Abstract
Background
The objective of this study was to test the hypothesis that maternal blood glucose excursions correlate with deviation from optimized birth weight.
Methods
Patients were recruited for 3-day continuous glucose monitoring (CGM) plus self-blood glucose monitoring followed by routine diabetes screening at 26-28 weeks gestation. Patients and caregivers were blinded to CGM results. The magnitude and duration of blood glucose (BG) excursions were measured as a “glycemia index.” A customized birth weight centile was calculated.
Results
Twenty-three patients consented, 21 completed the study: 5 diabetic and 16 nondiabetic individuals. The duration of CGM was 72 (±7.2) hours, and each patient performed self-BG monitoring ≥3 times per day. All diabetic and 10 nondiabetic patients had several measured BG excursions above 130 mg/dl. A positive correlation was observed between birth weight centile and glycemia index above 130 (p < 0.03); the trend persisted for nondiabetic patients alone (p < 0.05). No significant correlation was noted between birth weight centile and average 3-day CGM values, 3-day fasting BG, average 3-day self-BG monitoring values, or diabetes screening BG value.
Conclusions
The glycemia index has a better correlation with birth weight centile than BG measured by conventional methods in a mixed diabetic and nondiabetic population. Fetal exposure to maternal blood glucose excursions correlates positively with fetal growth, even in nondiabetic patients with apparently normal glucose tolerance.
Keywords: CGM, diabetes, fetus, glucose, growth, pregnancy
Introduction
Traditionally, metabolic impairment of carbohydrates (MI-CHO) has been regarded as a categorical disease entity. The hippocratic definition of diabetes persisted for more than two millennia until 20th-century scientists classified diabetes based on duration, pathology, therapy, and, most recently, pathoetiology categories (e.g., type 1, type 2, gestational diabetes). Recent investigations have further focused on studying “prediabetes” and “insulin resistance” disorders as pathologic entities in and outside of pregnancy. Insulin resistance (IR) is associated with an increased risk for obesity, diabetes, hypertension, coronary artery disease, and poor pregnancy outcome.1 IR can be defined as a categorical or a continuous variable.2,3 Recent investigations have focused on the continuous nature of MI-CHO4 similar to observations on impaired cholesterol metabolism and high arterial pressure. The incidence and prevalence of most carbohydrate metabolism disorders in the United States are on the rise5,6 and are attributed to population changes in ethnicity, senility, and obesity, as well as expanded screening.
Recognition of enhanced expression of MI-CHO in pregnancy has led to routine screening and diagnosis of gestational diabetes (GDM). Recognition of ethnic differences in susceptibility to diabetes7 and enhanced susceptibility to become frank diabetic following GDM8 point to a genetic and/or environmental susceptibility to MI-CHO in some individuals. Since its first recognition in 1964, criteria for screening, diagnosis, and management of GDM have evolved toward normalizing blood glucose (BG) to lower levels. However, the sanctioned 50-gram, 1-hour oral glucose tolerance screen and the diagnostic 2- or 3-hour glucose tolerance tests provide categorized diagnoses based on a limited number of BG values. While a categorical definition provides a practical means of diagnosis and management of the most pathogenic degrees of MI-CHO, it does not reveal the wide spectrum of abnormalities based on the frequency and duration of BG excursions over time. Maternal glucose crosses the placenta freely and stimulates fetal insulin production.9 A linear correlation exists between maternal and fetal BG,9 and evidence shows that maternal diabetes is associated with increased fetal insulin production.10,11 Insulin is the main fetal growth hormone. Diabetes is associated with fetal overgrowth and an increased rate of operative deliveries.12–15 It is not known, however, if concealed maternal hyperglycemia is associated with a higher birth weight and unplanned operative delivery. A recently published study showed that continuous glucose monitoring (CGM), compared to intermittent capillary self-BG monitoring (SBGM), provided additional information on BG control in the majority of GDM patients, which resulted in altered clinical management decisions.16
The purpose of this pilot study was to investigate if the level of concealed maternal BG excursions correlates with birth weight centile and rate of unplanned operative deliveries among diabetic and nondiabetic pregnancies.
