Abstract
Introduction
Hyperglycemia during critical illness is common, and intravenous insulin therapy (IIT) to normalize blood glucose improves outcomes in selected populations. Methods differ widely in complexity, insulin dosing approaches, efficacy, and rates of hypoglycemia. We developed a simple bedside-computerized decision support protocol (eProtocol-insulin) that yields promising results in the development center. We examined the effectiveness and safety of this tool in six adult and five pediatric intensive care units (ICUs) in other centers.
Methods
We required attending physicians of eligible patients to independently intend to use intravenous insulin to normalize blood glucose. We used eProtocol-insulin for glucose control for a duration determined by the clinical caregivers. Adults had an anticipated length of stay of 3 or more days. In pediatric ICUs, we also required support or intended support with mechanical ventilation for greater than 24 hours or with a vasoactive infusion. We recorded all instances in which eProtocol-insulin instructions were not accepted and all blood glucose values. An independent data safety and monitoring board monitored study results and subject safety. Bedside nurses were selected randomly to complete a paper survey describing their perceptions of quality of care and workload related to eProtocol-insulin use.
Results
Clinicians accepted 93% of eProtocol-insulin instructions (11,773/12,645) in 100 adult and 48 pediatric subjects. Forty-eight percent of glucose values were in the target range. Both of these results met a priori-defined efficacy thresholds. Only 0.18% of glucose values were ≤40 mg/dl. This is lower than values reported in prior IIT studies. Although nurses reported eProtocol-insulin required as much work as managing a mechanical ventilator, most nurses felt eProtocol-insulin had a low impact on their ability to complete non-IIT nursing activities.
Conclusions
A multicenter validation demonstrated that eProtocol-insulin is a valid, exportable tool that can assist clinicians in achieving control of glucose in critically ill adults and children.
Keywords: computerized decision support, critical care, glucose control, intensive insulin therapy
Introduction
A large single-center randomized trial of intravenous insulin with the goal to control blood glucose within the normal range (so-called intensive insulin therapy, IIT) reported reduced mortality in critically ill patients in a cardiac surgical intensive care unit (ICU).1 The beneficial effect on mortality was largest in the subset of patients with an ICU stay over 5 days (9% mortality reduction). However, subsequently studied medical ICU subjects demonstrated a benefit only in the subset with ICU stays of more than 3 days.2 Reported reductions in the development of acute kidney injury and neuromyopathy, as well as a strong pathophysiologic rationale for benefit, led the American College of Endocrinologists to recommend IIT.3,4 However, two more recent trials failed to confirm these initial findings.5,6
A recent review of published efforts to normalize blood glucose reported wide variations in insulin dosing approaches.7 Most of the protocols were implemented by ICU nurses, were complex, and required blood glucose monitoring every 30–60 minutes.7 Disparate rates of hypoglycemia have been reported from randomized trials in which 3 to 18% of patients in the IIT arms experienced one or more blood glucose values below or equal to 40 mg/dl.1,2,5,8 Some protocols imposed substantial burdens on nurses.10 Thus a safe, easy to use, and effective insulin protocol is needed for both research and clinical care.
Computer-based decision support tools can improve the conduct of complex clinical interventions.11–13 We developed a computerized-based decision support tool for conduction IIT (eProtocol-insulin) in critically ill patients at LDS Hospital in Salt Lake City, Utah, beginning in 2001.14 Our clinician compliance (acceptance of eProtocol-insulin instructions) at LDS Hospital was 93% (standard deviation = 5.6%). We successfully exported this tool for quality improvement purposes to adult ICUs in other hospitals.11 We now report results of a multicenter validation of this tool in adult and pediatric ICUs. We set thresholds for the proportion of protocol instructions that must be acceptable to clinicians, the proportion of blood glucose values in the target range, and the frequency of hypoglycemia that had to be met in order to consider the tool validated. In addition, we evaluated the impact of the protocol on nurse perceptions of quality of care and workload burden.
Methods
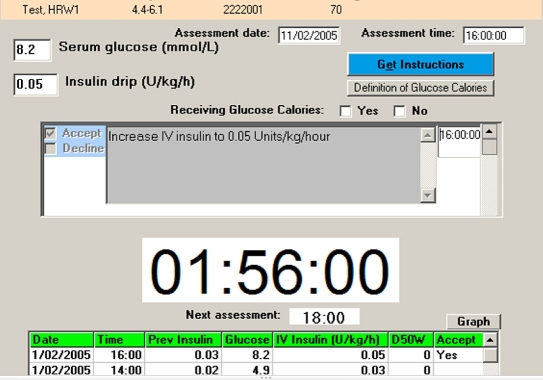
eProtocol-insulin is an open loop decision support system that runs on a laptop computer at the patient's bedside (see Appendix I). A bedside clinician uses a single computer screen to enter the blood glucose and receive an instruction that, if accepted, initiates a countdown timer for the next scheduled glucose measurement. eProtocol-insulin targets a blood glucose of 80–110 mg/dl. eProtocol-insulin had been iteratively refined by clinician–investigators with extensive experience with the development and implementation of computer-based decision support tools.12,13 After entering a blood glucose value, a bedside clinician receives an insulin infusion rate instruction. In the event of hypoglycemia, eProtocol-insulin generates instructions to discontinue insulin and administer intravenous glucose adjusted for patient weight. The clinician may accept or decline the instruction and enter an alternative treatment based on clinical experience or specific characteristics of a patient. If the instruction is declined, the clinician enters a reason captured by eProtocol-insulin. The computer then displays a digital timer for the next recommended glucose measurement. In most instances, the recommended interval between blood glucose measurements is 2 hours. Figure 1 shows an example of the single screen view for eProtocol-insulin. As part of the National Institutes of Health Roadmap initiative to improve clinical research, we assembled a multidisciplinary group of investigators to validate eProtocol-insulin and assess its exportability to other clinical environments (see Appendix II).
