Abstract
Background
The objective of this study was to evaluate computerized learning technology interventions that can empower patients in the self-management of diabetes and support diabetes education over a distance.
Methods
We searched Medline (1966–2006), CINAHL (1982–2006), and the Cochrane Central Register of Controlled Trials (first quarter 2007) databases. We also reviewed reference lists from included studies to identify additional studies. We included 25 articles representing 21 randomized controlled trials that evaluated a computerized learning technology and measured the outcome of patient care. We extracted patient sample, intervention, educational content topics, outcome measures, and statistical significance.
Results
Of 21 eligible trials, 18 trials (85.7%) reported significant positive outcomes. Almost 44% (43.8%) of the outcomes demonstrated significant improvements (49 of 112 outcomes).
Conclusions
Patient self-management behaviors are important in chronic disease management, and initial evidence suggests that computerized learning technology interventions can play a significant role in the future.
Keywords: computer-assisted instruction, diabetes mellitus, patient education, randomized controlled trials
Introduction
Diabetes self-management education is important in promoting health practices and in reducing risks of complications. The Behavioral Risk Factor Surveillance System survey conducted in 2001 and 2002 of 22,682 persons with type 2 diabetes found that only 52% had attended a diabetes self-management education program.1 Approximately one-third of Americans born in 2000 will develop diabetes in their lifetime.2
Studies have documented the poor health status of patients with chronic diseases when they are not adequately informed and involved in the management of their care. Patients without diabetes education are four times more likely to develop complications.3 A study focusing on diabetic eye examinations discovered that more than half of the subjects did not know that eye complications may be asymptomatic and that there are ways to lower the risk of eye problems, one-fifth did not know what type of health provider should perform an eye examination, and 17% did not know that annual eye examinations were recommended.4 Patient education plays an important role for continued good health5 and management of diabetes,6 but inadequate resources and time frequently limit the amount of education that can be provided in a face-to-face setting by qualified educators for a chronic disease such as diabetes.
Quality health care requires effective collaboration between patients and clinicians.7,8 Diabetes education is the cornerstone of effective diabetes care.9 Computerized knowledge management and education can enhance diabetes education and become an important component of quality diabetes care.10–12 Technology can assist with the provision of tailored and personalized education, feedback, and goal setting, thereby facilitating patient-centered care.
The objective of this study was to evaluate computerized learning technology interventions that can empower patients in the self-management of diabetes and support diabetes education over a distance. We systematically reviewed randomized controlled trials to evaluate the impact of computerized learning technology for persons with diabetes on health outcomes.
Methods
Data Sources
We searched Medline (1966–2006), CINAHL (1982–2006), and the Cochrane Central Register of Controlled Trials (first quarter 2007) for eligible trials using combinations of the following search terms: (1) diabetes mellitus [Medical Subject Headings (MeSH)], type 1 diabetes mellitus (MeSH), or type 2 diabetes mellitus (MeSH); (2) computer-assisted instruction (MeSH) or computer (truncated text word); and (3) randomized controlled trial (publication type). We also systematically searched the reference lists of included studies and relevant reviews.
Inclusion and Exclusion Criteria
Our inclusion criteria were any randomized controlled trial evaluating a computerized diabetes learning technology with assessment measured on patient outcomes. We excluded studies that were not randomized, had no control group, were planned studies, or were not in English.
Study Selection and Data Extraction
Two of the investigators (SAB and TLG) independently reviewed the titles and abstracts of the identified citations and applied a screening algorithm based on the inclusion and exclusion criteria described earlier. The investigators collected data from each relevant article, including patient sample, intervention, educational content topics, outcome measures, and statistical significance. The education content from all studies was organized and an education content list was developed. Each intervention included multiple topics. The investigators analyzed the articles to assess which interventions led to significant or nonsignificant results. For the purposes of this study, a trial was successful only if there was a significant outcome benefit (p < 0.05) for the intervention (computer-aided) group compared with the control group at follow-up. The investigators grouped the outcomes according to the diabetes self-management education core outcome measures continuum: learning, behavior change, clinical improvement, and improved health status.13 Satisfaction outcomes were also grouped.