Materials and Methods
This prospective observational study was approved by Stanford University's institutional review board. Consecutive patients, regardless of diabetic status, were recruited at the time of their first visit to the obstetric clinic at Lucile Packard Children's Hospital at Stanford University during the years 2005 and 2006. All patients had a teaching session when the study was described in detail and informed consent was obtained. At 26–28 weeks gestation volunteers underwent a 3-day CGM and SBGM followed by a routine conventional diabetes screen.
Blood Glucose Measurements
Patients were fitted with a Food and Drug Administration-approved Paradigm® CGM system (Medtronic MiniMed, Northridge, CA) and were instructed to also record their SBGM measures three or more times per day for the next 3 days using a FreeStyle Navigator® BG monitor (Abbott/TheraSense, Abbott Park, IL). The CGM system measures the interstitial fluid glucose concentration in 10-second intervals, averages the values every 5 minutes, and then calculates a correction factor when the patient enters the measured SBGM values into the CGM device and stores a calculated BG value. At the completion of each patient's study, digital data were downloaded into a designated secure personal computer in an Excel spreadsheet (Microsoft Corporation, Seattle, WA). CGM data remained undisclosed to the patient, the obstetric providers, and the investigators. SBGM data were transparent to the patients and their obstetric providers. Nondiabetic patients were scheduled for a routine 1-hour, 50-gram glucose load screen, and a venous BG of ≥140 mg/dl was followed by a 3-hour, 100-gram glucose tolerance test per established protocols.
Per the original study design, we employed a novel approach to standardize the magnitude of the concealed BG excursions. From the 3-day CGM values, we calculated the area under the curve for values above the predefined cutoffs of 110, 120, 130, and 140 mg/dl. Since the time on CGM slightly varied among the subjects, we normalized the aforementioned values to a 24-hour period and called it a “glycemia index” (GI). The GI values represent the product of mean BG value above the predetermined cutoff multiplied by the number of excursion episodes measured at 5-minute intervals within a 24-hour period.
Birth Weight Measurement
Birth weight centile was calculated through the open-source Gestation Network GROW program,17 which uses maternal height, gravid weight, ethnicity, parity, fetal gender, and gestational age. The GROW program assigns a value of 50 centile for optimal average birth weight with a range of 0–100.
Statistical Methods
Data were analyzed using t test, χ2, Kendall's tau_b correlation, and ANOVA.
Results
Twenty-three patients consented, 1 withdrew, and 1 was lost to follow-up. Twenty-one patients completed the study and were analyzed. Diabetes status, glycemia index, birth weight centile, and mode of delivery are shown in Table 1. Indications for unplanned cesarean delivery (CD) included failure to progress in labor, nonreassuring fetal heart rate pattern, or both. Among 16 nondiabetic patients, 1 had an elevated 50-gram glucose screen but a normal 3-hour glucose tolerance test and 1 had a strong family history of type 1 diabetes but normal diabetes screen. The latter patient was empirically placed on a 1-week-long capillary BG testing per her obstetrician (M.M.T.) and reported several 1-hour postprandial values above 180 mg/dl and was empirically placed on a diabetic diet. The sole GDM patient had a history of GDM in a prior pregnancy and required insulin therapy. All patients had an essentially uninterrupted CGM for 72 (±7.2, range 58–95) hours and each performed SBGM ≥3 times per day. Diabetic patients were treated per California State standard protocols. Two patients had a thyroid disorder and 1 had epilepsy; all were optimally treated. One fetus had gastroschisis diagnosed prenatally. No other fetal or neonatal anomalies were detected. All newborns had a 5-minute Apgar score of ≥7. Other maternal and neonatal characteristics are shown in Table 2.