Figure 1.
eProtocol-insulin screen. Single screen displayed to the bedside intensive care unit clinician (usually a nurse). The clinician enters patient-specific data [current blood glucose measurement (mg/dl or mmol/liter) and whether the patient is receiving at least 50% of predicted caloric intake]. eProtocol-insulin generates a patient-specific recommendation with a white background that turns gray when the clinician checks the “Accept” box, at which time the recommended insulin infusion rate is automatically entered in the “Insulin drip” box [Units/kg/h for children (as shown) and Units/h for adults]. A large countdown timer starts and indicates the time remaining until the next eProtocol-insulin mandated blood glucose measurement (1 hour, 56 minutes, and 0 second in the illustration). Past data are listed in the table on the bottom of the display (only the first two lines of the table are shown for clarity).
We conducted a multicenter study in six adult and five pediatric ICUs. Laptop computers with eProtocol-insulin were distributed to study sites. Study team members received limited, teleconference Web-based training. Study personnel then trained their local ICU nurses.
We required attending physicians of eligible patients to independently intend to use intravenous insulin to normalize blood glucose. We used eProtocol-insulin for glucose control for a duration determined by the clinical caregivers. In pediatric ICUs, we also required support or intended support either with mechanical ventilation (including noninvasive ventilation) for greater than 24 hours or with a vasocative infusion. In adult ICUs, we required an anticipated length of stay of 3 or more days.
We excluded pregnant patients, patients younger than 1 month, and patients with inborn errors of metabolism likely to affect glucose control, acute or chronic liver disease with a prior episode of glucose less than 60 mg/dl, or diabetic ketoacidosis. After informed consent and the collection of limited, deidentified baseline demographic data, patients were managed with eProtocol-insulin for a duration that was determined by the patient's ICU clinical care team. We relied on usual care methods for the measurement of blood glucose.
We used the percentage of accepted instructions as our primary efficacy measure and the percentage of blood glucose values ≤40 mg/d as our primary safety measure. To consider the eProtocol-insulin validated, all three of the following a priori criteria must have been met:
Clinician compliance with insulin dose instructions must exceed 90% (p = 0.05 one sided)
Percentage of glucose values in the normal 70- to 110-mg/dl range must be equivalent to the prior LDS experience of 55%
Percentage of all glucose values ≤40 mg/dl must be less than 0.5% (p = 0.05 one sided)
We originally chose a sample size of 200 patients (100 adults and 100 children) to obtain a multicenter estimate of the percentage of eProtocol-insulin instructions accepted by bedside clinicians. We subsequently reduced the pediatric sample to 50 subjects due to slow enrollment. Based on an analysis of the hypoglycemia rates in our pilot studies, with an estimated 7500 glucose determinations in 150 patients, we had 90% power to demonstrate that the observed hypoglycemia rates were less than our safety-stopping threshold of 0.5% of glucose values. Because low values are often repeated immediately before or after treatment, we considered multiple low blood glucose values within 30 minutes to represent one episode of hypoglycemia. The range of 70–110 mg/dl was chosen for our efficacy measure as it more closely represents the normal fasting range for blood glucose. The lower bound of the target range for eProtocol-insulin (80 mg/dl) was set higher than the lower bound of normal as an added safety measure.
The Glycemic Control Nurse Questionnaire was distributed randomly to bedside nurses who completed the paper survey at the end of their shift. We targeted nurses during the day shift and no attempt was made to control for nurse:patient ratio, prior experience with eProtocol-insulin, or the number of patients assigned to the survey nurses who were on the protocol. The questionnaire was adapted from a survey instrument used by Ingle and colleagues to assess nursing perceptions of a paper and computerized protocols.15 In addition to years of ICU nursing experience, survey elements included a workload comparison of eProtocol-insulin use burden to that of common clinical workloads, such as mechanical ventilation, usability of eProtocol-insulin, and perceived impact of eProtocol-insulin on the quality of care of all the patients assigned to the nurse. The workload associated with eProtocol-insulin was compared to five common intensive care unit interventions.16,17
We calculated the mean and standard deviation (SD) of patient age and the percentages of various dichotomous and categorical outcomes for variables reported in Tables 1–3. We made comparisons using a two-sided t test for age and a two-sided χ2 test for the categorical outcomes. P values of less than 0.05 were considered statistically significant.