Results
Comprehensive literature searches identified 89 articles (Figure 1). The titles and abstracts of these articles were read and 33 articles were determined to be potentially relevant. After reading the full articles, 8 additional articles were excluded because there was not a computerized diabetes self-management education intervention or patient outcomes were not measured. Twenty-five articles representing 21 trials met the eligibility criteria (Table 1).14–38
Figure 1.
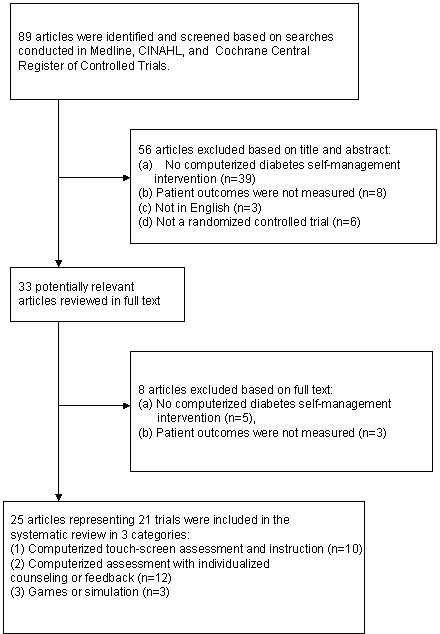
Trial flow diagram
Table 1.
Patient and Intervention Characteristics
| Trial | Diabetes type | Sample type | Sample size | Age (mean) | Gender (% males) | Study designa | Intervention descriptionb | Dose frequency | Study length (months) | Education environment |
|---|---|---|---|---|---|---|---|---|---|---|
| Barrera et al. (2002)14 | 2 | Adults | 160 | 59 | 47 | RCT | CF | Varied | 3 | Internet |
| Bloomfield et al. (1990)15 | 1 | Children | 48 | 9 | 55 | RCT | CAI | 10 sessions, 3.5 hours each | 24 | education Center |
| Brown et al. (1997)16 | 1 | Children | 59 | NA | NA | RCT | GS | 34 hours total | 6 | Home |
| Estabrooks et al. (2005)17 | 2 | Adults | 422 | 62 | 48 | RCT | CF | 30–45 minutes | 6 | Outpatient clinic |
| Gerber et al. (2005)18 | 1 and 2 | Adults | 244 | 55 | 34 | RCT | CF | 19 lessons, 10–20 minutes each | 12 | Outpatient clinic |
| Glasgow et al. (1996,1997)19,20 | 2 | Adults | 203 | 63 | 48 | RCT | CF | 1 session | 12 | Outpatient clinic |
| Glasgow et al. (2004,2005)21,22 | 2 | Adults | 886 | 63 | 47 | RCT | CF | 2 sessions, 30 minutes each | 6 | Outpatient clinic |
| Glasgow and Toobert (2003),23 Glasgow et al. (2000)24 | 2 | Adults | 320 | 60 | 43 | RCT | CAI | NR | 6 | Home |
| Graue et al. (2005)25 | 1 | Children | 101 | 14 | 53 | RCT | CF | 3 sessions, 3 hours each | 15 | Outpatient clinic |
| Levetan et al. (2002)26 | 2 | Adults | 128 | 59 | 33 | RCT | CF | 10 minutes or less per month | 6 | Home |
| Lo et al. (1996)27 | 1 and 2 | Adults | 36 | 57 | 36 | RCT | CAI | 4 sessions, 1 hour each | 3 | NR |
| McKay et al. (2001)28 | 2 | Adults | 78 | 52 | 47 | RCT | CF | Varied | 2 | Internet |
| McMahon et al. (2005)29 | 2 | Adults | 104 | 63 | 99 | RCT | CAI | 2.3 hours average | 12 | Home |
| Nebel et al. (2004)30 | 1 and 2 | Adults | 120 | NA | 27 | RCT | GS | Varied | Varied | NR |
| Sheldon (1996)31 | 1 | Adults | 13 | NA | NA | RCT | CF | NR | 3 | NR |
| Smith and Weinert (2000)32 | 2 | Adults | 30 | 47 | 0 | RCT | CF | NR | 10 | Home |
| Tatti and Lehmann (2003)33 | 1 | Children | 24 | 30 | 50 | RCT | GS | 1 lesson per week for 6 weeks | 1.5 | NR |
| Turnin et al. (1992)34 | 1 and 2 | Adults | 105 | 45 | 54 | RCT | CAI | 6 sessions per month, 15 minutes each | 12 | Home |
| Wheeler et al. (1983,1985)35,36 | 2 | Adults | 32 | 53 | 32 | RCT | CAI | 2 sessions, 30 minutes each | 1 | NR |
| Wise et al. (1986)37 | 1 and 2 | Adults | 174 | 50 | NA | RCT | CAI | NR | 5 | Outpatient clinic |
| Yeh et al. (2006)38 | 2 | Adults | 274 | 63 | 51 | RCT | CAI | Varied | 8 | Internet |
RCT, randomized controlled trial.
