Abstract
Background
DUROS® delivery technology consists of sterile, nonbiodegradable, single-use devices for continuous, subcutaneous administration of therapeutic molecules at steady rates. DUROS delivery technology is capable of delivering a wide range of therapeutic molecules for durations ranging from 3 to 12 months. Administration of therapy via DUROS devices may facilitate patient compliance with treatment since the DUROS device does not require self-injections. Consistent delivery of drug levels within a targeted therapeutic window achievable with DUROS delivery technology avoids exposure to high initial drug concentrations that can result from bolus injections and that may be associated with certain adverse drug effects.
Methods
Several approaches have been taken to assess the suitability of DUROS devices for delivery of the therapeutic molecules leuprolide acetate, glucagon-like peptide-1 (GLP-1), and omega interferon (omega IFN). Testing includes determining protein stability and measuring in vitro protein release rates.
Results
Three peptides or proteins were formulated into either a solution (leuprolide) or Intarcia's proprietary DUROS suspension formulation (GLP-1, omega IFN) and filled into DUROS devices. The devices demonstrated reliable start-up and continuous steady drug delivery in in vitro studies. Stability of the molecules was maintained for 3 years at 37°C (leuprolide), 2 years at 30°C (omega IFN), or 6 months at 37°C (GLP-1). Patients in clinical studies of a 1-year DUROS device found the device to be comfortable and convenient.
Conclusions
Multiple studies demonstrated that peptides or proteins remain stable in DUROS devices and that delivery at a steady rate can be achieved over a wide range of delivery rates.
Keywords: continuous peptide delivery, drug delivery, DUROS® delivery technology, GLP-1, omega interferon, leuprolide, Viadur® delivery device
Introduction
DUROS® delivery technology has been used in a marketed commercial pharmaceutical product (Viadur® delivery device) since March 2000. DUROS devices can be designed to deliver therapy for lengths of time ranging from several weeks to as long as 1 year. A broad array of therapeutic molecules may be delivered by the DUROS device, and it is particularly suitable for potent peptides and proteins that require chronic dosing. After the device has reached the end of its designed life span, it is removed and may be replaced with a new one. Based on its zero-order pharmacokinetics for drug delivery, DUROS delivery technology may reduce acute bolus injection-related side effects and, with increased convenience of dosing, may increase treatment compliance. Issues such as short drug half-life, frequent injections, and Cmax-related toxicities may be overcome.
The DUROS device consists of a cylindrical titanium alloy reservoir capped at one end by a controlled-rate, water-permeable membrane and capped at the other end by a diffusion moderator through which drug formulation is released from the drug reservoir. The drug formulation, piston, and osmotic engine are contained inside the cylinder. The piston separates the drug formulation from the osmotic engine and seals the osmotic engine compartment from the drug reservoir. The diffusion moderator is designed, in conjunction with the drug formulation, to prevent body fluid from entering the drug reservoir through the orifice. The external dimensions of the Viadur delivery device are 4 mm in diameter by 45 mm in length (Figure 1) but larger devices are possible.
Figure 1.
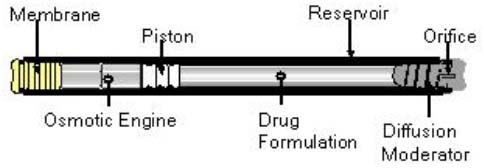
Schematic of DUROS delivery system.
The DUROS device releases a therapeutic agent at a predetermined rate based on the principle of osmosis. Extracellular fluid enters the DUROS device through a semipermeable membrane directly into a salt engine that expands to drive the piston at a slow and even delivery rate. Movement of the piston forces the drug formulation to be released through the orifice or exit port. The devices can be designed to provide an additional 10 to 14 days of continuous delivery of drug to allow scheduling flexibility for patients while avoiding interruption in drug delivery.
With a titanium outer cylinder, the DUROS device is designed to be removed quickly and safely. With calculated yield pressures of nearly 30,000 psi (based on a yield strength of 132,000 psi for the titanium alloy used), the device can withstand high pressures without structural failure. Successful start-up and continuous delivery of drug from the devices have been demonstrated in multiple animal studies and in in vitro models.
