Abstract
Background
Inpatient glycemic control is a constant challenge. Institutional insulin management protocols and structured order sets are commonly advocated but poorly studied. Effective and validated methods to integrate algorithmic protocol guidance into the insulin ordering process are needed.
Methods
We introduced a basic structured set of computerized insulin orders (Version 1), and later introduced a paper insulin management protocol, to assist users with the order set. Metrics were devised to assess the impact of the protocol on insulin use, glycemic control, and hypoglycemia using pharmacy data and point of care glucose tests. When incremental improvement was seen (as described in the results), Version 2 of the insulin orders was created to further streamline the process.
Results
The percentage of regimens containing basal insulin improved with Version 1. The percentage of patient days with hypoglycemia improved from 3.68% at baseline to 2.59% with Version 1 plus the paper insulin management protocol, representing a relative risk for hypoglycemic day of 0.70 [confidence interval (CI) 0.62, 0.80]. The relative risk of an uncontrolled (mean glucose over 180 mg/dl) patient stay was reduced to 0.84 (CI 0.77, 0.91) with Version 1 and was reduced further to 0.73 (CI 0.66, 0.81) with the paper protocol. Version 2 used clinician-entered patient parameters to guide protocol-based insulin ordering and simultaneously improved the flexibility and ease of ordering over Version 1.
Conclusion
Patient parameter and protocol-based clinical decision support, added to computerized provider order entry, has a track record of improving glycemic control indices. This justifies the incorporation of these algorithms into online order management.
Keywords: CPOE, diabetes, insulin, glycemic control, quality improvement
Introduction
The challenge of glycemic control among hospitalized diabetic inpatients is a significant one and creates a tremendous morbidity burden in terms of increased lengths of stay, higher infection rates, and hypoglycemic events.1 In order to regulate insulin usage and to facilitate optimal glycemic control, many institutions have looked to automated solutions to guide insulin ordering in a way that will produce steadier glycemic control and reduce both hyperglycemic and hypoglycemic values.2 The theory and practice behind algorithm-based insulin ordering and its effect on glucose readings have been described elsewhere.3
To promote patient safety and provide high-quality care, hospitals have looked to computerized provider order entry (CPOE) as a powerful tool to standardize practice and to improve patient outcomes. While large-scale prospective trials on CPOE are still lacking, there have been several excellent studies on the capacity of CPOE to improve process and clinical outcomes.4 Traditionally, CPOE interventions have focused on a series of alerts and reminders to guide providers when ordering a medication or test that might put the patient at risk or when omitting a key element in best practice.5 A novel paradigm is to have the application guide the provider through describing the clinical condition of the patient and then have the application suggest the best regimen.
This article describes the design of “indication-based” subcutaneous insulin orders. It also describes how this innovation arose as part of ongoing efforts to improve the care of inpatients with hyperglycemia at our institution.
Early Efforts to Improve Glycemic Control and Insulin Management
The University of California San Diego Medical Center (UCSDMC) is a 425-bed academic tertiary care hospital spanning two campuses, with a full suite of adult medical and surgical specialties.
A hospitalist-led multidisciplinary team was created in early 2003 with the goal of improving glycemic control, reducing hypoglycemia rates, and facilitating best-practice insulin use on the adult medical–surgical wards. The team's first tasks included broad educational efforts, designing structured subcutaneous insulin orders, and formulating metrics to assess the impact of their efforts. In anticipation of the transition to CPOE, we introduced a paper-based structured subcutaneous insulin order set (SQIO) in November 2003. This order set encouraged the use of scheduled basal and nutritional insulin and provided some guidance on insulin dosing, as well as an algorithm to treat hypoglycemia.
In a parallel effort, UCSDMC was developing a CPOE module for our comprehensive clinical information system, Invision (Siemens Medical Systems, Malvern, PA), that heretofore had focused primarily on result review, patient schedule management, and nursing documentation. We introduced our first standardized CPOE subcutaneous insulin order set in January 2004 at the smaller of our two campuses. We then completed full deployment across both campuses, in all adult medical–surgical care areas, by September 2004. All providers wishing to order anything but a one-time insulin order were compelled to use the structured CPOE insulin order set.