Table 1.
Baseline Demographics
| Adult (n = 100) | Pediatric (n = 48) | |
|---|---|---|
| Age | 56.9 ± 18.6 | 10.2 ± 5.9 |
| Gender | 46% female | 41% female |
| Type of ICU | ||
| Medical intensive care unit | 80% | NA |
| Pediatric intensive care unit | NA | 100% |
| Burn/trauma | 20% | 0 |
| Primary admitting category | ||
| Pulmonary | 51% | 52% |
| Neurological | 13% | 11% |
| Cardiovascular | 13% | 11% |
| Gastrointestinal | 5% | 0% |
| Multisystem | 16% | 21% |
| None identified | 2% | 5% |
| Comorbidities | ||
| Diabetes mellitus | 6% | 6.8% |
| Chronic renal failure | 12% | 2.2% |
Table 3.
Safety of eProtocol-Insulin
| Adult | Pediatric | Total | |
|---|---|---|---|
| Total glucose measurements | 8269 | 4617 | 12,886 |
| Measurements ≤60 (%) | 1.6% | 1.1% | 1.42% |
| Measurements ≤40 (%) | 0.10% | 0.32% | 0.18% |
| Patients with BGa ≤40 | 7/97 (7%) | 9/46 (20%) | 16/143 (11%) |
| Reasonb for BG ≤40 | |||
| Interruption of calorie source | 4 | 4 | 4 |
| Withdrawal of corticosteroids | 0 | 2 | 2 |
| Withdrawal of vasopressors | 0 | 0 | 0 |
| Interruption/withdrawal of renal replacement therapy | 0 | 0 | 0 |
| No reason identified | 4 | 6 | 10 |
Blood glucose.
More than one reason was recorded for some events. Glucose values in mg/dl.
The study was reviewed and approved by local institutional review boards. Monthly reports of study results were reviewed by an independent data and safety monitoring board (DSMB) that met twice per year by teleconference. Further, blood glucose values ≤40 mg/dl were reported to the coordinating center within 24 hours and a detailed review of each episode was sent to the DSMB and to investigators for local institutional review boards within 7 days. The protocol stated that if the safety threshold of hypoglycemia (0.5% or more of glucose values below 40 mg/dl) was reached, then enrollment would cease pending review by the DSMB.
Results
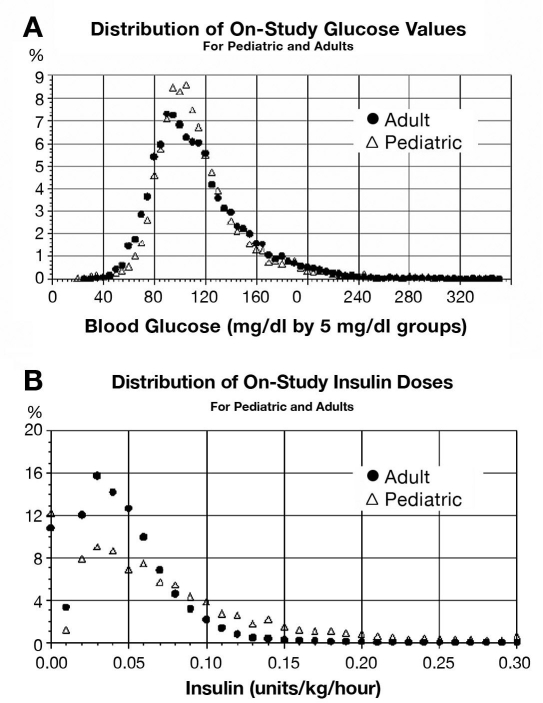
We enrolled 100 adults and 48 children between February 2006 and January 2007. The six adult centers enrolled 25, 24, 20, 20, 10, and 1 patient, respectively. The five pediatric sites enrolled 17, 13, 8, 7, and 3 patients, respectively. All trauma and burn patients were enrolled from a single site. Baseline characteristics of the subjects are summarized in Table 1. eProtocol-insulin generated a mean of 83 insulin instructions per adult and 96 per child. Adult and pediatric clinician compliances with eProtocol-insulin instructions were 95 and 91%, respectively (Table 2). Adult clinicians were usually ICU nurses, and pediatric clinicians were primarily residents. The overall clinician compliance was 93%. The mean (± SD) adult patient baseline blood glucose value was 157 ± 71 mg/dl and fell to 116 ± 38 mg/dl with eProtocol-insulin. The mean (± SD) pediatric patient baseline blood glucose value was 180 ± 87 mg/dl and fell to 118 ± 39 mg/dl with eProtocol-insulin (p < 0.0001 for both comparisons). The proportion of glucose values in the normal 70- to 110-mg/dl range was 47% for adults and 48% in children (47% overall) and fell within the confidence bounds necessary to meet this validation criterion. The distributions of blood glucose values in adults and children were similar (Figure 2).
Table 2.
