Abstract
Background
Advances in insulin pen technology continue to improve the usability of these devices for patients with diabetes. In this study, ergonomic features and injection force, as measured by glide force (GF) and glide force variability (GFV), were evaluated for the new Humalog® Mix75/25 KwikPen™ (KwikPen) and compared with the NovoLog® Mix 70/30 FlexPen® (FlexPen).
Methods
Fifty prefilled insulin pen devices (25 of each type) were measured for diameter at the cartridge holder and dose window, length and weight with cap attached, and thumb reach at 30 and 60 units. GF was also determined for 100 devices (50 of each type); GFV at 30 and 60 unit doses was calculated for the plateau portion of the force curve based on the minimum and maximum force measured in that portion of the curve.
Results
While FlexPen was lighter in weight than KwikPen, and presented a slightly smaller diameter at the cartridge holder and dose window, KwikPen had a shorter overall pen length compared to FlexPen, with a shorter thumb reach at both the 30- and 60-unit dose settings. The maximum GF for KwikPen was less than FlexPen at both the 30-unit (3.42 vs 5.36 lb, p <0.0001) and 60-unit doses (3.61 vs 5.62, p <0.0001). KwikPen GFV was lower across both doses (mean difference: −0.46 lb at 30 units, −0.44 lb at 60 units; p <0.0001 for both).
Conclusions
While FlexPen was lighter with a slightly smaller cartridge holder and dose window diameter, KwikPen was shorter in length with less thumb reach than FlexPen. KwikPen also demonstrated lower GF and GFV, resulting in a smoother injection profile than FlexPen. These features of KwikPen's design and function may offer important advantages for the user during insulin administration.
Keywords: ergonomic, glide force, injection force, insulin pen
Introduction
Since it was introduced in 1985, the “insulin pen” has been shown in most studies to be a viable and preferred alternative to the vial/syringe injection method of insulin delivery, contributing broadly to diabetes management.1–4 Technological advancements in insulin pen devices continue to provide design improvements that take into consideration factors such as patient lifestyle, age, preference, and potential physical limitations (i.e., limited joint mobility and neuropathology).5–9 In addition to industrial design features that affect ergonomic functionality, clinical experience and recent empirical data have demonstrated that the overall insulin pen delivery system (comprising internal design and mechanics) strongly influence injection force dynamics—objective measures of the relative smoothness and physical effort required for the injection process—and thus the experience of the patient during insulin administration.5,10,11
In this study, we evaluated a select set of ergonomic and injection force characteristics of a new, prefilled insulin pen, Humalog® Mix75/25 KwikPen™ (KwikPen) (Trademark of Eli Lilly, Indianapolis, IN), and a currently available prefilled pen, NovoLog® Mix 70/30 FlexPen® (FlexPen) (Tademark of Novo Nordisk, Princeton, NJ) (Humalog® Mix25 KwikPen™ and NovoMix® 30 FlexPen®, respectively, outside of the United States).
Methods and Study Design
This study involved ergonomic and force-measurement evaluations of KwikPen and FlexPen prefilled insulin devices. Respective devices tested are presented in Figure 1.
Figure 1.
Photographs of (A) KwikPen and (B) FlexPen prefilled insulin devices.
Ergonomic Testing
Ergonomic testing of 25 KwikPen devices (Lot no. A414346) and 25 FlexPen (Lot nos. SP51812, SP52026, TP50862, TP51077, TP51003, and SP51441) was performed by Eli Lilly and Company Pharmaceutical Delivery Systems (Indianapolis, IN). An optical comparator was used to measure overall length. A digital caliper was used to measure the diameter of the pen near the midpoint of the cartridge holder, the diameter of the pen at the dose window, and the thumb reach of the pen when the dose knob was dialed out to 30 units and 60 units. Weight of the full pen device containing 3 ml of insulin with the cap attached was measured by a digital scale.