This first version (hereafter labeled “Version 1”) of the CPOE insulin orders was modeled directly from the paper order set immediately preceding it. Version 1 encouraged the use of basal/bolus insulin regimens and placed the terms basal, nutritional (premeal), and adjustment dose insulin in the order sets and the medication administration record (Figure 1; note that all screen shots depict test mode and that all displayed patient names and identifiers are fictitious). With the initiation of Version 1, the ordering clinician had to specifically opt out of ordering scheduled premeal and basal insulin to order a sliding scale-only regimen. The first screen also ensured that appropriate point of care (POC) glucose monitoring was ordered and that a standing hypoglycemia protocol order was endorsed. Version 1 drove the user to basal, nutritional, and correction dose screens as indicated by the choices on the first screen. Guidance for preferred insulin regimens for patients in different nutritional situations was not inherent in Version 1; all basal and nutritional insulin options were offered as equally acceptable choices. This version did not calculate insulin doses or assist in apportioning insulin between basal and nutritional components. While the nutritional and correction insulin types were linked (to be identical), only a single adjustment dose scale was offered, leaving appropriate modifications up to the user.
Figure 1.
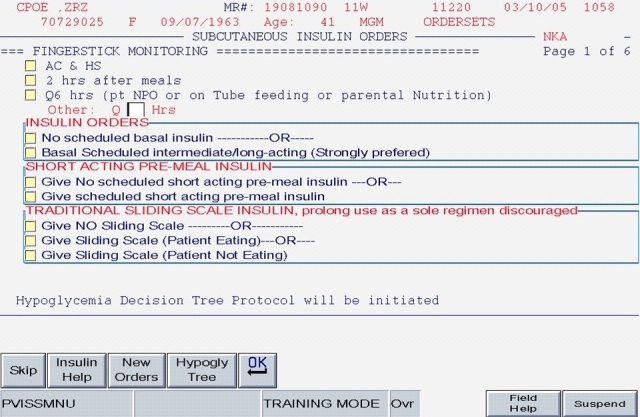
SQIO Version 1: initial screen encouraging the use of scheduled basal and nutritional insulin.
The key limitation of this initial order set, from a user standpoint, was its fundamental lack of dynamic flexibility. If any adjustment was required, the user was forced to manually discontinue all insulin orders and reenter the entire order set; no guidance for adjustment was offered. In short, Version 1 inherited all of the limitations of the paper version: gains that were made from eliminating legibility errors and unapproved abbreviations were offset by a lack of user-friendliness and flexibility.
Despite limitations of the paper order set and Version 1, significant improvements occurred in insulin use patterns, glycemic control, and hypoglycemia (reviewed later), but these improvements reached a plateau.
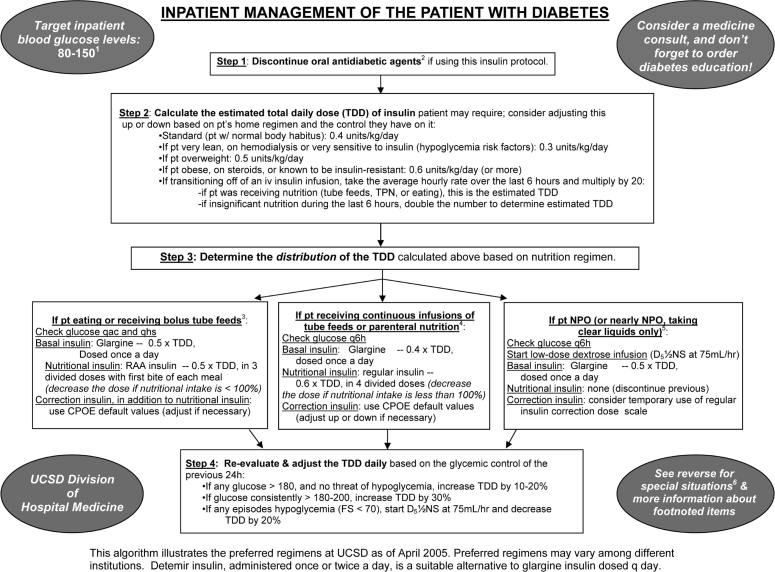
After a period of development and small-scale piloting, we introduced a new insulin management protocol, displayed in Figure 2, in an attempt to accelerate improvement. This protocol provided prompts to establish a glycemic target, discontinue oral antihyperglycemic agents, and arrive at a safe total daily dose (TDD) of insulin. It also provided a single preferred insulin regimen for the nil per os (NPO, “nothing through the mouth”) patient, the eating patient, and the patient receiving continuous tube feedings or parenteral nutrition. This simple protocol was used to reinforce key concepts in case-based teaching sessions with house staff and at surgical and medical grand rounds.