CAI, computerized touch-screen assessment and instruction; CF, computerized assessment with individualized counseling or feedback; GS, games or simulation; NR, not reported.
The total number of patients in the trials was 3561 (3329 adults and 232 children). Adults were subjects in 17 trials and children were subjects in 4 trials. Five trials focused on type 1 diabetes, 11 trials focused on type 2 diabetes, and 5 trials involved both type 1 and type 2 diabetes. The average trial duration was 7.7 months (range 1 to 24 months). The length varied in 1 trial.30 The dose frequency of the intervention was not reported in 4 trials23,24,31,32,37 and 4 other trials simply reported that the dose frequency varied.14,28,30,38 The number of sessions ranged from 119,20 to 1918 and the duration of the sessions ranged from 10 minutes18 to 3.5 hours.15 All trials except one provided a form of usual care to the control group. The one exception provided an attention control condition with an entertainment video game.16
Three computerized approaches were observed in these trials: computerized touch-screen assessment and instruction,15,23,24,27,29,34–38 computerized assessment with individualized counseling or feedback,14,17–22,25,26,28,31,32 and games or simulation.16,31,33 Computerized touch-screen assessment and instruction includes any trial that utilized a computer in its instruction, but did not give personalized counseling or feedback. Trials grouped into the computerized assessment with individualized counseling or feedback, however, are defined by the feedback available. Some of the trials used group visits,25 telephone follow-up,26 online tailored “personal coaches,”28 and computerized feedback and education.27,29–32,34–36 Trials included in the games or simulation category represent an educational video game with role playing,16 a simulation for hypoglycemia problem solving,30 and an interactive educational diabetes simulator that could be downloaded from the Internet.33 There were 24 diabetes education topics included in the trials (Table 2).
Table 2.
Diabetes Education Content
| Knowledge and prevention |
| 1. Understanding diabetes18,27,37,38 |
| 2. Self-care and monitoring25,38 |
| 3. Prevention and management of complications15,18,27,37,38 |
| 4. Emergencies30 |
| 5. Foot and skin hygiene18,26,37,38 |
| 6. Oral hygiene18 |
| 7. Regular eye examination18,26 |
| 8. Smoking cessation21,22 |
| Glucose level |
| 9. Blood glucose monitoring and recording16,18,25–27,29,33,37,38 |
| 10. Urine testing27,37 |
| 11. Insulin adjustment and administration16,18,27,33,37,38 |
| 12. Medication18,26,37,38 |
| Diet and activity |
| 13. Diet and nutrition15–24,26,27,31,34–38 |
| 14. Food purchasing and meal planning27,34–36 |
| 15. Exercise and physical activity17,18,21,22,26–28,31,33,34,38 |
| Management and coping |
| 16. Alcohol27 |
| 17. Goal setting17,19–26,28,35,36,38 |
| 18. Problem solving19,20,28 |
| 19. Self-motivation28 |
| 20. Social support25,32 |
| 21. Stress management18 |
| 22. Social activities15 |
| 23. Coping25 |
| 24. Traveling27,33 |
Using the definition for success described in the Methods section—significant benefits for the intervention group compared with the control group at follow-up—18 of 21 trials (85.7%) were successful. Three of the trials were not successful because they failed to show significant beneficial differences between the intervention and the control groups on any outcome measure.17,26,32 All 3 of these trials used a computerized assessment with individualized counseling or feedback intervention.
One hundred twelve outcomes were measured in the 21 trials. This was an average of 5.3 outcomes per trial. Almost 44% (43.8%) of the outcomes demonstrated significant improvements (49 of 112 outcomes).