Efficacy of eProtocol-Insulina
| Adult (n = 100) | Pediatric (n = 48) | Total (148) | |
|---|---|---|---|
| Total instructions | 8230 | 4603 | 12,833 |
| Instructions per patient | 83 | 96 | 87 |
| % instructions accepted (range for individual ICUs) | 95% (90–100%) | 91% (85–95%) | 93% |
| Mean baseline glucose | 157 ± 71 | 180 ± 87 | 164 ± 77 |
| Mean on-study glucose | 116 ± 38 | 118 ± 39 | 117 ± 39 |
| Mean interval between recommended glucose measurements (hours) | 1.8 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.4 |
| Mean interval between actual glucose measurements (hours) | 2.1 ± 0.8 | 1.7 ± 0.8 | 1.95 ± 0.8 |
| % glucose values 70–110 (range for individual ICUs) | 47% (37–58%) | 48% (41–52%) | 47% |
| Duration of eProtocol (hours) | 176 ± 142 | 168 ± 161 | 173 ± 148 |
| Range of eProtocol use (hours) | 12–691 | 10–688 | 10–691 |
Values are mean ± standard deviations; ranges of mean values given of the six adult and the five pediatric sites. Glucose values in mg/dl.
Figure 2.
Distributions of on-study blood glucose values (a) and insulin doses (b) are shown for adult and pediatric patients. Use of eProtocol-insulin resulted in a similar distribution of blood glucose, although the distribution of insulin infusion rates (normalized to body weight) differed between adults and children.
The percentage of glucose values ≤40 mg/dl was below the protocol-specified safety threshold of 0.5% (Table 3). Nineteen patients had one or more glucose values ≤40 mg/dl (one pediatric patient had three measurements ≤40 mg/dl in a single hypoglycemia episode). The overall rate of all glucose values of ≤40 mg/dl was 0.18%, and the rate of episodes of values less than 40 mg/dl (remeasurements within 30 minutes were counted as one episode) was 0.17%. Rates were higher in children but the reasons for hypoglycemia were similar to those in adults. In half of the hypoglycemic episodes, no reason was identified (Table 3). All the episodes were asymptomatic, without reported residua, and were reviewed by the DSMB, which recommended no changes to the protocol.
Twenty-one nurses were selected at random to complete a survey and all responded (Table 4). On average, respondents reported 6 years of ICU experience, had managed six patients with eProtocol-insulin, and thought that the eProtocol was easy to use (mean 2.6, with 0 = easy and 10 = difficult). However, over half reported that eProtocol-insulin consumed as much or more time as managing mechanical ventilation or a single vasoactive medication. Most nurses were caring for two ICU patients and reported that eProtocol-insulin had a low impact on their ability to complete other nursing activities for their patient on eProtocol-insulin or their other patient (mean 2.6; 0 = no impact, 10 = significant impact on nursing care). Finally, nurses reported that eProtocol-insulin did not impact their work-related stress.
Table 4.
Nurse Perceptions from a Random Sample of 21 ICU Nurses
| Average years working in ICU | 6 (1–30)a |
| Average number of patients managed on eProtocol insulin | 6 (1–250)a |
| As much work as: | |
| Intravenous medications | 9 |
| Mechanical vent support | 4 |
| Single vasoactive drug | 4 |
| Multiple vasoactive drug | 2 |
| Dialysis (all, e.g., peritoneal dialysis, CVVHb) | 2 |
| Dialysis: | |
| Ease of use (0 = easy, 10 = difficult) | 2.6 (0–8) |
| Impact on ability to complete non-IIT-related nursing care to the patient on eProtocol-insulin (0 = no impact, 10 = significant impact) | 4.0 (0–10) |
| Impact on work-related stress (0 = no added stress, 10 = significantly more stress) | 3.8 (0–8) |
| Impact on ability to complete non-IIT-related nursing care to other patients assigned to you today (0 = no impact, 10 = significant impact) | 2.6 (0–6) |
Median and range. All other values reported as mean and range.
Continuous venovenous hemofiltration.