Table 1.
Glycemia Index, Birth Weight Centile, and Pregnancy Outcome
| Case # | Diabetes status | GI-130 | Birth weight centile | Outcome |
|---|---|---|---|---|
| 1 | Nondiabetic | 0 | 1.2 | Vaginal delivery, Epi/Lac |
| 2 | Nondiabetic | 0.1 | 15.6 | SVD |
| 3 | Nondiabetic | 0 | 20.8 | SVD |
| 4 | Nondiabetic | 0 | 32.5 | Planned CD |
| 5 | Nondiabetic | 0 | 40.3 | Vacuum delivery |
| 6 | Nondiabetic | 1.5 | 41 | Vaginal delivery, Epi/Lac |
| 7 | Nondiabetic | 6 | 49.1 | Planned CD |
| 8 | Nondiabetic | 6.3 | 56.3 | Planned CD |
| 9 | Nondiabetic | 437.7 | 62.1 | Vaginal delivery, Epi/Lac |
| 10 | Nondiabetic | 0 | 66.5 | SVD |
| 11 | Nondiabetic | 0 | 76.1 | SVD |
| 12 | Nondiabetic | 0.1 | 77.7 | Unplanned CD, gastroschisis |
| 13 | Nondiabetic | 0.2 | 86.9 | Vacuum delivery |
| 14 | Nondiabetic | 31 | 97.1 | Vacuum delivery, SD |
| 15 | Nondiabetic | 8.3 | 98.9 | SVD |
| 16 | Nondiabetic | 0.5 | 99.3 | Planned CD |
| 17 | T1-DM | 240.6 | 61.1 | Vacuum delivery, SD |
| 18 | T1-DM | 221.6 | 82.2 | Unplanned CD |
| 19 | T1-DM | 1089.2 | 100 | SVD |
| 20 | T2-DM | 0.9 | 21.1 | Planned CD |
| 21 | GDM | 6.1 | 17.7 | SVD |
T1-DM, type 1 diabetes mellitus; T2-DM type 2 diabetes mellitus; Epi, episiotomy; Lac, perineal laceration; SVD, spontaneous vaginal delivery; CD, cesarean delivery; SD, shoulder dystocia.
Table 2.
Maternal and Neonatal Characteristics of the 21 Study Patients
| Age (years) | 29.7 (±5.5) |
| Nulliparous | 57% |
| Caucasian | 67% |
| Hispanic | 14% |
| Asian | 10% |
| Indian/Pakistani | 10% |
| Pregravid body mass index | 29.4 (±6.6) |
| Male | 48% |
| Birth weight (g) | 3393 (±543) |
Continuous glucose monitoring disclosed BG excursions above 130 mg/dl in 15 (5 diabetic and 10 nondiabetic) patients; 11 of whom also had episodic BG excursions above 130 mg/dl detected by SBGM (p = nonsignificant).
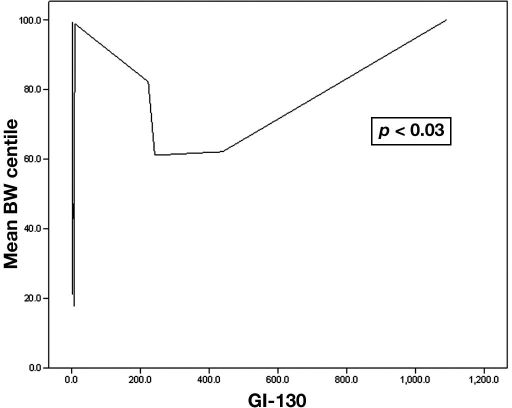
As continuous variables, a positive correlation was seen between the birth weight centile and the GI above all BG cutoffs tested. The strongest correlation was seen for GI above 130 (GI-130) (p < 0.03) (Figure 1). Furthermore, a positive correlation was seen between the birth weight centile and the GI-130 among the 16 nondiabetic individuals (p < 0.05). No significant correlation was seen between birth weight centile and average 3-day CGM values, 3-day fasting BG, average 3-day self-BG monitoring values, or diabetes screening BG value.