Injection Force Evaluation
Injection force (as measured by glide force and glide force variability in this study) is the peak force attained when pushing the dose knob of a pen device during injection. As injection force increases, a user would need to exert proportionately greater effort in self administering a prescribed dose. Glide force variability is the variation of force during steady-state dosing. In this study, the glide force variability measurement is used to quantify “smoothness” of injection as a means of translating a technical measure into clinical application.
Fifty KwikPen devices containing Humalog® Mix75/25 [75% insulin lispro protamine suspension and 25% insulin lispro injection (rDNA origin)] were compared with 50 FlexPen devices containing NovoLog® Mix 70/30 [70% insulin aspart protamine suspension and 30% insulin aspart injection (rDNA origin)] for injection force characteristics (mean plateau, maximum-dose glide force, and maximal/minimal glide force variation throughout the injection stroke) at 30- and 60-unit dose sizes. Pens were fitted with needles consistent with the device manufacturer's label—Becton Dickinson 31G needles (Franklin Lake, NJ) for the KwikPen devices, and NovoFine® (Novo Nordisk) 31G needles for the FlexPen devices.
Under ambient conditions (23 ± 5 °C), all pen devices were primed to a stream and then inserted into a Zwick TC-FR2.5TS Test System (Zwick testXpert 11.02 software, Kennesaw, GA) with a 100 N load cell zeroed prior to commencing each dose, using procedure PDS2078-AC (Operation of the Zwick Test System), as a reference.12 Dosing speed was set such that each pen would deliver the dose at 10 units/s into the air. Force was applied to the dose knob until the force reached a fixed value, to ensure that the full stroke of the device was utilized for each dose setting. Injection force was measured for the duration of the dosing, generating force curves for each dose. Each pen was dosed three times at 30 units and three times at 60 units, using a randomized dosing sequence. In addition to capturing the peak and average values in the plateau region of the force curve, variability (i.e., the maximal and minimal force variations at the plateau portion of the curve) of the force curves for each dose setting was determined using the Zwick software.
Statistical Methods
To determine whether the mean injection forces generated from the KwikPen and FlexPen testing were significantly different from each other, data were analyzed using SAS JMP 5.1.1 (SAS Institute, Cary, NC). Data were evaluated for normality, and an analysis-of-variance (ANOVA) was performed. If the test for homogeneity of variances did not pass at the 95% confidence level, the Welch ANOVA test was applied for unequal variances between groups. If the test passed for equal variances, the Student's t-test was used. In all cases, both the Welch ANOVA test and Student's t-test were indicated to test for the robustness of the analysis.
Results
Ergonomic Evaluation
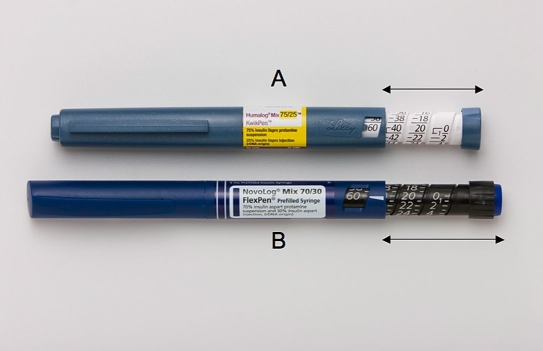
Laboratory measurements demonstrated that KwikPen devices, compared to FlexPen devices, averaged 0.54 inches shorter (5.69 vs 6.23 in, respectively) and 7.14 g heavier (31.12 vs 23.98 g), with a slightly greater diameter across the cartridge holder (0.56 vs 0.51 in) and dose window (0.66 vs 0.60 in). In addition, the “thumb reach” distance required for activating the dose knob, with the pen device dialed out to 30 or 60 dosage units (Figure 2), was notably less for KwikPen relative to FlexPen (at 30 units: 0.95 vs 1.20 in; at 60 units: 1.50 vs 1.83 in).
Figure 2.