Approximately one-half of the outcomes in studies of persons with type 2 diabetes (49.1%, 27 of 55 outcomes) or studies with a combination of persons with type 1 or type 2 diabetes (50.0%, 10 of 20 outcomes) demonstrated significant improvement. In contrast, only 32.4% (12 of 37) outcomes demonstrated significant improvement in the studies of persons with type 1 diabetes.
Among the computerized touch-screen assessment and instruction trials, 57.8% of outcomes (26 of 45) improved significantly. Fifty percent of the outcomes (5 of 10) for the games or simulation trials improved significantly. In contrast, only 31.6% (18 of 57) of outcomes of the computerized assessment with individualized counseling or feedback trials improved significantly.
Of the types of outcome measures, 10 outcomes measured learning (60.0% were improved significantly),15,16,18,27,30,35–37 28 outcomes measured behavior change (53.6% were improved significantly),16–25,28,31,32,34–36 46 outcomes measured clinical improvement (41.3% were improved significantly),15–27,29,31–37,38 and 25 outcomes measured health status (28.0% were improved significantly).14–16,18,21–25,32 In addition, 3 outcomes measured satisfaction (66.7% were improved significantly).19,20,23,24,28
Learning
The 10 learning outcomes were measured in eight different trials15,16,18,37,30,34–37 in various manners. Some were broad and categorized their outcomes under knowledge18,27,37 or learning results.30 However, others were more specific in stating that factual diabetic knowledge,15 problem-solving diabetic knowledge,15 exchange lists knowledge,35,36 recognition of foods containing concentrated carbohydrates,35,36 dietetic knowledge,34 and child diabetes knowledge test score16 were assessed.
Behavior Change
Ten trials measured 28 outcomes,16–24,31,32,34–36 of which 15 yielded significant improvements (53.6%). Seventeen of the outcomes were a measure of eating.17,19,20,23,24,34–36 Twelve of these outcomes were significant.19,20,23,24,34–36 Although many of these measured dietary activity using a self-assessment tool such as a food habits questionnaire,19,20 a 4-day food record,19,20 and one that measured “overall dietary behavior,”19,20 others drilled down to more specific measures. Three trials studies yielded significant improvements in these specific dietary measures, which included the Kristal fat and fiber behavior (FFB) fat composite,23,24 Kristal FFB fruit and vegetable scale,23,24 percent fat in diet,34 caloric excess,34 subjects with a carbohydrate deficit in diet,34 subjects with excess fat in diet,34 and fat intake.35,36 Other measures that did not yield significant change included food portioning skills,35,36 calorie consumption compliance,35,36 and the Block fat screener.23,24
Other behavioral outcomes that produced significant results include diabetes self-care rating scales,16 communication with parents about diabetes,16 and patient-centered activities completed (goal setting, eating, testing blood glucose).21,22 One trial measured moderate and vigorous activity, but did not have significant results.17 Additionally, three trials measured self-efficacy in some fashion, but none yielded significant results.16,18,23,24 Other outcomes measured that failed to show significant improvements included depression21,22,31 and coping as measured by the personal resource questionnaire.32
Clinical Improvement
Forty-six outcomes were measured in 17 trials,15,16,18–27,29,31,38 with 19 outcomes showing significant improvement (41.3 %). Hemoglobin A1c was measured in 13 of the trials,15,16,18,23–27,29,31–34,37 yielding significant improvements in only 315,18,29 (Table 3). Of the remaining trials, 5 did not demonstrate a significant difference,16,18,23–25,31,34 3 provided within-group significance but no analysis for between groups,26,27,33 and the significance level was not calculated for 2 trials.32,37
Table 3.