All materials used in the manufacture of components for the DUROS device have a prior history of use in long-term human implants and have passed United States Pharmacopeia 23 Class VI 50°C and/or International Organization for Standardization 10993 biocompatibility tests. All of the inactive excipients have been used in prior pharmaceutical and parenteral drug products and are compendial grade. The excipients have been shown to be compatible with the drug as well as with the components of the DUROS device.
The DUROS device can be inserted subcutaneously, for example, in the space in between the biceps and the triceps, during an in-office procedure, in as little as 5 minutes by a physician or physician's assistant.
The Viadur delivery system (the commercial device employing the DUROS delivery technology) is designed to deliver the therapeutic peptide leuprolide acetate for 12 months. New extensions of the DUROS drug delivery concept have been developed with different therapeutic agents and different delivery rates. The Viadur delivery system contains a solution formulation of leuprolide acetate dissolved in solvent, and the DUROS drug delivery platform has been expanded to include suspension formulations. These extensions make the DUROS device an even more versatile platform for delivering treatment to patients.
Experimental Methods
Delivery of three peptides or proteins is presented in this article, and they are discussed in chronological order. The DUROS device was used to stabilize and deliver the smaller peptide leuprolide acetate before expanding to glucagon-like peptide-1 (GLP-1) and the larger protein omega interferon (omega IFN). (1) Leuprolide acetate is a synthetic analog of a naturally occurring gonadotropin-releasing hormone. Leuprolide is a 9 amino acid peptide with a molecular mass of 1209 daltons.1 It is currently in the Viadur delivery system to treat prostate cancer.2 (2) GLP-1, an insulinotropic peptide, decreases serum levels of glucose and is being studied for the treatment of type II diabetes.3 GLP-1 is a 30 amino acid peptide with a molecular mass of 3298 daltons.4 (3) The third molecule is omega IFN, a recombinant form of a naturally occurring human protein with a molecular mass of 25,000 daltons. Omega IFN is an investigational drug that is being evaluated in clinical studies for the treatment of chronic hepatitis C.5–7 The injectable dosage form of omega IFN has been studied in over 200 patients with chronic hepatitis C, including a study in which omega IFN was administered in combination with ribavirin. In that study, 36% (24/67) of patients achieved a sustained viral response 24 weeks after 48 weeks of combination therapy. Omega IFN administered via the DUROS device is the subject of an ongoing phase 1b study.
In Vitro Drug Release Rate
Devices were incubated in 37°C water baths throughout the in vitro drug release rate studies. The membrane side of the device was immersed in buffer at neutral pH, and the exit port side of the device was either immersed in aqueous buffer or contained in a clean, dry vial. The buffer at the exit port side was exchanged every 7 days, or the formulation was collected from dry vials, and the protein concentration was measured by reversed-phase high-performance liquid chromatography (RP-HPLC).
Protein Stability Studies
For all three molecules, chemical stability was measured by RP-HPLC. The physical stability of omega IFN (% monomer) was measured by size-exclusion chromatography (SEC). The bioactivity of omega IFN was determined through an in vitro cytopathic effect bioassay.
Leuprolide1: Chromatography was performed using a binary gradient Waters HPLC system equipped with a HaiSil C-18 4.6 × 250-mm column. GLP-18: In the RP-HPLC analysis, a C-8 5-μm, 4.6 × 250-mm column was used. For both leuprolide and GLP-1, columns were from Higgins Analytical, and ultraviolet photodiode detection occurred at 210 nm. For omega IFN: RP-HPLC was performed using a Zorbax 300 SB-C8, 3.5-μm, 4.6 × 50-mm column (Agilent Technologies, Inc.). SEC was run using a 4-μm, 4.6 × 300-mm column (Toso Haas). The detector wave-length was 220 nm.