Figure 1.
Correlation between glycemia index above 130 mg/dl and birth weight (BW) centile.
Five patients had planned cesarean delivery, 10 patients had unassisted vaginal delivery with or without episiotomy or laceration, and 6 patients had assisted vaginal delivery or unplanned CD. No significant differences were seen in birth weight centile or GI values among the 3 modes of delivery groups.
Discussion
The rate of diabetes has increased among the general population in the United States, including among pregnant women.5,7 Maternal diabetes, when not optimally controlled, is associated with higher rates of fetal macrosomia and operative deliveries.18 However, the relationship between maternal BG levels and fetal overgrowth has not been studied among nondiabetic pregnants. We designed our study to include both diabetic and nondiabetic pregnancies to evaluate the effect of maternal hyperglycemia on birth weight. Fetal BG levels have a linear correlation with maternal BG levels.9 We hypothesized that exposure to BG excursions may have a significant effect on fetal body mass, even in those patients without the diagnosis of diabetes or GDM. To test this hypothesis, we chose the fetus as a biologic model. In this study, average 3-day maternal BG as measured by CGM was not a predictor of birth weight centile, nor were BG values obtained through conventional tests of maternal diabetes. The majority of diabetic and nondiabetic patients in this study demonstrated several episodes of BG excursions above 130 mg/dl detectable by CGM and by SBGM. The magnitude of BG excursions could be quantified more clearly with CGM than with SBGM, as was shown elsewhere.19 Our study suggests that BG excursions correlate positively with fetal growth. We obtained frequent maternal BG measurements by CGM over a 3-day period at 26–28 weeks gestation and found a significant correlation between the magnitude and the duration of BG excursions above 130 mg/dl and birth weight centile. Our study population consisted of 25% diabetic and 75% nondiabetic patients. The correlation between BG and birth weight is well known among diabetic patients. In our study, the correlation between the level of maternal BG excursions and birth weight centile remained significant after removal of the diabetic patients.
Our study failed to show a correlation between the degree of maternal BG excursions and assisted vaginal or unplanned cesarean delivery. A nonsignificant positive correlation was present between the degree of BG excursions and unplanned operative deliveries. A Canadian study showed that presence of a “borderline GDM” is associated with increased rates of fetal macrosomia and CD. However, the same study showed that the rate of CD remained elevated among known borderline GDM patients who carried a nonmacrosomic fetus.20 The relationship between milder degrees of maternal hyperglycemia and operative delivery remains unknown.
Our pilot study has several limitations. We did not measure glycosylated hemoglobin levels in nondiabetic patients. CGM systems measure interstitial fluid glucose as a surrogate measure of BG. Three days of monitoring may not accurately represent the whole duration of the pregnancy. While we used a validated tool to calculate a customized birth weight centile, the small number of subjects in this pilot study did not allow us to test for all confounding factors of fetal growth. The size of the study population further did not allow for calculation of a cutoff glycemia index for prediction of large fetuses and operative deliveries, nor were we able to prove a significant correlation between maternal BG excursions and unplanned operative deliveries. While the diabetic pregnant women in an Australian study reported use of the CGM system as “easy” and worth the inconvenience,21 the same may not apply to nondiabetic individuals until a clear benefit for pregnancy outcome is shown. It remains unknown at this time if intervention for hyperglycemia in nondiabetic patients will change the outcome of the pregnancy. Findings of this study merit larger studies to address the aforementioned inadequacies. The present CGM technology, while highly valuable in the care of diabetics, is expensive and cumbersome as a screening tool. For now, CGM in nondiabetic individuals should be reserved for research purposes.