Figure 2.
Paper insulin management algorithm used in conjunction with SQIO of Version 1 and designed into Version 2.
Impact of Version 1 and the Paper Insulin Management Protocol
We devised metrics to monitor insulin use patterns, hypoglycemia rates, and glycemic control to gauge the impact of our interventions. While the improvement team generally followed longitudinal results with run charts, this section describes the impact in terms of three time periods for ease of statistical comparisons. Pearson χ2 [with relative risks and 95% confidence intervals (CI)] values were calculated to compare glycemic control and hypoglycemia rates between the baseline period and the periods related to our two key interventions. The intervention periods for data reporting were defined as follows:
“Baseline” – (Nov. 2002–Oct. 2003) time period 1 (TP1)
“Structured order set—Version 1” – (Nov. 2003–Apr. 2005) time period 2 (TP2)
“Protocol plus structured order set” – (May 2005–Dec. 2005) time period 3 (TP3)
Insulin Usage
Thirty to 90 insulin orders were sampled by the pharmacy each month (a convenience sample representing 3–4 days of insulin orders per month) to assess basal insulin usage. The percentage of insulin regimens incorporating basal insulin improved from a range of 25–29% in TP1 to 71% by September 2004. This improvement has been sustained and sometimes reaches over 80% in spot checks performed every 3–4 months. While no formal statistical analysis was performed for this parameter, we feel confident that a consistent and positive change has occurred.
Hypoglycemia
We monitored 11,057 patients with POC glucose testing over 53,466 patient days. The percentages of monitored patient days with hypoglycemia (any glucose ≤60 mg/dl) and severe hypoglycemia (any glucose ≤40 mg/dl) were calculated. The percentage of patient days with hypoglycemia improved from 3.68% in TP1 to 2.8% in TP2 and 2.59% in TP3, representing a relative risk for hypoglycemic day in TP3 vs TP1 of 0.70 (CI 0.62, 0.80). Similar reductions were seen in severe hypoglycemic days and the risk of a hypoglycemic patient stay (Figure 3).
Figure 3.
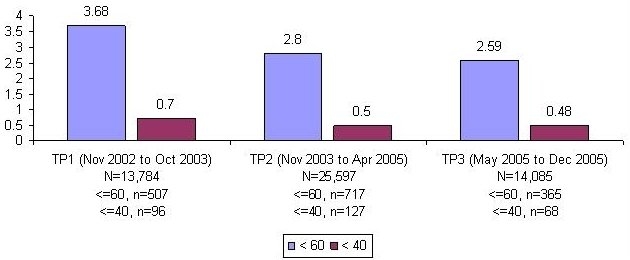
Percentage of patient days with hypoglycemia/extreme hypoglycemia decreases by 30 and 31%, respectively (Pearson χ2, p < 0.02).
Glycemic Control
Prior to data analysis, the improvement team established that patients being monitored for glycemic control would have to have ≥8 POC glucose values. Patients with fewer than 8 POC values were dropped from the analysis on the basis that legitimate summary estimates of inpatient glycemic control required several readings to meaningfully reflect inpatient efforts. Over the three time periods, 5800 patients, representing 37,516 monitored days, had 111,473 POC glucose tests.
All POC glucose values from hospital days 1 to 14 were used to calculate a mean glucose value for each patient's hospital stay. Hypoglycemic values (≤60 mg/dl) were excluded from calculation of mean glucose levels to avoid equating frequent hypoglycemia with optimal glycemic control. Patients with a mean glucose of over 180 mg/dl were defined as having a patient stay with uncontrolled hyperglycemia. The percentage of patients with an uncontrolled patient stay was 38% in the baseline period. The relative risk of an uncontrolled patient stay was reduced to 0.84 (CI 0.77, 0.91) in TP2 and to 0.73 (CI 0.66, 0.81) in TP3.