Discussion
Because hypoglycemia rates with IIT insulin therapy were reported inconsistently, we reported both the percentage of all blood glucose measurements ≤40 mg/dl and the percentage of patients with at least one glucose measurement ≤40 mg/dl. eProtocol-insulin recommendations for insulin therapy were accepted by bedside clinicians over 90% of the time and led to 48% of blood glucose values falling within the normal (70–110 mg/dl) range. eProtocol-insulin was well accepted by critical care nurses, and the rates of hypoglycemic episodes compared favorably with published values. Krinsley and colleagues8 reported a rate of 0.35% of blood glucose values in a single center experience in 800 patients. In a large trial of insulin therapy in adult surgical patients, the rate of hypoglycemia was 6.2% of subjects and similar to our experience in adults (7%).1 In a subsequent report of insulin therapy in medical ICU patients, hypoglycemia was reported in 18% of patients.2
There is limited published experience with IIT in children. Although no controlled trials of insulin therapy have been reported in children, it appears that hypoglycemia with or without insulin therapy occurs in greater frequency in children. One study comparing results before and after a commitment to normalize blood glucose in pediatric burn patients revealed an increase in survival, but no difference in average blood glucose values.18 In that study, insulin therapy was associated with a 27% incidence of hypoglycemia requiring treatment (glucose value <50 mg/dl), although there was no comment regarding its impact on patient outcomes. Srinivasan and colleagues19 demonstrated hypoglycemia in 7% of children but no association with insulin therapy. Wintergerst et al.20 reported that hypoglycemia was associated with increased mortality, but that hypoglycemia was defined as glucose <65 mg/dl and its association with insulin therapy not determined.20 We surveyed pediatricians and discovered that many pediatricians have extrapolated from the adult literature and now attempt to normalize glucose values for their patients.21
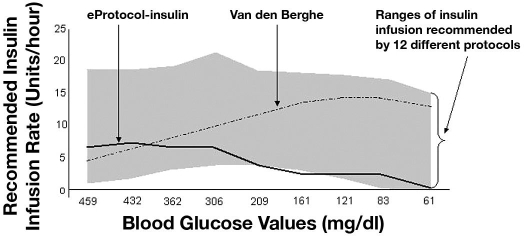
Wilson and colleagues7 reviewed 12 published insulin therapy protocols for critically ill patients and compared starting insulin doses and the method of subsequent insulin dose adjustments for the rising of falling glucose values. Most protocols used prior glucose values to titrate insulin without insulin sensitivity factors or strict titration based on caloric intake, with the latter being quite variable in critically ill patients. In a simulation based on glucose values from a patient managed with the Van den Berghe protocol, the different protocols generated subsequent 9-hour doses of insulin that varied from 27 to 115 units (mean = 67 units). The authors concluded that there is a wide variability in insulin dosing approaches and called for close attention to the choice of a protocol (Figure 3).7
Figure 3.
The range of intravenous insulin infusion rates (in units of insulin per hour) over 9 hours for 12 protocols is shown in gray. The patient was managed by the Van den Berghe protocol (dashed line). Wilson and colleagues7 then plotted the starting insulin dose and subsequent dose adjustments for a dozen published protocols to demonstrate the wide variability in insulin dosing recommendations. eProtocol-insulin values are plotted using a solid line for a 70-kg adult (initial infusion rate for a blood glucose of 459 mg/dl = 5.6 units per hour). Figure modified from Wilson et al.7
The Van den Berghe protocol delivered 115 units of insulin over 9 hours and instructed no dose reduction as glucose values approached the lower bound of the target range and only a minimal dose reduction when glucose fell from 83 to 61 mg/dl (insulin dose reduced from 15 to 14.5 units/hour). For the same blood glucose data, eProtocol-insulin instructed a total dose of 29 units and decreased the insulin dose by over 50% when the glucose value fell from 161 to 83 mg/dl (Figure 3), as did five other protocols (Figure 3). All protocols except the Van den Berghe protocol stopped insulin when the glucose value was 61 mg/dl.7
Differences in patient demographics or practice patterns in the ICUs where these protocols were developed, such as varying prevalence of diabetes or obesity or different approaches to nutrition or corticosteroid use, may explain this variation in insulin dosing regimens. If so, then protocol performance would depend on the population and clinical environment in which it is used. The Van den Berghe protocol may be one example of such a context-specific performance. This protocol was developed and refined in a cardiac surgical ICU where high rates of parenteral dextrose are infused routinely.1 When this protocol was subsequently studied twice (in three medical ICUs or in a multicenter trial of patients with severe sepsis) the rates of hypoglycemia increased from 6.2% to 18.7% and 17%, respectively.1,2,5 An additional explanation may be that in the medical ICU study, the Van den Berghe protocol was applied by clinical care nurses alone,2 without the research nurse and physician support available in the surgical ICU study.1 These two methods of application by Van den Berghe et al. may, therefore, have been different. Clinician compliance with protocol instructions may help resolve this question, but as is commonly the case, no clinician compliance data were provided.1,2,5
Critically ill patients may tolerate wide ranges of insulin doses with similar effects, which might also explain protocol variability. Alternatively, clinician oversight acts to correct deficiencies when they decline troublesome instructions. It is unclear how often the 12 protocols reviewed by Wilson et al.7 generated such instructions. Clinician compliance with protocol instructions is commonly not included in reports of protocol performance. Without clinician compliance data, it is difficult to evaluate how the protocols are actually implemented.12,13 Because each patient state and each protocol response depend on previous patient states, protocol performance is a sequentially determined outcome. Therefore, a rigorous comparison of different protocols would likely require a randomized comparison.