Photographs of thumb reach at 60 units: (A) KwikPen and (B) FlexPen. Arrows indicate distance measured.
Injection Force Testing
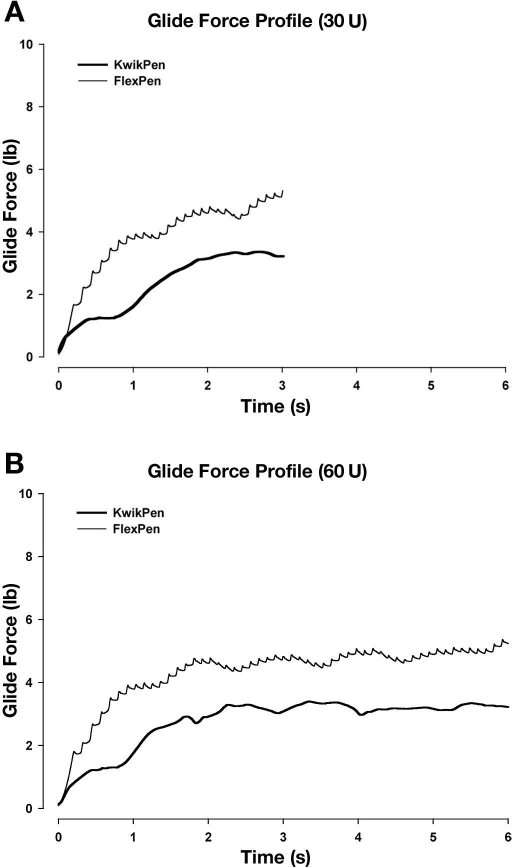
Across both dose sizes tested, KwikPen demonstrated a lower maximum glide force (mean difference: −1.94 lb at 30 units, −2.01 lb at 60 units; p <0.0001 for both), as well as a lower average glide force (mean difference: −1.64 lb at 30 units, −1.70 lb at 60 units; p <0.0001 for both). Glide force variability was also significantly lower with KwikPen devices compared to FlexPen devices across both doses (mean difference: −0.46 lb at 30 units, −0.44 lb at 60 units; p <0.0001 for both) (Table 1). Figure 3 provides a graphical representation of glide force variability with respect to time (force fluctuations exhibited over the course of the injection process), wherein it is demonstrated that KwikPen achieved and sustained a lower and smoother (less variable) injection-curve profile for the 30- and 60- unit insulin dose tests.
Table 1.
Comparison of KwikPen and FlexPen Injection Force Characteristics
| 30-unit dosea | p value | 60-unit dosea | p value | |||
|---|---|---|---|---|---|---|
| KwikPen | FlexPen | KwikPen | FlexPen | |||
| Maximum glide force (lb) | 3.42 ± 0.35 | 5.36 ± 0.76 | p <0.0001 | 3.61 ± 0.40 | 5.62 ± 0.77 | p <0.0001 |
| Average glide force (lb) | 3.31 ± 0.35 | 4.95 ± 0.65 | p <0.0001 | 3.39 ± 0.38 | 5.08 ± 0.66 | p <0.0001 |
| Glide force variability at plateau curve (lb) | 0.30 ± 0.06 | 0.76 ± 0.32 | p <0.0001 | 0.57 ± 0.15 | 1.01 ± 0.42 | p <0.0001 |
Data represent the mean ± standard deviation.
Figure 3.
Comparison of glide force profiles for a representative KwikPen and FlexPen at (A) 30 units and (B) 60 units.
Discussion
KwikPen is a new prefilled (3 ml) insulin pen approved in the United States (2007) for the administration of Humalog® (insulin lispro injection (rDNA origin)), Humalog® Mix75/25, and Humalog® Mix50/50 (50% insulin lispro protamine suspension, 50% insulin lispro injection (rDNA origin)). In this study, FlexPen was found to be lighter in weight and smaller in diameter at the cartridge holder and dose window than KwikPen. The KwikPen device was more compact, being shorter in overall length, and provided a shorter thumb reach to activate the injection mechanism than FlexPen. Additionally, injection force analysis demonstrated that KwikPen required approximately 36% less effort to eject the specified doses of insulin (mean maximum glide force), while providing for a smoother, less variable delivery throughout the injection stroke (44% less at 30 units, and 60% less at 60 units).