Iterative Design and Version 2
The team was now convinced that Version 1 had improved care and that the paper protocol facilitating proper use of the order set improved care even further. The team then wanted to bring the guidance offered in the one-page protocol to a larger percentage of clinicians, as frontline observation and pharmacy feedback suggested opportunities for further improvement. At that time, the challenge before the team was to provide as much guidance from the protocol into the next CPOE version as possible, while ensuring that the application remained user-friendly. The team wished to place guidance about the following best practices and key concepts into the order set.
Selected Best Practices and Key Concepts
Establish a glycemic target.
Use a hemoglobin A1c level to assess outpatient control.
Coordinate glucose monitoring with nutrition and insulin administration.
Oral hypoglycemic agents should generally be discontinued on admission (insulin is preferred).
Suggested insulin starting doses and adjustment strategies should be incorporated into institutional protocols and order sets.
“Basal–nutrition–correction dose” insulin regimens are preferred.
Institutional guidance for different nutritional circumstances and special situations should be provided in protocols and educational efforts.
Correction insulin scales (sliding scales) should vary based on insulin sensitivity.
Hypoglycemia management protocols should be included automatically with insulin orders.
The ordering physician using Version 2 would be prompted to discontinue all oral diabetes medications, establish a glycemic target, and order a hemoglobin A1c test (Figure 4).
Figure 4.
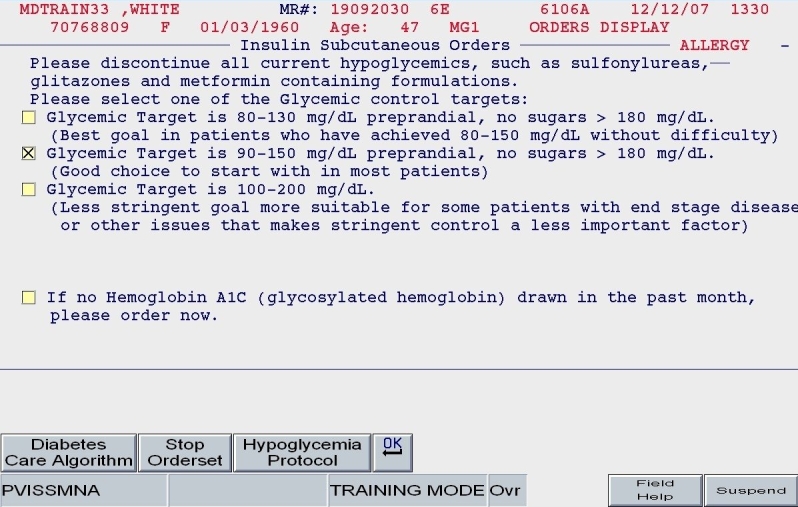
SQIO Version 2: glycemic target; prompts to stop oral agents and order a hemoglobin A1c.
We sought to enhance the ordering experience by using patient-based characteristics and the insulin management protocol to seamlessly guide the user, offering calculated values in place of relying on a paper worksheet.
To guide the user in initiating insulin in the diabetic or hyperglycemic patient, several clinical parameters were required.
Patient weight and general body habitus: Body habitus was defined subjectively by the entering provider. It was not based on a precise calculation of body mass index (BMI), as we knew that BMI would not always be available at the moment insulin orders were placed. This assessment of body habitus was used primarily to estimate insulin sensitivity: the insulin-sensitive patient had an estimated requirement of only 0.3 units/kg/day, whereas the obese patient had an estimated requirement of 0.6 units/kg/day.
Total daily dose: Based on the patient's body habitus and weight, a reasonable estimate for a total daily dose of insulin was calculated. Alternate methods for calculating the TDD were offered. Also, the practitioner could reject the default TDD and enter a TDD based on the patient's past experience with insulin and other clinical factors.
Nutritional intake: Patients were categorized in one of three nutritional intake patterns, as outlined in the paper protocol: eating regular meals (or bolus tube feeds), NPO, or receiving continuous enteral tube feeding.