Hemoglobin A1c (HbA1c) Measurement
| Source | Notes | HbA1c% Intervention vs control | Change in HbA1c% Intervention vs control | Significance |
|---|---|---|---|---|
| Bloomfield et al. (1990)15 | 10.4 (1.2) vs 10.5 (1.4) | NR | p < 0.01 | |
| Brown et al. (1997)16 | 9.33 vs 8.94 | (+) 0.86 vs (+) 0.66 | NS,bp = 0.67 | |
| Gerber et al. (2005)18 | Overall | NRa | NR | NS |
| High-literacy subjects | NR | (−) 0.9 vs (−) 1.3 | NS, p = 0.548 | |
| Low-literacy subjects | NR | (−) 2.1 vs (−) 0.3 | p = 0.036 | |
| Glasgow and Toobert (2003),23 Glasgow et al. (2000)24 | NR | (−) 0.2 vs (−) 0.1 | NS | |
| Graue et al. (2005)25 | End of intervention | NR | (−) 0.35 (1.59) vs (+) 0.09 (1.19) | NS, p = 0.15 |
| 24 months after study start | NR | (−) 0.37 (1.52) vs (−) 0.08 (1.31) | NS, p = 0.15 | |
| Levetan et al. (2002)26 | 7.78 (2.22) vs 7.79 (1.91) | (−) 1.08 vs (−) 0.6 | Significant w/in group, but no between-group p value | |
| Lo et al. (1996)27 | NR | NR | Significant w/in group, but no between-group p value | |
| McMahon et al. (2005)29 | NR | (−) 1.2 (1.4) vs (−) 1.6 (1.4) | p < 0.05 | |
| Sheldon (1996)31 | NR | NR | NS | |
| Smith and Weinert (2000)32 | NR | (−) 1.6 vs (+) 1.0 | NR | |
| Tatti and Lehmann (2003)33 | 6.4 (0.7) vs 7.0 (0.8) | NR | Significant w/in group, but no between-group p value | |
| Turnin et al. (1992)34 | 10.1 (0.4) vs 11.0 (0.2) | NR | NS | |
| Wise et al. (1986)37 | IDDMc group 3 vs group 1 | 8.1 (0.4) vs 8.8 | (−) 1.2 vs (−) 0.1 | NR |
| IDDM group 4 vs group 1 | 8.6 (0.3) vs 8.8 | (−) 0.7 vs (−) 0.1 | NR | |
| NIDDMd group 3 vs group 1 | 7.9 (0.4) vs 8.5 | (−) 1.3 vs (−) 0.2 | NR | |
| NIDDM group 4 vs group 1 | 7.9 (0.6) vs 8.5 | (−) 0.8 vs (−) 0.2 | NR |
NR, not reported.
NS, not significant.
IDDM, insulin-dependent diabetes mellitus.
NIDDM, noninsulin-dependent diabetes mellitus.
Blood pressure was measured in three trials18,29,37 using five measures, but only one revealed significant change.29 Body mass index and/or weight was measured in seven trials,18–20,23,24,26,31,34–36 but with improvement in only one measure.35,36 Cholesterol was measured in five trials,19,20,23,24,29,31,38 with five of the six outcomes (83.3%) showing significant improvements.19,20,23,24,29,31,38 Hypoglycemic events were measured in two trials, with both revealing significant change.15,33 Other clinical outcomes that were measured and successfully revealed significant improvement included overall physiologic outcomes,19,20 laboratory procedures completed (blood pressure, dilated eye examination, foot examination, microalbumin),21,22 glycosylated hemoglobin levels,27 triglycerides,29 hip/waist circumference,31 and fasting blood glucose.38 However, a number of outcomes failed to show such significant improvement, including insulin dose,15 lipid ratio,23,24 lipids,26 self-monitoring blood glucose,33 and fructosamine.34
Improved Health Status
Eight trials assessed a health status measure.14–16,18,21–25,32 Seven of 25 health status outcomes were successful. One trial used the CHQ-CF87 scale, as well as the Diabetes Quality of Life (DQOL) scale to measure health status.35,36 With the CHQ-CF87 tool, however, they were able to see significant improvements only in the family activities category; they failed to see significant improvements in bodily pain, change in health, family cohesion, general behavior, general health, mental health, physical functioning, role behavioral, role emotional, role physical, and self-esteem. The DQOL scale revealed a significant improvement in impact, but not in worry. Other trials found other scales to be useful. Qualify of life was assessed using the PAID-2 instrument, but failed to show a significant improvement.21,22 However, Glasgow and colleagues23,24 were able to see significant improvements when assessing diabetes intrusiveness through the Illness Intrusiveness Scale. Smith and Weinert32 used the Psychosocial Adaptation to Illness Scale and quality of life index, but failed to reveal any significant improvements with either tool. Additionally, Barrera and colleagues14 revealed significant improvements through their use of the diabetes support scale, as well as the Interpersonal Support Evaluation List. The four remaining trials measured health status through absences from school,15 hospital admission,15 urgent visits for diabetes in the past 3 months,16 and perceived susceptibility to complications survey.18 Significant improvements were seen in absences from school,15 as well as with the perceived susceptibility to complications survey.18
Satisfaction
Although only three trials assessed patient satisfaction,19,20,23,24,28 two revealed significant improvements.19,20,28 The two that revealed significant improvements measured patient or client satisfaction broadly,19,20,28 whereas the trial that failed to show a significant improvement measured satisfaction with the program using an illness intrusiveness scale.23,24
Discussion
This systematic review analyzed computerized learning technology interventions evaluated in randomized controlled trials. Eighteen of the 21 trials (85.7%) indicated at least one outcome that was significantly better in the intervention group than in the control group. We observed a steady decrease in the percentage of significantly improved outcomes (from 60.0 to 28.0%), as the outcome measures progressed through the continuum from immediate (learning) to long term (improved health status).