Results
In vitro release rate of leuprolide
Leuprolide acetate was delivered from DUROS devices that operated under in vitro conditions at 37°C, as shown in Figure 2.9 In vitro testing of the devices was performed using the experimental set-up described in the previous section to assess the overall functionality of the devices. The devices delivered leuprolide at a steady rate for the full year of testing (360 days). The devices were tested shortly after manufacturing and were tested after storage at 25°C for 3, 6, 9, 12, and 18 months. Wright et al.9 found that the release rate was approximately 130 μg/day of leuprolide, and devices stored at 25°C for up to 18 months had similar release profiles to the devices stored for shorter time periods.
Figure 2.
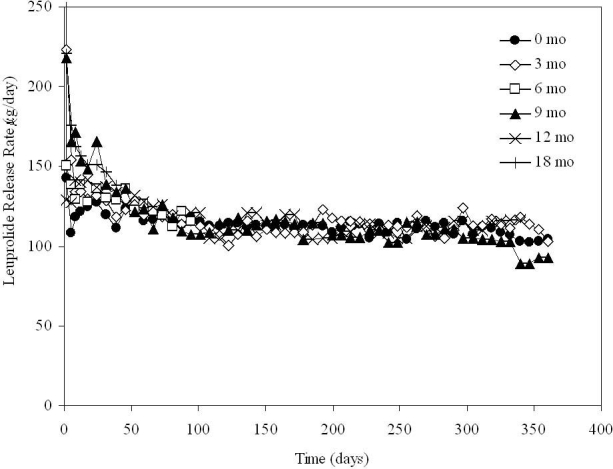
In vitro release rate of leuprolide from DUROS at 37°C. Storage occurred at 25°C for up to 18 months prior to testing. N = 6 systems per group.
Biochemical Stability of Leuprolide
Leuprolide acetate remains stable for at least 3 years when in the DUROS device solution formulation at 37°C; see Figure 3.9 Stability samples composed of 370 mg/ml leuprolide in DMSO contained at least 92% leuprolide remaining after 3 years of storage in titanium reservoirs. Three years at 37°C is significantly longer than the 1-year operating period of the Viadur delivery system.
Figure 3.
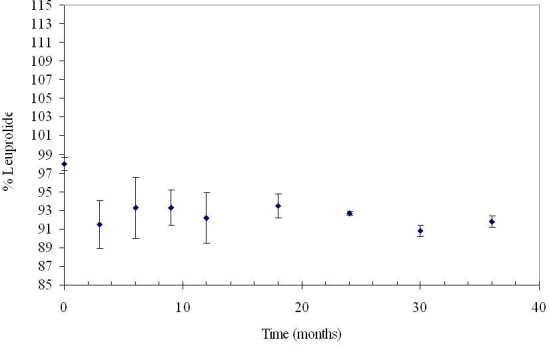
Stability of 370 mg/ml leuprolide in DMSO at 37°C for 3 years. N = 3. Error bars represent 1 standard deviation.
Patient Experience with Viadur Device
The Viadur device is implanted subcutaneously into the inner aspect of the upper arm of patients and remains there for 12 months as leuprolide is released. The experience of patients using this device with respect to effect on daily activities, health-related quality of life, and general comfort and convenience was studied by J. E. Fowler Jr.10 Eighty men being treated for prostate cancer with the Viadur delivery device were studied. Twelve months after initial implantation, 70 of 73 (96%) men who were eligible chose to proceed with reimplantation. More than 90% of patients were extremely satisfied or satisfied with the overall treatment when polled at both 24 and 52 weeks after implantation. Similarly, at 24 and 52 weeks after implantation, more than 90% of patients were only occasionally aware of or forget about the device most of the time, felt the device was very or somewhat comfortable, and felt the device was convenient.
In Vitro Release Rate of GLP-1
The release rate of GLP-1 from DUROS devices was determined under in vitro conditions by Berry et al.8 and is shown in Figure 4. The quantity of GLP-1 released was measured at days 2, 5, and 7 of device operation and then approximately weekly thereafter. After the first week of device operation, the rate of protein release was steady throughout the duration of the study (82 days). Approximately 450 μg of GLP-1 was released per day, which is consistent with the target of 480 μg per day.
Figure 4.