Once the TDD estimate was either endorsed or altered by the ordering clinician and once the nutritional intake category was chosen (as depicted in Figures 5 and 6), the subsequent screens reflect protocol-driven insulin choices and dosing. Patients who were eating would be offered basal, nutritional, and correction insulin, while the NPO patient would be offered basal and correction insulin only.
Figure 5.
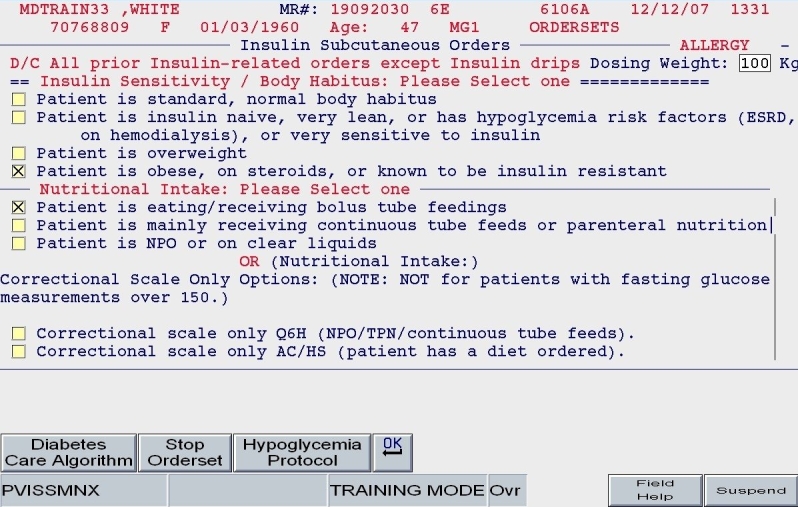
SQIO Version 2: user input of key clinical parameters.
Figure 6.
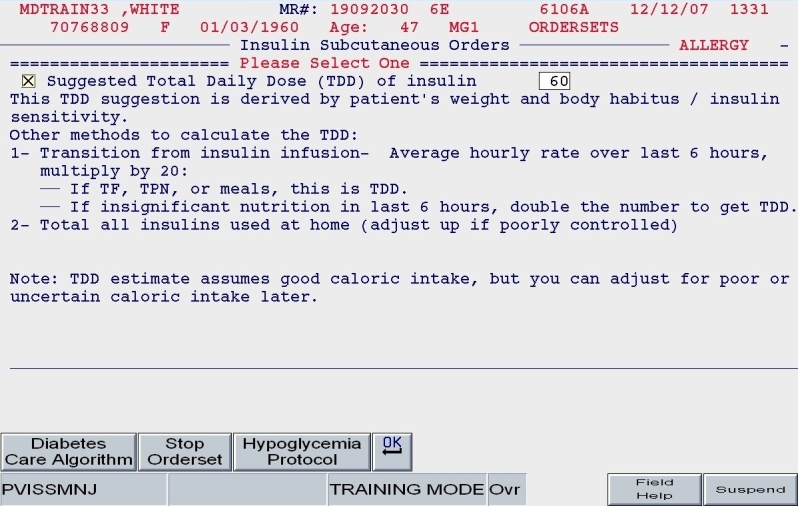
SQIO Version 2: calculation and validation of total daily dose.
This was the key innovation of indication-based ordering: by simply selecting the patient's weight, subjective body habitus, and nutritional pattern, all further clinical “decisions” were defined by the algorithm. The application embedded “constituent logic” on each screen such that one combination of clinical choices would take the user to one screen as opposed to another, whereas a different combination of entries would generate a different path. Thus, it was unlikely for the user to order the incorrect form of insulin, as the insulin offered was predicated on his or her first clinical description choices on the first screen. This methodology is in contradistinction to classic online order sets, which offer numerous alternatives, relying on clinician expertise to choose optimal combinations.