Although insulin pen device studies reporting injection force are limited, this dynamic of insulin delivery performance appears to vary widely across insulin pens and positively impacts patient satisfaction and the likelihood of continued insulin pen usage—with patients preferring devices with lower injection forces.5,6,10,13 Several factors that influence injection force dynamics have been reported, including plunger mechanism differences, a device's internal frictional properties, and injection needle characteristics, however, the primary concern for a patient, at a practical performance level, is the overall effect of a pen's delivery system on the injection force required to deliver a desired dose.5,10
Conclusions
In summary, the ergonomic design, glide force, and glide force variability characteristics of KwikPen may offer important insulin administration advantages and improve the injection experience in patients with diabetes. Further research to evaluate the potential clinical significance of the KwikPen design features is warranted.
Acknowledgments
The authors would like to express their appreciation to Tom Miyakawa for assistance with the statistical data analysis and to Jeffrey Gates for assistance with the development of the manuscript.
Abbreviations
- ANOVA
analysis-of-variance
- GF
glide force
- GFV
glide force variability
- rDNA
recombinant DNA
References
- 1.Cobden D, Lee WC, Balu S, Joshi AV, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(7):948–962. doi: 10.1592/phco.27.7.948. [DOI] [PubMed] [Google Scholar]
- 2.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27(10):2495–2497. doi: 10.2337/diacare.27.10.2495. [DOI] [PubMed] [Google Scholar]
- 4.Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28(3):173–177. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 5.Clarke A, Spollett G. Dose accuracy and injection force dynamics of a novel disposable insulin pen. Expert Opin Drug Deliv. 2007;4(2):165–174. doi: 10.1517/17425247.4.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Haak T, Edelman S, Walter C, Lecointre B, Spollett G. Comparison of usability and patient preference for the new disposable insulin device Solostar versus Flexpen, Lilly disposable pen, and a prototype pen: an open-label study. Clin Ther. 2007;29(4):650–660. doi: 10.1016/j.clinthera.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Thurman JE. Insulin pen injection devices for management of patients with type 2 diabetes: considerations based on an endocrinologist's practical experience in the United States. Endocr Pract. 2007;13(6):672–678. doi: 10.4158/EP.13.6.672. [DOI] [PubMed] [Google Scholar]
- 8.Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Da Costa S, Brackenridge B, Hicks D. A comparison of insulin pen use in the United States and the United Kingdom. Diabetes Educ. 2002;28(1):52–56. 59–60. doi: 10.1177/014572170202800106. [DOI] [PubMed] [Google Scholar]
- 10.Toraishi K, Yuizono Y, Nakamura N, Kato S, Aoki T, Ashida K, Sako Y. Force requirements and insulin delivery profiles of four injection devices. Diabetes Technol Ther. 2005;7(4):629–635. doi: 10.1089/dia.2005.7.629. [DOI] [PubMed] [Google Scholar]
- 11.Liepmann D. Analysis: Actuation force requirements for glucose pens. Diabetes Technol Ther. 2005;7(4):636–637. doi: 10.1089/dia.2005.7.636. [DOI] [PubMed] [Google Scholar]
- 12.Zwick Roell Instruction Manual for Materials Testing Machines, TC-FR2-5TS-D09. 2005. Oct,
- 13.Martin JM, Llewelyn JA, Ristic S, Bates PC. Acceptability and safety of a new 3.0 ml re-usable insulin pen (HumaPen) in clinical use. Diabetes Nutr Metab. 1999;12(5):306–309. [PubMed] [Google Scholar]