While eProtocol-insulin satisfied the a priori efficacy target for glucose control, a substantial number of glucose values were above the target range (Table 2, Figure 3). One potential approach for better controlling blood glucose would be to condition insulin dosing on additional patients and practice factors, such as the dose of corticosteroids and vasopressors, quantity of nutritional support, and degree of insulin resistance estimated from the preceding insulin–glucose relationships for a given patient. Such approaches are more complex and some have been published recently.22,23 One of these, a computerized protocol using an insulin resistance factor (GlucoStabilizer), achieved 73% of glucose values in the 70- to 110-mg/dl range with a mean glucose of 107 mg/dl and a frequency of glucose values less than 50 mg/dl of 0.4%. It is unclear if the ICU case mix, nutrition practices, or insulin dosing rules, including a resistance factor, account for this more favorable performance.23
We do not understand clearly how to assess the efficacy of glucose control for improving important clinical outcomes. One analysis suggested that the benefit from insulin therapy for mortality and prevention of neuromuscular weakness derived from glucose control, as opposed to insulin exposure, and described superior outcomes when glucose was normalized.3 Another report suggested superiority of the “glycemic index,” a time-weighted average of values above the normal range.24 However, an observational cohort study of 7049 critically ill patients suggested that reduced variability in blood glucose, measured by the standard deviation, was equally predictive of adverse outcomes as the mean glucose.25 We chose the proportion of values in the normal range, which approximated the goal of eProtocol-insulin per se as our primary glucose control efficacy measure. Our standard deviation of mean glucose was 38 mg/dl in adults and 39 mg/dl in children. In a large cardiac surgical ICU cohort where insulin2 therapy was particularly effective, the SD was 19 mg/dl.1 In a follow-up medical ICU study with the same protocol, the SD was 29 mg/dl overall and 25 mg/dl in the subgroup with improved outcomes (ICU length of stay >3 days). Further research is needed to determine the optimal goal for insulin therapy and to establish cause and effect.
Nursing workload is an important contributor to patient outcomes. Nurses found eProtocol-insulin intuitive and relatively easy to use with minimal formal training, although our sample was relatively small. However, over half of the nurses equated IIT with the same or greater workload as that for managing mechanical ventilation or a single vasoactive infusion. Similar to findings reported by nurses using a computerized information management system in England,15 our IIT had little impact on nonglucose control-related nursing activities. This is an important observation, as Sochalaski26 noted that 40% of the variation in nurse-perceived quality of care was associated with the number of tasks left undone at the end of a nurse's work shift.
Intensive insulin therapy requires a change in clinical behavior and resource use.15 Aragon10 observed 21 ICU nurses performing scheduled blood glucose monitoring and making adjustments in insulin therapy. Nurses spent a median of 4.67 minutes per blood work-titration cycle with more time spent when glucose monitoring supplies were not available or functioning properly. In contrast, in their survey of nurses before and after implementation of a computerized IIT system, Vogelzag and colleagues24 reported a time savings; specifically, time was saved by avoiding the need to call the physician with each blood glucose result and treatment change. Nurses reported that the time saved was then devoted to other patient care activities. Additional and more comprehensive evaluations of the impact of IIT approaches on nursing care are needed.
Conclusion
eProtocol-insulin generated clinically acceptable instructions in 93% of instances with similarly high clinician compliance in both adult and pediatric ICUs in multiple hospitals. The performance in adults mirrored that in the development hospital, demonstrating that eProtocol-insulin is an exportable decision-support tool and is a replicable method for modulating blood glucose. Published insulin dosing methods vary widely and a given method may perform differently when used outside the ICU in which it was developed. Careful evaluation of insulin dosing protocols should be undertaken before adopting externally developed protocols in ICU usual care practices or clinical research.
Acknowledgments
Participants in the\ Reengineering Critical Care Clinical Research Investigators group writing committee take full responsibility for the content of this manuscript.
We are indebted to our many nurse and physician colleagues who enabled the completion of this work. This study is registered with ClinicalTrials.gov, study number NCT00655460.
Abbreviations
- D10w
dextrose 10% in water
- D50w
dextrose 50% in water
- DSMB
data and safety monitoring board
- ICU
intensive care unit
- IIT
intensive insulin therapy
- SD
standard deviation
Appendix I: Intensive Insulin Therapy Protocol Developed by Clinicians at LDS Hospital, 2002
Goals
A specific blood glucose is targeted. Currently the JEq4 Infusion Protocol targets 95 mg/dl, but the intent is to allow this value to be variable.
Definition of Terms
δ: measured glucose – target value
Interval: time period between glucose checks
Desired rate of change: Flexible desired rate of change. Desired rate of change decreases as the blood glucose approaches the target. This concept is used to modify the insulin infusion changes based on the most recent rate of change as well as the most recent value of the blood glucose.
Mitigating factor: a factor that adjusts the desired rate of change based on δ in the equation given later
Target range: 80–110 mg/dl
Overview
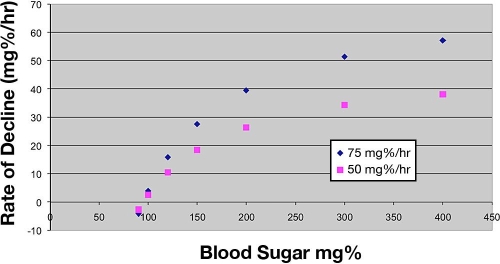
Measured glucose values are classified as above range, in range, or below range. The equation uses a specific glucose value (midpoint of the range, 95 mg/dl) as the target. The initial insulin infusion depends on the measured glucose and body weight. Subsequent infusion instructions depend on the current infusion rate, the measured glucose, and the rate of change of the measured glucose over the last two measurements. The graph shown here shows variation of the desired rate of decline for two different maximum rates of change (50 and 75 mg/dl/hr) as the measured glucose changes. The protocol in use at LDS hospital used 75 mg/dl/hr as the maximum rate of change before the refinement process.