A clinical example is depicted in Figures 5 and 6. For a 100-kg patient with an obese body habitus eating regular meals, the TDD is estimated to be 100 × 0.6 units/kg/day, or 60 units. Once the provider endorses this TDD, 50% (30 units) of glargine insulin becomes the default basal insulin regimen, and the eating patient automatically has orders entered for POC glucose monitoring before meals and at bedtime (Figure 7). The remaining 30 units of the TDD is divided equally among the three meals as lispro insulin (as opposed to regular insulin), and further guidance to decrease the nutritional insulin dose in the setting of reduced intake is offered (Figure 8). The correction dose scale presented was dependent on the TDD, thus providing scales of varying insulin sensitivities (Figure 9). If, however, this same patient was described as being on continuous tube feeds, the nutritional component would be offered as regular insulin every 6 hours with a correctional scale to supplement.
Figure 7.
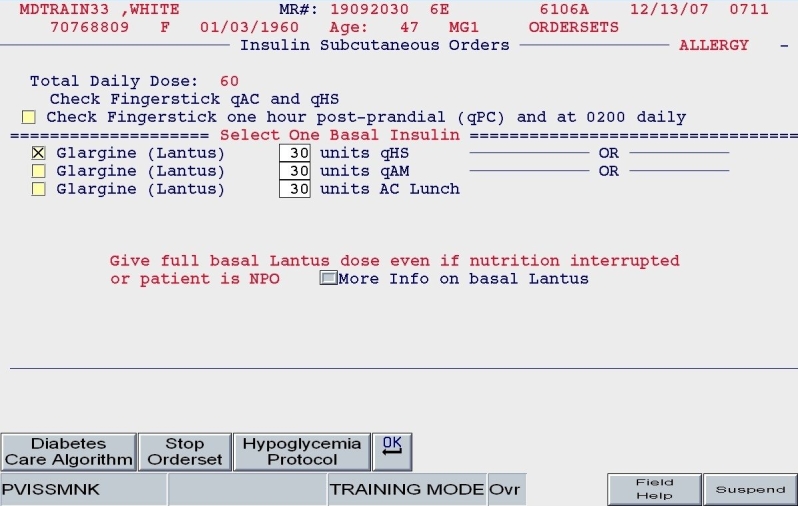
SQIO Version 2: guided apportionment of preferred basal insulin.
Figure 8.
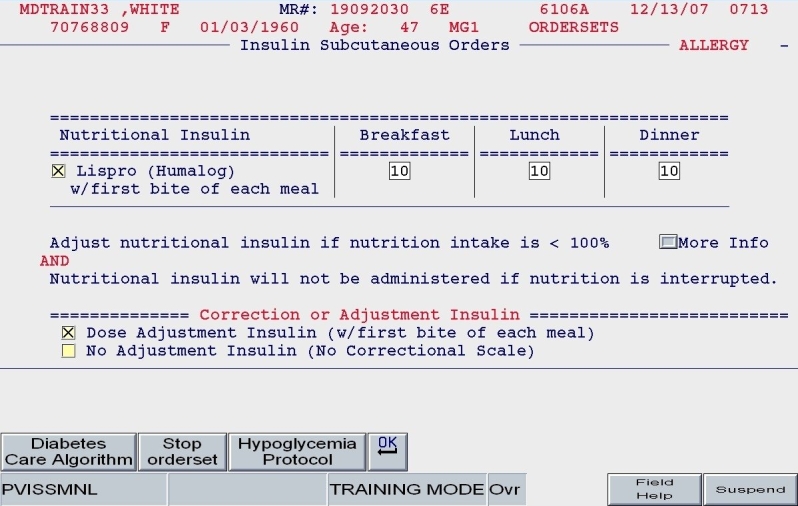
SQIO Version 2: guided apportionment of preferred nutritional insulin.
Figure 9.
