA1c 6.6%.
Initiation of Insulin Pump Therapy: Appropriate Patient Selection
The decision to commence CSII is made jointly between an individual patient, his/her family, and the treating physician and allied health team. Our experience suggests that patient (as opposed to parent or family) motivation and enthusiasm for pump therapy are of utmost importance when considering CSII. The simple question of “who wants the pump?” can often reveal a lack of cohesion within families regarding readiness for the initiation of pump therapy. Commencement of CSII is often associated with a significant shift in responsibility for “control” over diabetes management from parent to child, which can have associated attendant difficulties for both parties. The ability to cope with the increased focus on diabetes and more frequent insulin administration varies from child to child. While there are reports of favorable outcomes in young children using CSII,14 improved glycemic control and less hypoglycemia have not been universal findings in this age group.12 In our experience, the practicalities of frequent bolus dosing in a child care or primary school setting require intensive input from parents, teachers, and other child care staff. Unless this intensive input is logistically feasible, our preference is to defer initiation of CSII until the child is older or more independent.
Realistic expectations as to outcomes with the use of CSII are also key factors in determining suitability for pump therapy. Patients and families commonly report a perception that insulin pump therapy is superior to other forms of insulin administration; as mentioned previously, this has yet to be borne out in long-term RCTs. While there are many associated benefits reported with CSII,6–9 families need to appreciate that CSII is the most intensive insulin administration regime currently available. Potential benefits are therefore often only attained with increased input into daily diabetes management.
Frequency of blood glucose level (BGL) testing has been shown in a large observational study15 to be predictive in terms of outcomes and persistence with CSII. Our clinic experience mirrors that finding. We regard frequency of BGL testing as an equal or better surrogate for an individual's “commitment” to their diabetes management and their ability to intensify their insulin regime than their current HbA1c. As shown in Figure 1, HbA1c can improve significantly in a short period of time with CSII, even in those with poor baseline glycemic control. It is our experience that a patient with poor control despite regular BGL testing is more likely to accept the increased intensity of effort and to succeed with CSII than a counterpart with “good” glycemic control (as judged by HbA1c), despite minimal daily monitoring. While we have no HbA1c “inclusion” criteria for CSII at our center, regular BGL testing (at least four times daily) must be established prior to consideration for pump therapy.
Further smaller subgroups of patients who may benefit from early consideration of CSII include infants with neonatal diabetes, children or adolescents with eating disorders, and those with severe needle phobia. Our center has reported positive experiences with CSII in very young infants.15 In this cohort, frequent small feeds are the norm. CSII allows for precise dose titration and delivery of tiny insulin volumes, which are difficult to achieve with intermittent subcutaneous injections. The use of temporary basal rates and the potential for pump suspension can also prevent hypoglycemia in the event of decreased oral intake.
We also have experience with initiation of CSII in a patient with an established eating disorder (ED), as well as patients using CSII who developed an ED and have found CSII to have attendant benefits for managing this patient group. Health care workers can utilize the pump memory to explore the possibility of insulin omission to facilitate weight loss. When patients embark on a refeeding program, CSII also allows for more accurate bolus dose administration based on meal composition. Protracted meal duration is common in patients with ED and may result in postprandial hypoglycemia. Conventional methods of treating hypoglycemia [jelly beans or other fast-acting carbohydrates (CHO)] are abhorrent to this patient group; the use of a combination or “dual-wave” prandial bolus may help minimize this complication. In our patients, insulin pump therapy was associated with good glycemic control, allowing the focus of care priorities to shift from diabetes to management of the ED.
Similarly, albeit in small numbers of patients, families of children with severe needle phobia at our center report improved QOL and less conflict surrounding day-to-day diabetes care with CSII. Patients in these subgroups who otherwise meet the motivation and BGL testing “criteria” for CSII may therefore benefit from its early consideration. Short-term use of CSII may also be appropriate in individual circumstances. We have experience of commencing CSII in a patient with poorly controlled cystic fibrosis-related diabetes who required surgical resection of an aspergilloma. CSII afforded the opportunity for tight perioperative glycemic control, which was crucial in the setting of invasive fungal infection.