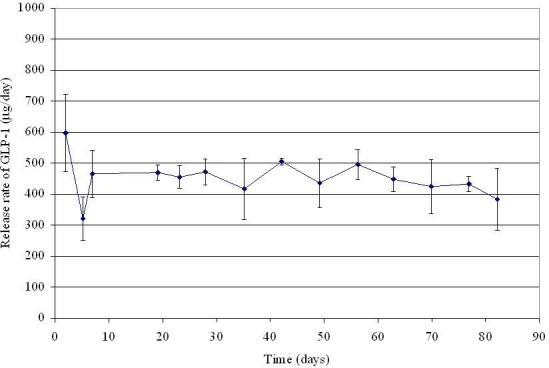
In vitro release rate of GLP-1 from DUROS devices at 37°C. N = 7 systems. Error bars represent 1 standard deviation.
Stability of GLP-1
Glucagon-like peptide-1 shows promising stability when suspended in a DUROS formulation at 37°C; see Figure 5. The same GLP-1 suspension as described earlier for in vitro release rate studies was stored at 37°C. Greater than 90% purity of GLP-1 was maintained throughout 6 months of storage.
Figure 5.
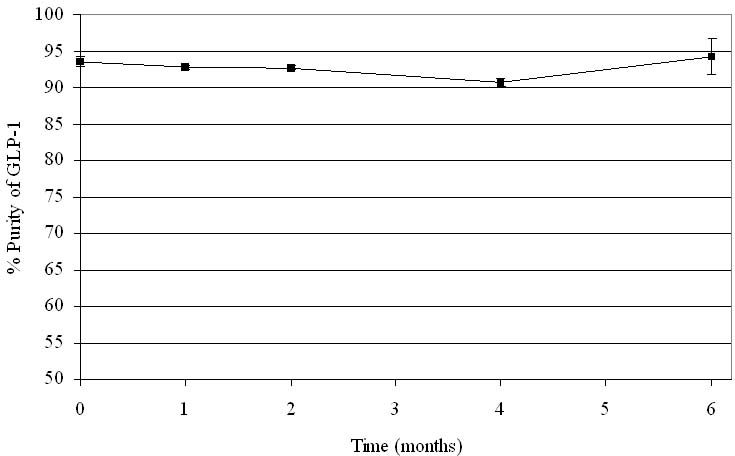
Stability of GLP-1 in suspension at 37°C for 6 months. N = 3. Error bars represent 1 standard deviation.
In Vitro Release Rate of Omega IFN
Delivery of omega IFN from DUROS devices is shown in Figures 6 and 7. We tested three groups of devices: two groups contained a protein concentration in the formulation corresponding to a target release rate of 22 μg/day, and one group contained a protein concentration in the formulation corresponding to a 9-μg/day target release rate. The two groups with the higher protein loading differed in their storage times prior to testing. One of the 22-μg/day groups was tested shortly after manufacturing, and the other 22-μg/day group was stored at 5°C for 1 year before operating.
Figure 6.
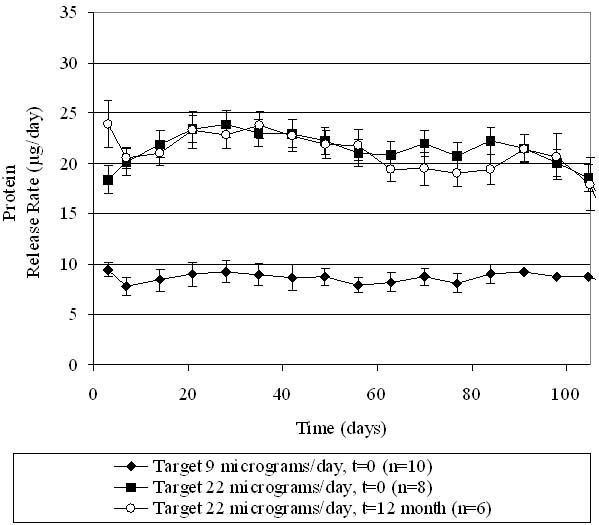
In vitro release rate of omega IFN, with protein loadings in the device corresponding to two different target drug delivery rates. The “t = 0” group was tested shortly after manufacturing, and the “t = 12 month” group was stored at 5°C for 1 year before testing. Data points represent averages, and error bars represent 1 standard deviation of data.