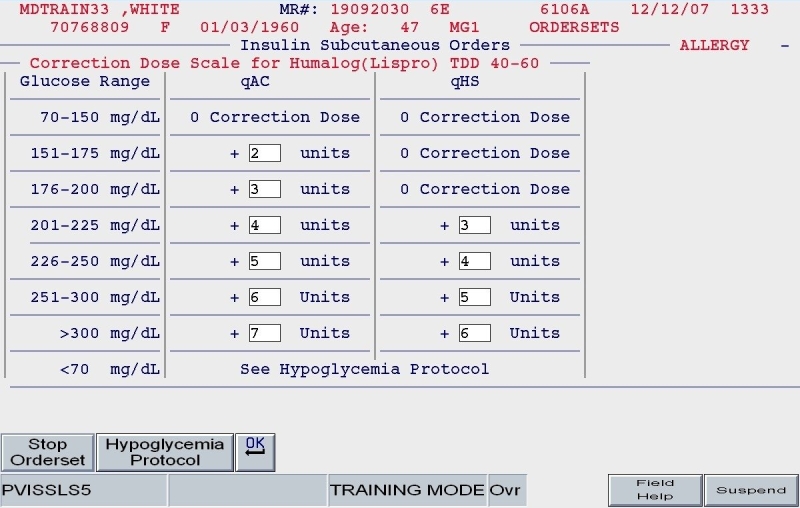
SQIO Version 2: correctional scale insulin based proportionally on total daily dose.
Version 2 offered additional benefits with respect to usability. When the user revised the insulin orders, parameters such as the patient's weight and TDD would be “remembered” so that the user could adjust accordingly. This concept is known as “object permanence” and is taken from the developmental literature; the application “remembers” a fact about a patient, defined by the user, and brings it back to the forefront when needed, e.g, the next time that insulin orders need revision. Traditionally, rule-based or alert-based order sets would have the user reenter clinical parameters the next time of revision and offer a warning if the user “got it wrong.” In our Version 2, upon revision, the user would have the opportunity to update the degree of insulin sensitivity or food intake parameters if these clinical characteristics had changed, producing a new set of calculations and new apportionment of basal and nutritional insulin doses. Constituent logic would be invoked to properly guide the user to the next screen. To assist novice providers in appropriate adjustment of insulin regimens, a series of prebuilt “adjustment sets” were embedded in the application. Again, this relied on “indication-based” choices, as users were prompted to consider the patient's control in the past 24 hours prior to revision (as depicted in Figure 10).
Figure 10.
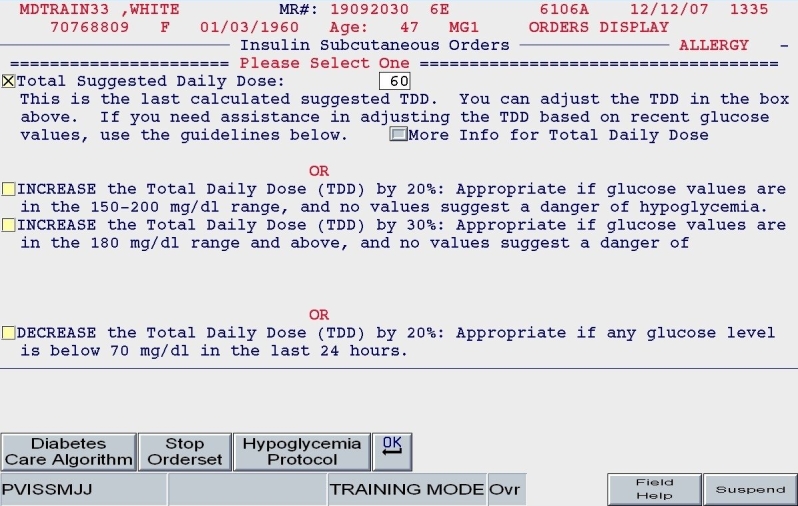
SQIO Version 2: global adjustment of insulin dose options.
One of the most significant informatics innovations in “Version 2” was the optimized handling of orders to prevent duplicates and to facilitate quick revisions. By grouping all medications with the classification of subcutaneous insulin as a unique subgroup of medication orders, the user had the option of discontinuing all prior insulin orders when revising the patient's regimen. This would ensure that no duplicate insulin orders were generated when various users ran the full subcutaneous insulin order set. At the same time, users were given the option to simply revise individual insulin orders (Figure 11). For example, if only the prelunch lispro insulin required adjustment, the user would have the option to revise a single insulin dose, providing significant time savings for minor adjustments. At the same time, the baseline hypoglycemic protocol orders that were put in place in the initial order set were unchanged and would persist as long as the patient was receiving insulin.
Figure 11.