Continuous Subcutaneous Insulin Infusion Education Program
The increasing demand for CSII places significant pressures on multidisciplinary diabetes education services. Our initial education program introduced in 2002 was derivative of that in place at Yale University in the United States, where CSII use in children and adolescents is well established. This involved a 2-night/3-day hospital admission for intensive education and adjustment of insulin infusion rates and pump settings. Over time, this program has been fine-tuned to now comprise 1.5 days of education, with close daily telephone follow-up and adjustment of rates and settings as required thereafter. Our current CSII practice is broadly in keeping with a recently published consensus statement on this topic.16 Because CSII is the most intensive form of insulin administration available, it is incumbent upon diabetes health care providers to ensure that the young person and his/her family are fully equipped to effectively manage all aspects of the pump. Patients commencing CSII at our institution receive all of their pump-related education from our diabetes nurse educators; educational support from individual pump companies is not readily available for our patients. At present, approximately two patients per week commence CSII at our institution. Although our waiting list for pump initiation is currently approximately 12 months, resource limitations in terms of diabetes nurse educator and diabetes team dietitian availability have prevented an increase in the rate of pump starts.
Preparation prior to Initiation
The CSII education process commences ∼6–8 weeks prior to the initiation date with introductory sessions for the patient and his/her family. Currently available insulin pump models and their respective features are discussed at this session. Patients are also encouraged to access related Web sites to familiarize themselves with the various pump models. Features that may influence the decision include the ability for small basal rate increments for infants or toddlers where total daily dose (TDD) is low, alarm features for missed BGL or mealtime bolus, total reservoir capacity, waterproof casing, and potential for use with other technological components such as a real-time glucose sensor. To enhance patient enthusiasm and readiness to accept CSII, we recommend that where age permits, the young person or child should make the ultimate decision regarding device selection.
At our center, children and adolescents using twice-daily insulin regimes are changed to MDI with long and rapid-acting insulin analogues in the weeks prior to CSII commencement. This serves two purposes. First, the young person will be familiar with using rapid-acting analogue pens, which will serve as their “backup” should their pump device malfunction and fail to deliver insulin. Perhaps more importantly, MDI trains the young person to think about insulin administration prior to each of their main meals, paving the way for the introduction of bolus insulin before all food and snacks on insulin pump therapy. The importance of attention to bolus dose administration has been shown in studies documenting elevated HbA1c in those who missed mealtime bolus doses.17
It is our practice to emphasize the importance of all aspects of accurate meal- and snack-time bolus administration prior to CSII initiation. Our experience is that the biggest hurdle to accurate prandial insulin dosing is inaccurate CHO and portion size estimation. All patients commencing CSII have prepump education sessions with the diabetes team dietitian for further intensive education regarding accurate CHO counting, CHO portion size estimation, and label reading for CHO content. Regular review of this process once established on CSII is critical to successful pumping. Practical interactive group workshops on CHO counting and bolus delivery have been introduced as part of our ongoing pump program.
Approximately 1 week prior to CSII initiation, a further “button-pushing” session is conducted, which gives the young person and his/her family an opportunity to familiarize themselves with their chosen insulin pump device. We have introduced a mock catheter site insertion to this session also, which has benefits, particularly for younger children, in reducing anxiety around being “attached” to a pump device.
Continuous Subcutaneous Insulin Infusion Commencement
Education sessions at the time of CSII commencement last approximately 8 hours total (duration can vary, depending on an individual's age and ability to absorb the information provided). Our practice is to divide the sessions over 2 consecutive days, as both patients and educators find that this helps minimize “information overload” in 1 day. The shorter second day offers the opportunity to revise initial pump settings and to supervise a further site insertion. Patients also meet with the team dietitian on the second day to review CHO gram counting and portion size estimation.