Figure 7.
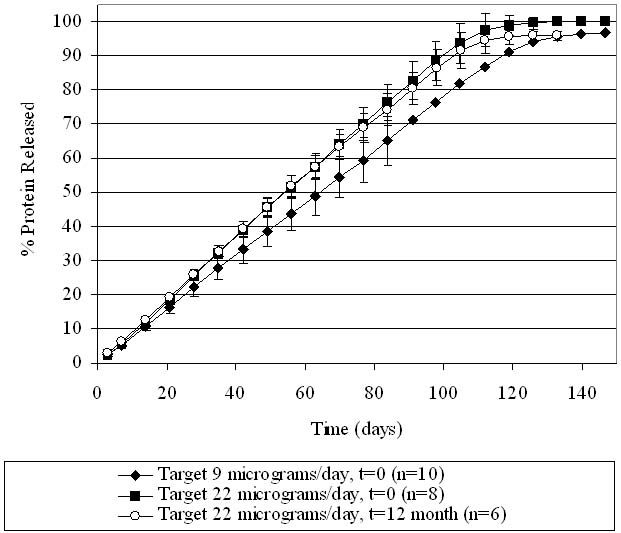
Cumulative percentage of omega IFN released. Protein loadings in the device correspond to two different target drug delivery rates. The “t = 0” group was tested shortly after manufacturing, and the “t = 12-month” group was stored at 5°C for 1 year before testing.
As seen in Figures 6 and 7, all omega IFN groups demonstrated a consistent delivery of the protein in agreement with target levels for at least 100 days. A stable delivery rate was reached after a quick start-up. The two target release rates (22 and 9 μg/day) were achieved, demonstrating that the rate of drug delivered can be adjusted based on the design of the product. The drug delivery rate was not affected by storage of the devices for 1 year at 5°C. The cumulative drug release from the devices is shown in Figure 7, demonstrating that at the end of the operational lifetime of the device, all of the drug has been released from the device. The linearity of drug release over time further demonstrates the steady release of drug from the DUROS devices.
Biochemical Stability of Omega IFN
The stability of omega IFN was monitored over a 2-year period, and results are shown in Figure 8. Levels of purity and monomer were relatively unchanged after 2 years of storage at 30°C. Throughout the 2-year period of incubation, omega IFN purity was approximately 96–97% and monomer content remained above 99.5%.
Figure 8.
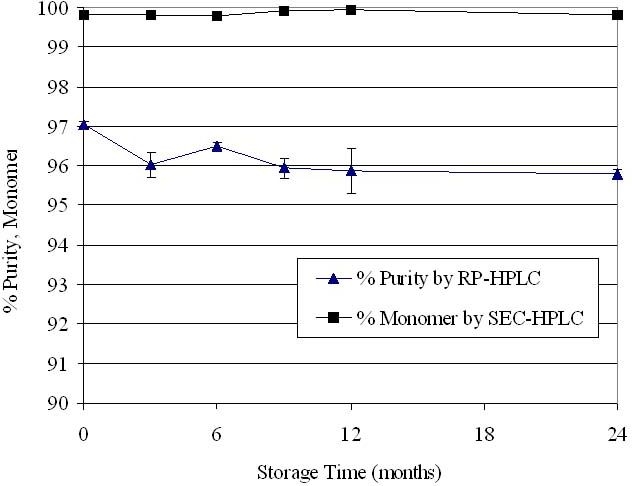
Stability of omega IFN in DUROS devices. After storage at 30°C, the protein was extracted from the formulation and analyzed. N = 3 per data point. Data points represent averages, and error bars represent 1 standard deviation of data.
Bioactivity of Omega IFN
Figure 9 shows the bioactivity of omega IFN in the incoming, bulk drug and after incorporation into the formulation and filling into a device. Over a 1-year period of incubation in the DUROS device, the bioactivity remained unchanged. The different storage temperatures of 5, 30, and 40°C did not affect bioactivity levels.