Results of this systematic review should be interpreted with limitations in mind. First, only published articles written in the English language were reviewed. Second, the heterogeneity of the studies prevented a meta-analysis, which could have allowed for a more quantitative assessment. Third, the control groups were not uniform in how usual care was defined.
The cited trials studied a wide variety of interventions generalized into three approaches. Many of the trials also featured interventions with telephone follow-up, educational sessions, feedback, and other resources. It is not surprising that there was a greater success among behavioral outcomes than clinical or health status outcomes for this behavioral intervention. None of the trials in this review provided cost information and only a few provided information about utilization. Any analysis of cost or utilization would also be confounded by the poor reporting of the dose of the intervention in several of the trials. For those trials that did report the dose of the intervention, there was great variation and much can still be learned in future research about the most effective dose for computerized learning technologies.
The education content areas described in the articles are a mix of things to know (i.e., declarative knowledge) and things to do (i.e., procedural knowledge). Results indicate that the most common education content areas were diet and nutrition, exercise and physical activity, blood glucose monitoring and recording, prevention and management of complications, and goal setting. These areas are the most frequently addressed ways to control diabetes.39 Goal setting and feedback are also important patient-centered care activities for the long-term management of diabetes.17,40
The key information abstracted from the trials for this systematic review was not described consistently across all studies. A taxonomy for diabetes self-management education could facilitate the description of these behavioral, self-management, and educational types of interventions in future trial reports. As ongoing education, support, and follow-up are now included in the National Standard for Diabetes Self-Management Education guidelines,39 there will be a greater opportunity for computer-aided diabetes education to play a significant role in the future.
Acknowledgement
This research was supported by a Department of Veterans Affairs VISN 15 Research Award (S.A.B.) and by research assistance from the University of Missouri Center for Health Care Quality (T.L.G.).
Abbreviations
- DQOL
diabetes quality of life
- MeSH
Medical Subject Headings
References
- 1.Strine TW, Okoro CA, Chapman DP, Beckles GLA, Balluz L, Mokdad AH. The impact of formal diabetes education on the preventive health practices and behaviors of persons with type 2 diabetes. Prev Med. 2005;41:79–84. doi: 10.1016/j.ypmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Basic diabetes information [cited 2007 Ap 18]. Available from: http://www.diabetes.org/
- 3.Nicolucci A, Cavaliere D, Scorpiglione N, Carinci F, Capani F, Tognoni G, Benedetti MM. A comprehensive assessment of the avoidability of long-term complications of diabetes. A case-control study. SID-AMD Italian Study Group for the Implementation of the St. Vincent Declaration. Diabetes Care. 1996;19:927–933. doi: 10.2337/diacare.19.9.927. [DOI] [PubMed] [Google Scholar]
- 4.Pasagian-Macaulay A, Basch CE, Zybert P, Wylie-Rosett J. Ophthalmic knowledge and beliefs among women with diabetes. Diabetes Educ. 1997;23:433–437. doi: 10.1177/014572179702300408. [DOI] [PubMed] [Google Scholar]
- 5.Mullen PD, Simons-Morton DG, Ramirez G, Frankowski RF, Green LW, Mains DA. A meta-analysis of trials evaluating patient education and counseling for three groups of preventive health behaviors. Patient Educ Couns. 1997;32:157–173. doi: 10.1016/s0738-3991(97)00037-2. [DOI] [PubMed] [Google Scholar]