The education sessions focus specifically on the principles of insulin pump therapy, with particular emphasis on differences from twice-daily or MDI insulin regimes. The change to using CSII often requires families to alter their perspective with regard to diabetes management. Where individual pre- and postprandial targets are often elusive on intermittent injection regimes, these targets are realistically attainable with the intensive use of CSII. Features taught at initiation include the roles of basal and bolus insulin and the principles behind calculation and adjustment of individual dose requirements. Although initial changes to pump settings will be made in consultation with the diabetes team, we ultimately aim to empower patients to make changes themselves, based on their observed blood glucose profiles. Differences in management of “sick days” and exercise on CSII are also highlighted. The impact of administration of only rapid-acting insulin on both the management of hypoglycemia and the potential for rapid development of ketoacidosis is particularly emphasized; the use of temporary basal rates and the need for frequent blood ketone checks are also discussed. Practical issues of navigating and running the pump, site management, catheter changes, and so on are also taught and practiced.
In general terms, our policy is to commence a TDD of ∼80% of prepump TDD; this is individualized based on the patient's prepump HbA1c, adherence to previous regime, and reasons for pump initiation. At initiation, 50% of the proposed TDD is administered as basal insulin in a “flat” rate over 24 hours. This is then tailored over subsequent days and weeks based on circadian variation and glycemic response. The “500” and “100” “rules” are used for initial estimation of insulin:CHO and insulin sensitivity factor, respectively. All patients are encouraged to perform self-monitoring of blood glucose (SMBG) at least eight times/24-hour period in the days immediately after CSII commencement: blood glucose measurements 2–3 hours after meals guide fine-tuning of mealtime bolus indices, whereas overnight, fasting, and premeal checks aid in the adjustment of basal insulin rates.
Follow-up Post-CSII Initiation
Following initiation of CSII, patients make daily telephone contact with our diabetes nurse educators for 3 days. Thereafter, we suggest weekly contact (more frequent if necessary) to allow for the revision of pump settings until a stable pattern emerges. Practically speaking, the frequency and duration of contact vary across the CSII-using cohort. In general terms, frequent contact tends to diminish after 3–4 weeks, with patients seeking advice on an ad hoc or “troubleshooting” basis thereafter. Patients using CSII are seen by a physician every 3–4 months in the general diabetes clinics at our institution.
Technological Advances in Insulin Pump Therapy
In the early years of our CSII program, the insulin pump devices used by our patients did not contain bolus dose calculators, necessitating manual calculation of mealtime insulin bolus doses by the user. Bolus dose calculators minimize the potential for error in manual calculations, allow for regular corrections of elevated BGL where necessary, and help avoid insulin dose “stacking” by accounting for active insulin on board. Newer generation pump models incorporate this feature routinely and its use is now taught and encouraged from initiation of CSII in our patient group.
Although a cause-and-effect link between postprandial glycemia and the development of complications in T1DM has yet to be established, the weight of emerging evidence suggesting a link between postprandial glycemia and cardiovascular disease in healthy adults18 and diabetic subjects19 suggests that efforts to minimize postprandial glycemic excursions should also be made in T1DM. The ability to vary mealtime insulin bolus delivery based on meal composition is an exciting technological advance in recent generation insulin pump models. Evidence surrounding the use of various premeal bolus types for different foods is limited in pediatrics; however, an extended dual-wave bolus may be beneficial for foods with high fat content such as pizza.20 Optimizing postprandial glycemic control and improving the advice we offer regarding the use of different meal bolus types with varying meal composition are current research focuses at our center.
Real-time continuous glucose monitoring incorporated into insulin pump therapy (sensor-augmented pump therapy) has become available in Australia. Pilot data with the use of this system suggest that it may have benefits in terms of glycemic outcomes over a short time period in pediatric patients with T1DM.21 Experience with its use is limited to a small number of our patients, as there is currently no refund system in place for the costs associated with its sensor and transmitter components.