Figure 9.
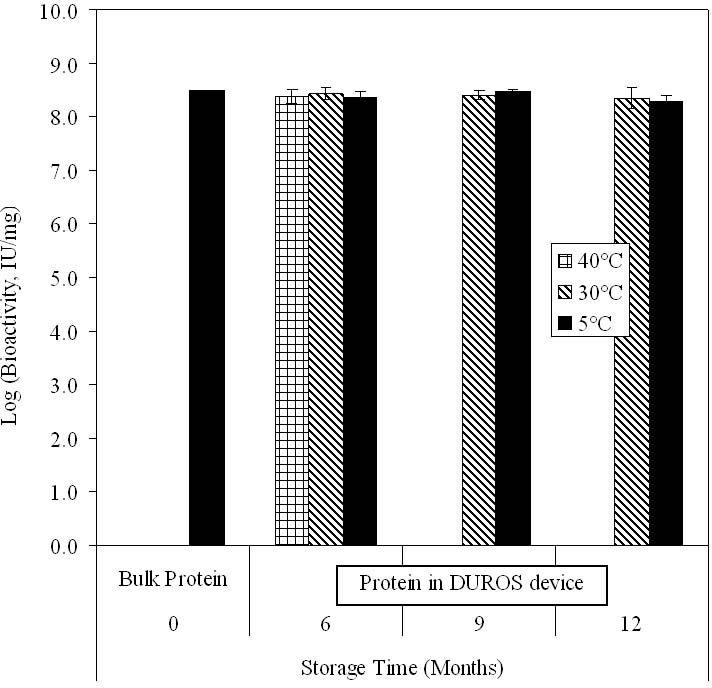
Specific activity of omega IFN in bulk form before formulating (“bulk protein”) and after formulating and storage in a DUROS device. Devices were stored at 5, 30, or 40°C. After storage, the protein was extracted from the formulation and its bioactivity was measured. Data bars represent averages, and error bars represent 1 standard deviation of data. N = 1 for measurement of bulk protein, and N = 5–10 at later time points.
Both the in vitro release rate and protein stability data of omega IFN support the conclusion that DUROS devices can be stored for at least 1 year without affecting the performance of the product.
Conclusions
The DUROS device is a subcutaneously implantable osmotic pump that has been shown to deliver uniform levels of therapeutic peptides and proteins continuously for 80 to 360 days. DUROS devices have successfully delivered therapeutic molecules as small as 1.2 kDa and as large as 25 kDa. DUROS delivery technology can also be used to deliver small molecules if the daily dosage requirements are in the appropriate range. The three examples described in this article demonstrate the suitability of DUROS devices for stabilizing and delivering therapeutic molecules.
When tested under in vitro conditions, leuprolide release rates are steady over a 1-year period and are not affected by storage of devices for up to 18 months prior to testing. Leuprolide remained stable for at least 3 years in DUROS devices at 37°C.
In an extension of the DUROS technology platform, delivery of both GLP-1 and omega IFN show potential. GLP-1 was released from DUROS devices at target levels for 80 days when tested under in vitro conditions. The stability of the GLP-1 molecule in DUROS formulations is promising, with GLP-1 purity above 90% after 6 months of storage at 37°C. Omega IFN delivery in vitro was steady and relatively unchanged after the devices were stored for 1 year prior to testing. No changes in the biochemical characteristics of omega IFN were detected after storage for 24 months at 30°C. The bioactivity of omega IFN was unchanged throughout processing and after 1 year at 30°C in the DUROS device as well as 6 months of storage at 40°C.
In all three examples of drug delivery from DUROS devices, start-up of the devices occurs rapidly, with drug delivery reaching steady state within the first few days of operation. The start-up profiles are smooth and do not contain the very high peaks typically associated with subcutaneously injected proteins.