Abbreviations
- BG
blood glucose
- CD
cesarean delivery
- CGM
continuous glucose monitoring
- GDM
gestational diabetes
- GI
glycemia index
- GI-130
GI above 130
- MI-CHO
metabolic impairment of carbohydrates
- IR
insulin resistance
- SBGM
self-blood glucose monitoring
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among us adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;288(3):342–350. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 5.National Diabetes Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health [homepage on the Internet] 2005. Total Prevalence of Diabetes in the United States, All Ages Bethesda (MD) [NIH Publication No. 06–3892, Nov 2005; cited 2007 Dec 29]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics/#7.
- 6.American Diabetes Association. Gestational diabetes mellitus (Position statement) Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara M, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstet Gynecol. 2004;103:526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 8.Peters RK, Xiang A, Kjos SL, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet. 1996;347:227–230. doi: 10.1016/s0140-6736(96)90405-5. [DOI] [PubMed] [Google Scholar]
- 9.Gordon MC. Pancreas and fuel metabolism. In: Gabbe SG, Niebyl JR, Simpson JL, editors. 5th. Orlando: Churchill Livingstone; 2007. pp. 78–79. Obstetrics–normal and problem pregnancies. [Google Scholar]
- 10.Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers: relationship to fetal hyperinsulinism. Diabetes Care. 1995;18(5):611–617. doi: 10.2337/diacare.18.5.611. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter MW, Canick JA, Hogan JW, Shellum C, Somers M, Star JA. Amniotic fluid insulin at 14-20 weeks' gestation: Association with later maternal glucose intolerance and birth macrosomia. Diabetes Care. 2001;24(7):1259–1263. doi: 10.2337/diacare.24.7.1259. [DOI] [PubMed] [Google Scholar]
- 12.De Valk HW, van Nieuwaal NH, Visser GH. Pregnancy outcome in type 2 diabetes mellitus: a retrospective analysis from the Netherlands. Rev Diabet Stud. 2006;3(3):134–142. doi: 10.1900/RDS.2006.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunne F. Type 2 diabetes and pregnancy. Semin Fetal Neonatal Med. 2005;10(4):333–339. doi: 10.1016/j.siny.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract. 2005;69(3):279–286. doi: 10.1016/j.diabres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Khatun N, Latif SA, Uddin MM. Pregnancy associated complications of mothers with gestational diabetes mellitus. Mymensingh Med J. 2005;14(2):196–198. [PubMed] [Google Scholar]
- 16.Kestilä KK, Ekblad UU, Rönnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res Clin Prac. 2007;77(2):174–179. doi: 10.1016/j.diabres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Gestation Related Optimal Weight (GROW) program [software on the Internet] Birmingham, UK: West Midlands Perinatal Institute, NHS Perinatal Institute for Maternal and Child Health, The Gestation Network; c2007 [cited 2007 Nov 30]. Available from: http://www.gestation.net/birthweight_centiles/birthweight_centiles.htm. [Google Scholar]
- 18.Coustan DR, Imarah J. Prophylactic insulin treatment of gestational diabetes reduces the incidence of macrosomia, operative delivery and birth trauma. Am J Obstet Gynecol. 1984;150:836–842. doi: 10.1016/0002-9378(84)90459-9. [DOI] [PubMed] [Google Scholar]
- 19.Cypryk K, Pertyńska-Marczewska M, Szymczak W, Wilcyński J, Lewiński A. Evaluation of metabolic control in women with gestational diabetes mellitus by the continuous glucose monitoring system: a pilot study. Endocr Pract. 2006;12(3):245–250. doi: 10.4158/EP.12.3.245. [DOI] [PubMed] [Google Scholar]
- 20.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA. 1996;275(15):1165–1170. [PubMed] [Google Scholar]
- 21.McLachlan K, Jenkins A, O'Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186–190. doi: 10.1111/j.1479-828X.2007.00716.x. [DOI] [PubMed] [Google Scholar]