Medium-Term Outcomes of Patients on CSII at Our Institution
We reviewed glycemic outcomes in 148 patients with T1DM who commenced CSII at our institution prior to the end of 2006. A statistically significant reduction in HbA1c of 0.7 ± 0.1% (mean ± SEM) was seen in the first 3 months following commencement of CSII (p < 0.001). This significant improvement in glycemic control was sustained until 15 months. Thereafter mean HbA1c was similar to pre-CSII levels at both 24 and 36 months. In this patient cohort, 9 patients required 11 admissions for treatment of diabetic ketoacidosis (DKA) while on CSII. Prior to commencing CSII, none of these patients had experienced DKA since the time of diagnosis. DKA was associated with noncompliance with care and SMBG in four cases (median HbA1c 10.5%), line occlusion in four cases (median HbA1c 7.7%), and intercurrent viral infection in three cases (median HbA1c 7.2%).
Discontinuing CSII
Since our CSII program began, eight children and adolescents who commenced CSII at our center have discontinued its use. In five cases, this decision was made on the basis of ongoing suboptimal glycemic control with significant deterioration in HbA1c from prepump values attained on MDI. One adolescent girl had recurrent problematic site infections necessitating discontinuation. Two further adolescents elected to discontinue CSII to return to a simpler regime; in one such case, the young man reverted to MDI use for 6 months around the time of high school exit examinations but has since recommenced CSII for perceived improvements in QOL. Discontinuation rates at our center are lower than those reported at a large U.S. center22: however this may change with prolonged follow-up.
Conclusions and Future Projections
The significant growth in the availability and use of CSII at our center in recent years has afforded us a greater insight into the practical aspects of CSII in a pediatric age group. As borne out in our recent audit, initial improvements in glycemic control have waned over time, which may reflect diminishing patient interest and intensity of effort in their “new” regime. Mean most recent HbA1c in our CSII patients remains below the overall clinic average; however, because patients commencing CSII are more likely to be motivated than those who do not consider changing from intermittent injections, this is not entirely unexpected. Patient selection is difficult, but increasing experience has highlighted some key factors that we suggest warrant consideration in determining suitability (see Table 1). Notwithstanding the lack of sustained metabolic improvement, the low rate of discontinuation of CSII (∼5%) suggests that it is an acceptable means of insulin delivery for young people with T1DM. Optimizing the use of ongoing technological advances, such as sensor-augmented pump therapy and “advanced” mealtime bolus administration, may further improve outcomes for young people committed to improving glycemic control on CSII.
Table 1.
Targeting Patient Selection for CSII: The “Recipe” for Success
| • Realistic expectations around the intensity of insulin pump therapy and glycemic outcomes |
| • Decision to initiate CSII made by the child/young person (age permitting) |
| • Established history of regular BGL testing (minimum of the time of diagnosis. 4/day) |
| • Enthusiastic, supportive family |
| • Proficient with CHO counting willingness to commit to applying these principles |
| • Ability to master the technological requirements of the pump device or willingness of parent and teacher/childcare provider to do so |
| • Willingness for close regular contact with the diabetes team |
Abbreviations
- BGL
blood glucose level
- CHO
carbohydrate
- CSII
continuous subcutaneous insulin infusion
- DKA
diabetic ketoacidosis
- ED
eating disorder
- HbA1c
hemoglobin A1c
- MDI
multiple daily injections
- QOL
quality of life
- RCTs
randomized controlled trials
- SMBG
self-monitoring of blood glucose
- TDD
total daily dose
- T1DM
type 1 diabetes mellitus
References