Based on clinical studies of the Viadur delivery device, feedback from patients is favorable with respect to comfort, quality of life, and overall satisfaction with the DUROS device. These results demonstrate that the DUROS delivery system offers a convenient alternative where there is a need for daily or weekly injections for treatment of chronic diseases. In addition, the steady, adjustable delivery rate and long duration of drug stability offered by DUROS devices make it a versatile delivery platform for a range of therapeutic molecules.
Acknowledgments
DUROS® is a registered trademark of ALZA Corporation (Mountain View, CA) licensed to Intarcia Therapeutics, Inc.
Viadur® is a registered trademark of ALZA Corporation licensed to Bayer Pharmaceuticals, Inc.
The Viadur® delivery system is developed and manufactured by ALZA Corporation and marketed and distributed by Bayer HealthCare Pharmaceuticals.
Abbreviations
- GLP-1
glucagon-like peptide-1
- omega IFN
omega interferon
- RP-HPLC
reversed-phase high-performance liquid chromatography
- SEC
size-exclusion chromatography
References
- 1.Hall SC, Tan MM, Leonard JJ, Stevenson CL. Characterization and comparison of leuprolide degradation profiles in water and dimethyl sulfoxide. J Pept Res. 1999;53(4):432–441. doi: 10.1034/j.1399-3011.1999.00069.x. [DOI] [PubMed] [Google Scholar]
- 2.Fowler JE, Jr, Gottesman JE, Reid CF, Andriole GL, Jr, Soloway MS. Safety and efficacy of an implantable leuprolide delivery system in patients with advanced prostate cancer. J Urol. 2000;164(3 Pt 1):730–734. doi: 10.1097/00005392-200009010-00026. [DOI] [PubMed] [Google Scholar]
- 3.Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22(7):1137–1143. doi: 10.2337/diacare.22.7.1137. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 5.Novozhenov V, Zakharova N, Vinogradova E, Nikitin I, Gorbakov V, Yakovlev A, Pak S, Rafalski V, Bogomolov P, Alessi T, Blanchett D, Lang W, Langecker P, McNally J, McHutchison J. Phase 2 study of omega interferon alone or in combination with ribavirin in subjects with chronic hepatitis c genotype-1 infection. 42nd Annual Meeting of the European Association for the Study of the Liver (EASL).2007. [Google Scholar]
- 6.Buckwold VE, Lang W, Scribner C, Blanchett D, Alessi T, Langecker P. Safety pharmacology, toxicology and pharmacokinetic assessment of recombinant human omega interferon produced from CHO-SS cells. Basic Clin Pharmacol Toxicol. 2006;99(1):62–70. doi: 10.1111/j.1742-7843.2006.pto_365.x. [DOI] [PubMed] [Google Scholar]
- 7.Buckwold VE, Wei J, Huang Z, Huang C, Nalca A, Wells J, Russell J, Collins B, Ptak R, Lang W, Scribner C, Blanchett D, Alessi T, Langecker P. Antiviral activity of CHO-SS cell-derived human omega interferon and other human interferons against HCV RNA replicons and related viruses. Antiviral Res. 2007;73(2):118–125. doi: 10.1016/j.antiviral.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Berry SA, Fereira P, Tan M, Kang L, Dehnad H, Chen G, Ayer R, Wright J, Stevenson C. To investigate zero order delivery profiles of protein/peptide suspension formulations from osmotically driven implantable pumps. American Association Pharmaceutical Scientists 2000 Annual Meeting; October 2000; Abstract 2248. [Google Scholar]
- 9.Wright JC, Tao Leonard S, Stevenson CL, Beck JC, Chen G, Jao RM, Johnson PA, Leonard J, Skowronski RJ. An in vivo/in vitro comparison with a leuprolide osmotic implant for the treatment of prostate cancer. J Control Release. 2001;75(1-2):1–10. doi: 10.1016/s0168-3659(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 10.Fowler JE, Jr Viadur Study Group. Patient-reported experience with the Viadur 12-month leuprolide implant for prostate cancer. Urology. 2001;58(3):430–434. doi: 10.1016/s0090-4295(01)01192-x. [DOI] [PubMed] [Google Scholar]