- 6.Padgett D, Mumford E, Haynes M, Carter R. Meta-analysis of the effects of educational and psychological intervention on management of diabetes mellitus. J Clin Epidemiol. 1988;41:1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine, Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 8.Wagner EH, Austin B, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 9.American Association of Diabetes Educators. Standards for outcomes measurement of diabetes self-management education. Diabetes Educ. 2003;29(5):804–816. doi: 10.1177/014572170302900510. [DOI] [PubMed] [Google Scholar]
- 10.Balas EA, Krishna S, Kretschmer RA, Cheek TR, Lobach DF, Boren SA. Computerized knowledge management in diabetes care. Med Care. 2004;42(6):610–621. doi: 10.1097/01.mlr.0000128008.12117.f8. [DOI] [PubMed] [Google Scholar]
- 11.Balas EA, Boren SA, Griffing G. Computerized management of diabetes: a synthesis of controlled trials. J Am Med Inform Assoc. 1998;5(S):295–299. [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna S, Balas EA, Spencer DC, Griffin JZ, Boren SA. Clinical trials of interactive computerized patient education: implications for family practice. J Fam Pract. 1997;45(1):25–33. [PubMed] [Google Scholar]
- 13.Mulcahy K, Maryniuk M, Peeples M, Peyrot M, Tomky D, Weaver T, Yarborough P. Diabetes self-management education core outcomes measures. Diabetes Educ. 2003;29(5):768–803. doi: 10.1177/014572170302900509. [DOI] [PubMed] [Google Scholar]
- 14.Barrera M, Glasgow RE, McKay HG, Boles SM, Feil EG. Do internet-based support interventions change perceptions of social support? An experimental trial of approaches for supporting diabetes self-management. Am J Commun Psychol. 2002;30(5):637–654. doi: 10.1023/A:1016369114780. [DOI] [PubMed] [Google Scholar]
- 15.Bloomfield S, Calder JE, Chisholm V, Kelnar CJ, Steel JM, Farquhar JW, Elton R. A project in diabetes education for children. Diabet Med. 1990;7:137–142. doi: 10.1111/j.1464-5491.1990.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown SJ, Lieberman DA, Germeny BA, Fan YC, Wilson DM, Pasta DJ. Educational video game for juvenile diabetes: results of a controlled trial. Med Inform. 1997;22(1):77–89. doi: 10.3109/14639239709089835. [DOI] [PubMed] [Google Scholar]
- 17.Estabrooks PA, Nelson CC, Xu S, King D, Bayliss EA, Gaglio B, Nutting PA, Glasgow RE. The frequency and behavioral outcomes of goal choices in the self-management of diabetes. Diabetes Educ. 2005;31(3):391–400. doi: 10.1177/0145721705276578. [DOI] [PubMed] [Google Scholar]
- 18.Gerber BS, Brodsky IG, Lawless KA, Smolin LI, Arozullah AM, Smith EV, Berbaum ML, Heckerling PS, Eiser AR. Implementation and evaluation of a low-literacy diabetes education computer multimedia application. Diabetes Care. 2005;28(7):1574–1580. doi: 10.2337/diacare.28.7.1574. [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, La Chance PA, Toobert DJ, Brown J, Hampson SE, Riddle MC. Long-term effects and costs of brief behavioural dietary intervention for patients with diabetes delivered from the medical office. Patient Educ Couns. 1997;32(3):175–184. doi: 10.1016/s0738-3991(97)00039-6. [DOI] [PubMed] [Google Scholar]
- 20.Glasgow RE, Toobert DJ, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care. 1996;19(8):835–842. doi: 10.2337/diacare.19.8.835. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, Rahm AK, Whitesides H. Randomized effectiveness trial of a computer-assisted intervention to improve diabetes care. Diabetes Care. 2005;28(1):33–39. doi: 10.2337/diacare.28.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, Rahm AK, Whitesides H, Amthauer H. A practical randomized trial to improve diabetes care. J Gen Intern Med. 2004;19(12):1167–1174. doi: 10.1111/j.1525-1497.2004.30425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasgow RE, Toobert DJ. Brief, computer-assisted diabetes dietary self-management counseling: effects on behavior, physiologic outcomes, and quality of life. Med Care. 2000;38(11):1062–1073. doi: 10.1097/00005650-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Glasgow RE, Toobert DJ, Hampson SE, Strycker LA. Implementation, generalization and long-term results of the “choosing well” diabetes self-management intervention. Patient Educ Couns. 