- 1.Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br Med J. 1978;1:204–207. doi: 10.1136/bmj.1.6107.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300:573–578. doi: 10.1056/NEJM197903153001101. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniatis AK, Klingensmith GJ, Slover RH, Mowry CJ, Chase HP. Continuous subcutaneous insulin infusion therapy for children and adolescents: an option for routine diabetes care. Pediatrics. 2001;107:351–356. doi: 10.1542/peds.107.2.351. [DOI] [PubMed] [Google Scholar]
- 7.Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr. 2003;143:796–801. doi: 10.1067/S0022-3476(03)00579-1. [DOI] [PubMed] [Google Scholar]
- 8.Deiss D, Hartmann R, Hoeffe J, Kordonouri O. Assessment of glycemic control by continuous glucose monitoring system in 50 children with type 1 diabetes starting on insulin pump therapy. Pediatr Diabetes. 2004;5:117–121. doi: 10.1111/j.1399-543X.2004.00053.x. [DOI] [PubMed] [Google Scholar]
- 9.Nimri R, Weintrob N, Benzaquen H, Ofan R, Fayman G, Phillip M. Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics. 2006;117:2126–2131. doi: 10.1542/peds.2005-2621. [DOI] [PubMed] [Google Scholar]
- 10.Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, Lilos P, Dickerman Z, Phillip M. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics. 2003;112:559–564. doi: 10.1542/peds.112.3.559. [DOI] [PubMed] [Google Scholar]
- 11.Wilson DM, Buckingham BA, Kunselman EL, Sullivan MM, Paguntalan HU, Gitelman SE. A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care. 2005;28:15–19. doi: 10.2337/diacare.28.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A randomized controlled trial of insulin pump therapy in young children with type 1 diabetes. Diabetes Care. 2005;28:1277–1281. doi: 10.2337/diacare.28.6.1277. [DOI] [PubMed] [Google Scholar]
- 13.Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27:1554–1558. doi: 10.2337/diacare.27.7.1554. [DOI] [PubMed] [Google Scholar]
- 14.Weinzimer SA, Ahern JH, Doyle EA, Vincent MR, Dziura J, Steffen AT, Tamborlane WV. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow-up report. Pediatrics. 2004;114:1601–1605. doi: 10.1542/peds.2004-0092. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha T, Brown J, McDonnell C, Gebert R, McDougall P, Cameron F, Werther G, Zacharin M. Neonatal diabetes mellitus: Insulin pump as an alternative management strategy. J Paediatr Child Health. 2005;41:522–526. doi: 10.1111/j.1440-1754.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 16.Phillip M, Battelino T, Rodriguez H, Danne T, Kaufman F European Society for Paediatric Endocrinology; Lawson Wilkins Pediatric Endocrine Society. International Society for Pediatric Adolescent Diabetes; American Diabetes Association; European Association for the Study of Diabetes Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30:1653–1662. doi: 10.2337/dc07-9922. [DOI] [PubMed] [Google Scholar]
- 17.Burdick J, Chase HP, Slover RH, Knievel K, Scrimgeour L, Maniatis AK, Klingensmith GJ. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113:e221–4. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 18.DECODE Study Group. the European Diabetes Epidemiology Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 19.Ceriello A. The possible role of postprandial hyperglycaemia in the pathogenesis of diabetic complications. Diabetologia. 2003;46(Suppl)(1):M9–16. doi: 10.1007/s00125-002-0931-5. [DOI] [PubMed] [Google Scholar]
- 20.Chase HP, Saib SZ, MacKenzie T, Hansen MM, Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19:317–321. doi: 10.1046/j.1464-5491.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 21.Halvorson M, Carpenter S, Kaiserman K, Kaufman FR. A pilot trial in pediatrics with the sensor-augmented pump: combining real-time continuous glucose monitoring with the insulin pump. J Pediatr. 2007;150:103–105. doi: 10.1016/j.jpeds.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 22.Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care. 2006;29:2355–2360. doi: 10.2337/dc06-1141. [DOI] [PubMed] [Google Scholar]