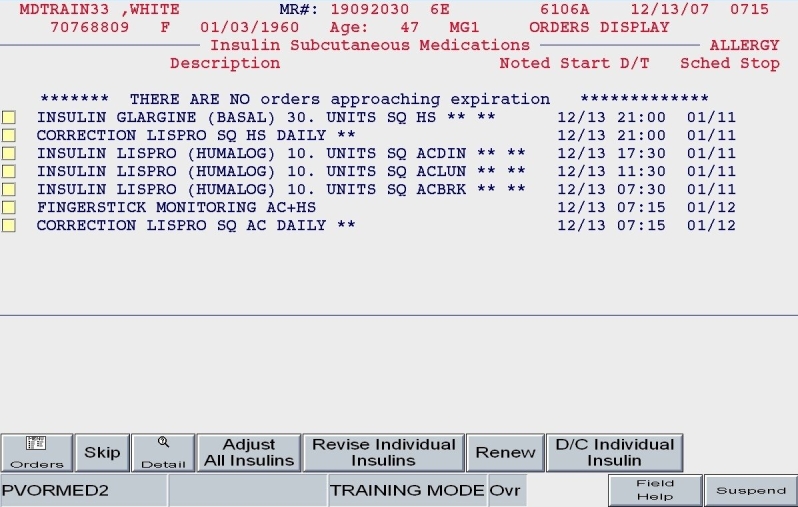
SQIO Version 2: capacity to revise individual insulin or entire regimen.
Caveats to the Protocol-Driven Approach to Insulin Orders
We endorse building the most protocol-driven insulin orders that a medical staff will accept, with the following two caveats. First, there must be extra efforts on the “back end” of the admission to ensure that the discharge regimen is tailored to the clinical and social needs of the patient. Second, a protocol-driven approach is no substitute for a good educational program or for sound clinical judgment. In fact, education should reinforce major concepts driving the protocol and highlight “exceptions to the rule,” subject to clinical expertise.
Conclusion
The use of indication-based ordering in subcutaneous insulin regimens in the hospitalized inpatient allows for improved glycemic control and reduced episodes of hypoglycemia. Our institution validated the use of standardized insulin orders and an insulin protocol before we embarked on implementing protocol-driven insulin orders. The use of user-generated clinical data in the ordering process also allowed for “remembrance” of clinical parameters and embedded logic, assisting in providing best-practice insulin regimens. By remembering prior TDD calculations and patients' body weight, the application also allowed the user to make more informed and rational decisions about regimen adjustments in the face of poor glycemic control. This success has implications not only for hospital medicine practitioners and diabetologists, but for informaticists as well. By use of the tool of constituent logic, the application uses real-time clinical parameters to drive decision making, as opposed to predetermined sets of rules. This is a new paradigm in real-time clinical decision support, as it requires the programming of dynamic ordering experiences rather than static ordering workflows. Developers need to consider this paradigm when planning new ordering algorithms for insulin management and other common hospital-based problems.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CPOE
computerized provider order entry
- NPO
nil per os
- POC
point of care
- SQIO
subcutaneous insulin order set
- TDD
total daily dose
- TP
time period
- UCSDMC
University of California San Diego Medical Center
References
- 1.Pomposelli JJ, Baxter LK, 3rd, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. J Pareneter Enteral Nutr. 1998;22(2):77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 2.Garber AJ, Moghissi ES, Bransome ED, Jr, Clark NG, Clement S, Cobin RH, Furnary AP, Hirsch IB, Levy P, Roberts R, Van den Berghe G, Zamudio V. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):77–82. doi: 10.4158/EP.10.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Society of Hospital Medicine [homepage on the Internet] Society of Hospital Medicine Glycemic Control Task Force: Optimizing glycemic control and reducing hypoglycemia at your medical center. Glycemic Control Quality Improvement Resource Room [cited 2007 Jul 27] Available from: http://www.hospitalmedicine.org.
- 4.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163(12):1409–1416. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 5.Jha AK, Kuperman GJ, Teich JM, Leape L, Shea B, Rittenberg E, Burdick E, Seger DL, Vander Vliet M, Bates DW. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998;5(3):305–314. doi: 10.1136/jamia.1998.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]