2002;48(2):115–122. doi: 10.1016/s0738-3991(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 25.Graue M, Wentzel-Larsen T, Hanestad BR, Sovik O. Evaluation of a programme of group visits and computer-assisted consultations in the treatment of adolescents with Type 1 diabetes. Diabet Med. 2005;22(11):1522–1529. doi: 10.1111/j.1464-5491.2005.01689.x. [DOI] [PubMed] [Google Scholar]
- 26.Levetan CS, Dawn KR, Robbins DC, Ratner RE. Impact of computer-generated personalized goals on HbA(1c) Diabetes Care. 2002;25(1):2–8. doi: 10.2337/diacare.25.1.2. [DOI] [PubMed] [Google Scholar]
- 27.Lo R, Lo B, Wells E, Chard M, Hathaway J. The development and evaluation of a computer-aided diabetes education program. Aust J Adv Nurs. 1996;13:19–27. [PubMed] [Google Scholar]
- 28.McKay HG, King D, Eakin EG, Seeley JR, Glasgow RE. The diabetes network internet-based physical activity intervention: a randomized pilot study. Diabetes Care. 2001;24(8):1328–1334. doi: 10.2337/diacare.24.8.1328. [DOI] [PubMed] [Google Scholar]
- 29.McMahon GT, Gomes HE, Hickson Hohne S, Hu TM, Levine BA, Conlin PR. Web-based care management in patients with poorly controlled diabetes. Diabetes Care. 2005;28(7):1624–1629. doi: 10.2337/diacare.28.7.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nebel IT, Klemm T, Fasshauer M, Müller U, Verlohren HJ, Klaiberg A, Paschke R. Comparative analysis of conventional and an adaptive computer-based hypoglycaemia education programs. Patient Educ Couns. 2004;53(3):315–318. doi: 10.1016/j.pec.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Sheldon AW. Computer-assisted diet and exercise training in the behavioral treatment of type i diabetes [dissertation] University of Miami; 1996. [Google Scholar]
- 32.Smith L, Weinert C. Telecommunication support for rural women with diabetes. Diabetes Educ. 2000;26(4):645–655. doi: 10.1177/014572170002600412. [DOI] [PubMed] [Google Scholar]
- 33.Tatti P, Lehmann ED. A prospective randomised-controlled pilot study for evaluating the teaching utility of interactive educational diabetes simulators. Diabetes Nutr Metab. 2003;16(1):7–23. [PubMed] [Google Scholar]
- 34.Turnin MC, Beddok RH, Clottes JP, Martini PF, Abadie RG, Buisson JC, Soulé-Dupuy C, Bonneu M, Camaré R, Anton JP, et al. Telematic expert system Diabeto. New tool for diet self-monitoring for diabetic patients. Diabetes Care. 1992;15(2):204–212. doi: 10.2337/diacare.15.2.204. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler LA, Wheeler ML, Ours P, Swider C. Use of CAI/video in diabetes patient nutritional education. Proc Annu Symp Comput Appl Med Care. 1983:961–964. [Google Scholar]
- 36.Wheeler LA, Wheeler ML, Ours P, Swider C. Evaluation of computer-based diet education in persons with diabetes mellitus and limited educational background. Diabetes Care. 1985;8:537–544. doi: 10.2337/diacare.8.6.537. [DOI] [PubMed] [Google Scholar]
- 37.Wise PH, Dowlatshahi DC, Farrant S, Fromson S, Meadows KA. Effect of computer-based learning on diabetes knowledge and control. Diabetes Care. 1986;9:504–508. doi: 10.2337/diacare.9.5.504. [DOI] [PubMed] [Google Scholar]
- 38.Yeh YT, Chiu YT, Liu CT, Wu SJ, Lee TI. Development and evaluation of an integrated patient-oriented education management system for diabetes. Stud Health Technol Inform. 2006;122:172–175. [PubMed] [Google Scholar]
- 39.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 40.Bauman AE, Fardy HJ, Harris PG. Getting it right: why bother with patient-centred care? Med J Aust. 2003;179(5):253–256. doi: 10.5694/j.1326-5377.2003.tb05532.x. [DOI] [PubMed] [Google Scholar]