Because the insulin infusion could be changed as a percentage of the existing infusion or as an absolute unit increase or decrease to the existing infusion (the usual paper protocol approach), we took an intermediate approach by dividing the computation by the square root of the insulin infusion. This makes changes at higher levels of infusion more like absolute unit changes and changes at lower levels more like /dl changes, e.g., compare an infusion rate of 1 U/hr vs 10 U/hr. If the computation calls for a 50/dl increase, dividing by the square root of the infusion rate will give a 0.5 unit increase at 1 U/hr and a 1.6 unit increase at 10 U/hr.
Insulin infusion instructions are given in units/hour for adults and in units/kg/hr or units/hr for pediatric patients.
Specifics
Starting insulin infusion for blood glucose values >110 mg/dl
Adult
Infusion = 0.0126 × measured glucose × patient weight/70 (units/hour)
Infusion rate instruction never <0.5 units/hr
All instructions to the tenth of a unit/hour
Give 1 unit (1 cc) to clear catheter dead space
Pediatric
Infusion = 0.0012 × measured glucose (units/kg/hr) × patient weight (units/hr)
Infusion rate instruction never <0.01 units/kg/hr
Instructions to 0.01 units/hr or units/kg/hr
No bolus
Adjustment of insulin infusion for blood glucose = 60 – 420 mg/dl
New infusion rate = current infusion rate * (1 + the equation)
{[(dg/dt – desired rate of change) / |desired rate of change|] × MF} / SqRoot of insulin infusion
If the equation ≤(−1), then new infusion rate = 0
where dg/dt is the difference between the current value (g2) and the preceding glucose value (g1) adjusted to the rate of change per 1 hour, that is, [(g2 – g1) / × minutes] * 60 min/hr
Desired rate of change = (g2 – g(target))/g2 * (−50)
Adjustment of insulin infusion for blood glucose = 41 – 60 mg/dl
Stop insulin and give intravenous dextrose 0.25 g/kg
Instructions in g/kg, cc of dextrose 50% in water (D50w) and cc of dextrose 10% in water (D10w)
Adjustment of insulin infusion for blood glucose ≤40 mg/dl
Stop insulin and give intravenous dextrose 0.50 g/kg
Instructions in g/kg, cc of D50w and cc of D10w
Timing of Glucose Monitoring
Above range: q2h
In range: q2h
Below range: If blood glucose concentration is <80 mg/dl, then q1h
If blood glucose is decreasing rapidly and approaching target range:
If g2 <140 mg/dl and dg/dt more rapid than –20 mg/dl/hr, then q1h
Nutrition
If enteral feeding or intravenous glucose administration (including parenteral nutrition) is stopped for more than 30 minutes, stop insulin infusion.
Check glucose in 1 hour and restart the protocol.
Appendix II: Reengineering Critical Care Clinical Research Investigators
The following persons and institutions are participants in the BAA Roadmap Initiative Reengineering Research in Critical Care
Principal Investigator and Steering Committee Chair—A. Morris.
Medical Informatics, University of Utah/LDS Hospital—Dean Sorenson (Principal Investigator), Kathy Sward, Homer Warner, Peter Haug.
Phase One Protocol and Writing Committee—B.T. Thompson (Chair), J. Orme, H. Zheng, P. Luckett, J. Truwit, D. Willson, D. Hite, R. Brower, G. B. Bernard, Martha Curley, Jay Steingrub, Dean Sorenson, Kathy Sward, E. Hirshberg, A. Morris.
Clinical Coordinating Center—D. Schoenfeld (Principal Investigator), B.T. Thompson (Medical Monitor), C. Oldmixon, H. Zheng, C. Bliss.
Data and Safety Monitoring Board—H. Wiedemann (Chair), G. Rubenfeld, M. Meade, S. Anand.
Clinical Centers
LDS Hospital**—A. Morris, J. Orme, T. Clemmer, C. Grissom;
Primary Children's Medical Center*—E. Hirshberg, G. Larsen;
Children's Hospital of Philadelphia*—V. Nadkarni, V. Srinivasan, C. Bayer Roth, L. Hutchins;
Vanderbilt University**—G. B. Bernard, S. Bozeman;
Wake Forest University**—R.D. Hite, A. Howard;
Massachusetts General Hospital—B.T. Thompson, C. Oldmixon;
Johns Hopkins University**—R. Brower, K. Boucher;
LDS Hospital—J. Orme, L. Baumann, T. Clemmer;
University of Virginia**—J. Truwit, M. Marshall;
University of Virginia Children's Hospital*—D. Willson, M. Ball;
Yale University*—C. Bogue, V. Faustino, I. Lazar;
Penn State Children's Hospital, Hershey—N. Thomas, J. Hess;
Baystate Medical Center**—J. Steingrub, M. Tidswell, L. Kozikowski;
Vanderbilt Children's—N. Patel, T. Shalaby;
Children's Hospital Central California—A. L. Graciano;
Hospital for Sick Children—P. Cox, A. Guerguerian;
St. Justine Hospital—J. Lacroix, G. Cannizzaro;
Dartmouth Hitchcock Medical Center—D. Levin, D. Jarvis;
Children's Hospital Minnesota/St. Paul—Kurachek, L. Blumberg;
Children's Hospital Michigan—S. Heidemann;
Children's Hospital Los Angeles—C. Newth, F. Fajardo;
Children's Medical Center Dallas—P. Luckett;
Baylor Children's—L. Jefferson;
Children's Hospital Boston—A. Randolph.
A single asterisk indicates the pediatric centers and a double asterisk indicates the adult centers that enrolled subjects in this multicenter validation exercise.
References
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters P, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, Moghissi ES, Bransome ED, Jr, Clark NG, Clement S, Cobin RH, Furnary AP, Hirsch IB, Levy P, Roberts R, Van den Berghe G, Zamudio V American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(Suppl 2):4–9. doi: 10.4158/EP.10.S2.4. [DOI] [PubMed] [Google Scholar]
- 5.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 6.Preiser J. Intensive glycemic control in med-surg patients (European Glucontrol trial). Program and abstracts of the Society of Critical Care Medicine 36th Critical Care Congress; 2007 Feb 17–21; Orlando, FL. [Google Scholar]
- 7.Wilson M, Weinreb J, Hoo G. Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care. 2007;30(4):1005–1011. doi: 10.2337/dc06-1964. [DOI] [PubMed] [Google Scholar]
- 8.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 9.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370–377. [PubMed] [Google Scholar]
- 10.Morris A, Orme J, Truwit J, Steingrub J, Grissom C, Lee K, Li GL, Thompson BT, Brower R, Tidswell M, Bernard G, Sorenson D, Sward K, Zheng H, Schoenfeld D, Warner H. A replicable method for blood glucose control in critically ill patients. Crit Care Med. In press 2008. [DOI] [PubMed]
- 11.Morris A. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med. 2000;132:373–383. doi: 10.7326/0003-4819-132-5-200003070-00007. [DOI] [PubMed] [Google Scholar]
- 12.Morris A. The importance of protocol-directed patient management for research on lung-protective ventilation. In: Dreyfuss D, Saumon G, Hubamyr R, editors. Ventilator-induced lung injury. New York: Taylor & Francis Group; 2006. pp. 537–610. [Google Scholar]
- 13.Saberi AA, Orme J, Clemmer T, Sorenson D, Skinner P, Green S, Warner H, Morris AH. A safe computerized protocol for lower ICU blood glucose. Am J Respir Crit Care Med. 2004;169(7):A38. [Google Scholar]
- 14.Ingle S, Underwood C, Blunt M, Mackenzie I. Tight glycaemic control: impact on nursing staff. Proceedings of the American Thoracic Society. 2005;2:A38. [Google Scholar]
- 15.Miranda DR, Nap R, de Rijk A, Schaufeli W, Iapichino G TISS Working Group. Therapeutic Intervention Scoring System. Nursing activities score. Crit Care Med. 2003;31(2):374–382. doi: 10.1097/01.CCM.0000045567.78801.CC. [DOI] [PubMed] [Google Scholar]
- 16.Reis Miranda D, Moreno R, Iapichino G. Nine equivalents of nursing manpower use score (NEMS) Intensive Care Med. 1997;23(7):760–765. doi: 10.1007/s001340050406. [DOI] [PubMed] [Google Scholar]
- 17.Pham T, Warren A, Phan H, Molitor F, Greenhalgh D, Palmieri T. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59(5):1148–1154. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5(4):329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 19.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118(1):173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 20.Hirshberg E, Lacroix J, Sward KP, Willson D, Morris AH. Blood glucose control in critically ill adults and children: A survey on stated practice. Chest. In press 2008. [DOI] [PubMed]
- 21.Wong X, Singh-Levett I, Hollingsworth L, Shaw G, Hann C, Lotz T, Lin J, Wong OS, Chase JG. A novel, model-based insulin and nutrition delivery controller for glycemic regulation in critically ill patients. Diabetes Technol Ther. 2006;8(2):174–190. doi: 10.1089/dia.2006.8.174. [DOI] [PubMed] [Google Scholar]
- 22.Juneja R, Roudebush C, Kumar N, Macy A, Golas A, Wall D, Wolverton C, Nelson D, Carroll J, Flanders SJ. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9(3):232–240. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 23.Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care. 2004;8(3):R122–R127. doi: 10.1186/cc2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105(2):244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Sochalski J. Is more better?: the relationship between nurse staffing and the quality of nursing care in hospitals. Med Care. 2004;42(2 Suppl):II67–II73. doi: 10.1097/01.mlr.0000109127.76128.aa. [DOI] [PubMed] [Google Scholar]